ABSTRACT
Vaccination programs against COVID-19 vary globally with estimates of vaccine effectiveness (VE) affected by vaccine type, schedule, strain, outcome, and recipient characteristics. This study assessed VE of BNT162b2 and ChAdOx1 vaccines against PCR positive SARS-CoV-2 infection, hospital admission, and death among adults aged 50 years and older in Wales, UK during the period 7 December 2020 to 18 July 2021, when Alpha, followed by Delta, were the predominant variants. We used individual-level linked routinely collected data within the Secure Anonymized Information Linkage (SAIL) Databank. Data were available for 1,262,689 adults aged 50 years and over; coverage of one dose of any COVID-19 vaccine in this population was 92.6%, with coverage of two doses 90.4%. VE against PCR positive infection at 28-days or more post first dose of any COVID-19 vaccine was 16.0% (95%CI 9.6–22.0), and 42.0% (95%CI 36.5–47.1) seven or more days after a second dose. VE against hospital admission was higher at 72.9% (95%CI 63.6–79.8) 28 days or more post vaccination with one dose of any vaccine type, and 84.9% (95%CI 78.2–89.5) at 7 or more days post two doses. VE for one dose against death was estimated to be 80.9% (95%CI 72.1–86.9). VE against PCR positive infection and hospital admission was higher for BNT162b2 compared to ChAdOx1. In conclusion, vaccine uptake has been high among adults in Wales and VE estimates are encouraging, with two doses providing considerable protection against severe outcomes. Continued roll-out of the vaccination programme within Wales, and globally, is crucial in our fight against COVID-19.
Introduction
As of July 2021 almost every country has introduced a vaccination programme against COVID-19, with variations in coverage.Citation1 There are a number of authorized vaccines currently available with further candidates in development, with consideration for changing SARS-CoV-2 variants and potential booster doses required.Citation2 Early data suggest good effectiveness of the available vaccines against the current circulating variants.Citation3–11 The COVID-19 vaccination program in Wales began on 8 December 2020, at a time when the second wave of the pandemic was reaching its peak locally. Individuals were invited for vaccination, through the NHS Wales electronic vaccination register, in a phased approach according to the priorities advised by the UK Joint Committee on Vaccination and Immunization (JCVI).Citation12 The Pfizer-BioNTech (BNT162b2) vaccination was available first and was the sole vaccine used throughout December 2020, being mainly distributed through mass vaccination centers (MVCs), due to the cold-chain and handling requirements of the vaccine at the time, and the limitations these posed for remote vaccination. The Oxford-AstraZeneca (ChAdOx1) vaccine was used from 4 January 2021 in a variety of settings, including care homes, general practices and (later) community pharmacies, which contributed to rapid increases in coverage among the elderly population including care home residents. The Moderna mRNA-1273 vaccine has been used in a limited way, mainly in one of seven Health Board areas, since 7 April 2021. All three of these vaccinations follow a two dose schedule, which from January 2021 used a dose interval of 8–12 weeks.
The COVID-19 vaccination program has received high levels of acceptance in the Welsh population. As of 18 July 2021, routine surveillance reported that 85.6% of the population aged 18 years and older had received one dose of any COVID-19 vaccine and 72.8% had received two doses.Citation13 Coverage in those aged 50 years of age and older was 93.6% and 91.6% for one and two doses, respectively. As of 18 July 2021, there had been cumulatively 232,672 PCR confirmed episodes in Wales with 5,589 associated deaths.Citation13 The Alpha variant of SARS-CoV-2 was dominant in Wales from December 2020 to end of May 2021, accounting for over 98% of all genomically confirmed and probable cases (n = 12,848).Citation13 Onset of circulation of the Delta variant of SARS-CoV-2 in Wales was first detected in late April 2021 and became the dominant virus type by the beginning of June.
Independently reviewed estimates of vaccine effectiveness (VE) have been published from a relatively small number of large post-implementation studies in Israel, Qatar, Canada, England, and Scotland, assessing effectiveness of different vaccines against a range of outcomes and taking different approaches to analysis.Citation3,Citation5–8 Large population based studies are important in strengthening the evidence for ongoing vaccination programmes and building capacity for ongoing monitoring of ‘real-world’ COVID-19 VE in different populations. The aim of this study was to provide estimates of VE of BNT162b2 and ChAdOx1 against PCR positive SARS-CoV-2 infection, hospital admission due to SARS-CoV-2 infection and death amongst adults aged 50 years and older in Wales.
Materials and methods
Analysis were carried out using individual-level linked routinely collected national-scale data available in the Secure Anonymized Information Linkage (SAIL) Databank, hosted by Swansea University.Citation14–16 The study population was all individuals aged 50 years and over, alive and resident in Wales as at 7 December 2020 and part of the SAIL Databank Con-COV e-cohort.Citation17 The population was identified on the basis of those registered for National Health Service (NHS) care in Wales on the Welsh Demographic Service Dataset (WDSD) within SAIL, which includes information on Lower-layer Super Output Area (LSOA) small geography of residence, date of birth, and sex. As care homes are prone to outbreaks and residents are subject to ongoing frequent virology testing in Wales, those identified as living at a residential care home address were excluded from this analysis to limit bias. Healthcare workers were included in the study population, but occupational status was controlled for. Although this population may be at increased risk of COVID-19 due to increased exposure, they are a fairly large cohort to exclude (n = 75,324) and level of risk may differ within this group and be challenging to define. Other occupational groups may be at comparable risk of infection to healthcare workers and may also be subject to increased testing.Citation18 Vaccination date and vaccine type were recorded in the COVID-19 Vaccination Data (CVVD) data, which originates from the all Wales Immunization System (WIS) population vaccination register for COVID-19. A second dose was considered valid when given at least 21 days after the first, doses with shorter time intervals were discarded and there was no upper exclusion limit to this interval.
The study population was described, and the odds of being vaccinated based on a number of characteristics, as at 18 July 2021 was calculated using univariate logistic regression.
Using a retrospective cohort study design, hazard ratios (HR) were calculated using an extended Cox regression model with vaccination status introduced as a time varying covariate. VE was calculated based on the HR, with VE = 1-HR. The baseline for all estimates is the unvaccinated population. Individuals entered the study at time zero (7 December 2020) and moved through categories based on the time since vaccination in 7-day intervals. In this design, individuals contribute unvaccinated time until the end of the observation period or point they are vaccinated. Individuals were censored if they moved out of Wales, died, reached the end of follow up (18th July 2021) or had the outcome of interest. VE was assessed against three outcomes: i) PCR positive infection, defined as any PCR positive SARS-CoV-2 test recorded in the Pathology COVID-19 Daily data (PATD) allowing for 90 days between episodes for those with potential repeat infection ii) Hospital admission due to SARS-CoV-2 infection, defined as any hospital admission recorded in the Patient Episode Dataset for Wales (PEDW) where the individual had a PCR positive test in the 28-days prior to admission, on the day of admission or the day after admission and COVID-19 was listed as the primary cause for admission, and iii) Death with COVID-19 recorded as an underlying cause on the death certificate, where the individual had a PCR positive SARS-CoV-2 test in the 28-days prior.
The unadjusted Cox regression models included vaccination status and variables associated with priority status, and therefore when someone would have had the opportunity to be vaccinated: age as at 31st March 2021 as a restricted cubic spline, individual identified as clinically extremely vulnerable (CEV) based on shielding list status and health and care worker status.Citation19 In the fully adjusted model, a number of additional variables were included: any previous PCR positive SARS-CoV-2 test prior to the cohort start date, number of SARS-CoV-2 PCR tests prior to the cohort start, QCOVID score, Health Board of residence, sex, ethnic group, deprivation quintile (a measure for deprivation for small areas in Wales), and urban/rural location of residence.Citation20 Adjustment was also made for previous vaccination against shingles, previous vaccination against pneumococcal polysaccharide, vaccination against influenza between 1 October 2020 and 31 March 2021 and number of days with a General Practitioner (GP) consultation recorded in the year prior to 1 February 2020, prior to the pandemic reaching Wales. As part of a sensitivity analysis, VE calculations also including inverse propensity score weighting (IPW).Citation21 All variables included in the adjusted model were included in the calculation of propensity score to be vaccinated with at least one dose of COVID-19 vaccine.
Health Board of residence, identification of care home residents (based on address) and health and care worker status (based on provision of occupational records from employing organizations) were as recorded in the CVVD. Level of deprivation was assigned at ecological level by linking LSOA of residence to the Welsh Index of Multiple Deprivation (WIMD) 2019, LSOA’s were then ranked according to overall WIMD score and divided into quintiles.Citation22 Urban/Rural location was assigned by linking LSOA of residence to the 2011 census rural urban classification data provided by the Office for National Statistics (ONS).Citation23 Shielding status for individuals was sourced from a central register of COVID-19 Shielding Persons (CVSP) who were advised to isolate due to high clinical risk, maintained by Digital Health and Care Wales (DHCW). Adjustment for important comorbidities was done using the QCOVID algorithm, which predicts the risk of being admitted to hospital or dying from COVID-19 in adults.Citation20,Citation24 Ethnicity data were sourced from 20 electronic health record (EHR) and administrative data sources, including the ONS Census, and collated into the five minority ethnic categories (White, Asian, Black, Mixed, and Other)).Citation25
Previous vaccination history, for pneumococcal, shingles, and influenza vaccination was assessed using Read coded primary care events data as part of the Wales Longitudinal General Practice (WLGP) data and included in the model as separate binary variables. WLGP includes data from ~80% of practices in Wales and was also used to determine the number of days with a GP consultation recorded in the year prior to the pandemic as a proxy for GP visits. These adjustments were made to account for differences in healthcare seeking behavior between the vaccinated and unvaccinated individuals. Where data were missing an unknown category was assigned.
SARS-CoV-2 PCR test data were taken from PATD which were obtained from Public Health Wales (PHW) Datastore and include all test data from NHS hospital laboratories (mainly hospital samples, with some community and other settings) and COVID-19 Lighthouse Laboratories (mainly community samples, with some other non-hospital setting). Hospital admissions were identified using PEDW where a primary cause for admission was recorded as COVID-19 (coded as ICD-10 U07.1 or U07.2) and linked to PATD to identify individuals who had a positive PCR test within the defined time period. The earliest of hospital admission date and positive PCR test sample date was used as the end point for follow up as this will be the closest to when the individual was first infected. COVID-19-related deaths were identified using the Consolidated Death Data Source (CDDS) held in SAIL (a consolidation of records from the Master Patient Index, the Office for National Statistics (ONS) Annual District Death Extract (ADDE) and the WDSD sources of all mortality records), linked to PATD.
Due to small numbers, estimates against death are presented for effectiveness of any COVID-19 vaccine type, whilst VE of one and two doses of vaccine against PCR confirmed infection and admission due to SARS-CoV-2 infection is additionally stratified by vaccine type.
All analysis were carried out using R version 4.0.4.
Results
There were 1,262,689 adults aged 50 years and over eligible for inclusion in the study, after excluding care home residents (n = 16,062) and individuals who had a vaccination other than BNT162b2 or ChAdOx1 (n = 5,406) or a mixed vaccine course (n = 551). As at 18 July 2021, coverage of one dose of any COVID-19 vaccine in this population was 92.6%, with coverage of two doses 90.4%. Over the course of the follow-up period 29.0% (n = 331,064) of those who received two doses, had received BNT162b2 and 71.0% (n = 810,771) had received ChAdOx1. In total, 36.8% (n = 464,455) were aged 70 years or over (). Over half (51.5%, n = 650,835) were female, with the odds of being vaccinated with a full two dose course 1.40 (95% CI 1.38–1.42) times higher compared to males (). Coverage varied by Health Board of residence, and coverage of a full two dose course was lowest in the most deprived areas (87.2%) compared to the least deprived areas (92.9%) and urban areas (92.4%) compared to rural areas (93.0%) (). The odds of being vaccinated with a complete two dose course for a person in a Black ethnic group was 0.21 (95% CI 0.20–0.23) compared to those in a White ethnic group ().
Table 1. Summary of study the study population used in the estimation of BNT162b2 and ChAdOx1 vaccine effectiveness and odds of being vaccinated based on select characteristics, Wales UK
Health and care workers (n = 75,324), and those on the list of individuals advised to shield due to increased clinical risk from COVID-19 (n = 94,531), were also more likely to be vaccinated ().
Additionally, those who had a record of receiving a herpes zoster, pneumococcal (PPV) or seasonal influenza vaccine in 2019/20 were more likely to be fully vaccinated as at 18 July 2021 than those who did not, as were those with a higher QCOVID score (). Overall, those who consulted their GP more frequently in the year prior to the pandemic were more likely to be vaccinated compared to those who did not. Fifteen percent (n = 193,039) of the study cohort had at least one SARS-CoV-2 PCR test prior to the cohort start date on 7th December 2020 and overall, the more SARS-CoV-2 PCR tests individuals had the more likely they were vaccinated with two doses of vaccine (). Of these, 26,778 (2.1%) individuals had a positive result prior to the cohort start date. Those with previous PCR confirmed infection, were more likely to be fully vaccinated (OR 1.18, 95% CI 1.13–1.23) ().
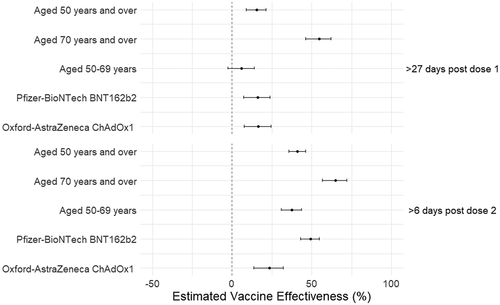
Over the course of follow up, 7 December 2020 to 18 July 2021, there were 38,163 individuals with SARS-CoV-2 PCR positive tests in the study population, 9,876 of which were in those aged 70 years and over (36.3 events per 1000 person years follow up) and 28,287 in those aged 50–69 years (60.8 events per 1000 person years follow-up) (, Supplementary Table S1). Adjusted estimates showed significant VE against SARS-CoV-2 PCR positive infection at 28-days or more post first dose of any COVID-19 vaccine to be 16.0% (95%CI 9.6–22.0) with effectiveness 7 or more days after a second dose 42.0% (95%CI 36.5–47.1) (). VE was higher in those aged 70 years and over (66.0%, 95%CI 57.8–72.6) compared to those aged 50 to 69 years (38.4%, 95%CI 31.8–44.4) (, Supplementary Table S1). A difference was also seen by vaccine type with VE for those who received two doses of BNT162b2 higher than those who received ChAdOx1, 50.1% (95%CI 44.0–55.5) vs. 24.9% (95% CI 15.4–33.3) (, Supplementary Table S1).
Table 2. Cox-regression estimates of outcomes from COVID-19 infection following vaccination with BNT162b2 or ChAdOx1 in those aged 50 years and over, Wales UK
Figure 1. Vaccine effectiveness estimates of COVID-19 vaccination with BNT162b2 or ChAdOx1 against PCR positive SARS-CoV-2 infection in those aged 50 years and over, Wales UK.1
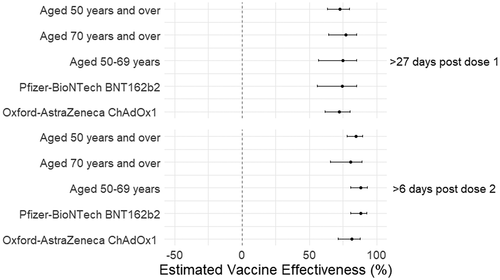
VE against hospital admission was higher at 72.9% (95%CI 63.6–79.8) 28 days post vaccination with one dose of any vaccine type and 84.9% (95%CI 78.2–89.5) at 7 or more days post two doses (). Estimates after two doses were lower in those aged 70 years and over (80.6% 95% CI 65.6–89.1) compared to those aged 50 to 69 years (88.4% 95% CI 80.9–93.0) (, Supplementary Table S2). VE in those who received two doses of BNT162b2 (88.2% 95% CI 80.6–92.8) was higher compared to ChAdOx1 (81.4% 95% CI 71.5–87.9) (, Supplementary Table S2). Neither differences were statistically significant.
Figure 2. Vaccine effectiveness estimates of COVID-19 vaccination with BNT162b2 or ChAdOx1 against hospitalization due to SARS-CoV-2 infection in those aged 50 years and over, Wales UK.1.
Estimates from models including inverse propensity weighting were higher at all-time points post vaccination, although estimates for 7 or more days after dose two were similar.
VE for one dose of any vaccine type against death with COVID-19, where COVID-19 was mentioned as a cause or contributing factor, was estimated to be 80.9% (95%CI 72.1–86.9).
Fewer than 10 individuals who had two doses of COVID-19 vaccine died between 7 December 2020 and 18 July 2021; therefore, VE estimates for two doses could not be produced due to potential disclosure of identity.
Discussion
The overall VE estimate 7 or more days post dose two of any vaccine type against SARS-CoV-2 PCR positive infection, in this large cohort study of the Welsh population was 42%. This estimate is lower compared to other published studies, which mainly include estimates for BNT162b2, whilst two thirds of this study population had received ChAdOx1.Citation3,Citation4,Citation11 Estimates against SARS-CoV-2 PCR positive infection were lower for ChAdOx1 compared to BNT162b2, as has been seen elsewhere.Citation9,Citation10
However, estimates of 85% against hospital admission due to SARS-CoV-2 PCR infection 7 or more days post dose two are encouraging, and in line with estimates from other studies. Haas EJ et al.Citation3 saw 97.2% (96.8–97.5) effectiveness of two doses of Pfizer-BioNTech BNT162b2 vaccine against COVID-19-related hospitalization 7 days after the second dose and data from Qatar 97.4% (92.2–99.5) for BNT162b2 against severe, critical or fatal disease 14-days after the second dose.Citation6 Interim data from a large population cohort in Scotland estimated 91% (95% CI 85–94) effectiveness of one dose BNT162b2 against hospitalization at 28 to 34 days post vaccination and 88% (95% CI 75–94) effectiveness of ChAdOx1 against the same outcome.Citation5
Due to small numbers of COVID-19 related deaths in the study population, it was not possible to obtain estimates of VE against a complete two dose course at this time; however, one dose was estimated to reduce deaths by 81%.
Follow-up in this cohort started on 7 December 2020 when the vaccination program began in Wales. At this time Wales was reaching the peak in a second outbreak wave where the dominant variant was Alpha.Citation13 This adds complexities in interpreting the impact of the vaccine compared to other factors that changed over time, including changes in infection prevalence and restrictions that were implemented to control the second wave.Citation26 By the end of March 2021, cases returned to low levels and remained low until the beginning of June 2021 when cases started to increase and Delta was the dominant variant.
The estimates in this study can be considered to be mainly against the Alpha variant and provide a baseline for how the vaccination is working over time, and can be useful in comparing VE in the face of new variants of SARS-CoV-2 arising. A systematic long-term approach to surveillance of VE is important due to changing variants, the large number of vaccine candidates and proposed schedules and booster doses. Evidence is still emerging, but recent studies from England and Scotland suggest effectiveness against sequenced symptomatic cases is slightly reduced for Delta compared to Alpha after two doses of BNT162b2 or ChAdOx1.Citation9,Citation10 Early data from Canada suggest similar VE following two doses of BNT162b2 when comparing Delta and Alpha, and similar VE for one dose against hospitalization or death.Citation8 It is too early to produce robust variant-specific estimates for Wales but the Delta dominant third wave does not appear to have resulted in a large increase in hospital admissions, as with waves 1 and 2 and mortality due to COVID-19 infection also appears lower than the previous two waves.Citation13 At time of writing, confirmed case incidence is consistently lower in the most highly vaccinated age-groups. Early evidence suggests VE against confirmed COVID-19 for BNT162b2 vaccination may decrease by more than ten percentage points 4 months post second dose.Citation27
Comparing estimates from different studies using different definitions and statistical methods should be done with caution due to differences in surveillance systems and study populations, schedule, strain, outcome, and recipient characteristics. Whist direct comparison of our VE estimates against those reported from other studies is difficult due to differences in approach and the samples used, we found that in general our VE estimates against positive PCR were lower but our estimates against hospitalization were similar.
Vaccine effectiveness against hospital admission was seen 0–6 days post dose 1. This apparent immediate effectiveness post vaccination may be explained by bias in those who are unwell or having received a positive test, not being vaccinated in line with policy for attending appointments. These individuals would then be more likely to be admitted to hospital due to a COVID-19-related illness than people who were well enough to be vaccinated.
Symptom information was unavailable for this study, and when looking at the outcome of SARS-CoV-2 PCR positive infection, data may include those with asymptomatic infection identified through community screening activities, and enhanced case finding in other closed settings, outbreaks and incidents. This is likely to lead to under estimates of COVID-19 VE. In this study, estimates for hospital admission with COVID-19 may also be underestimated, given the age of the cohort, although COVD-19 may have been listed as the primary cause for admission, reasons for admission can be complex and co-morbidities are likely. The addition of misclassified outcomes (hospital admissions due to reasons other than testing positive for COVID-19) to both vaccinated and unvaccinated groups will tend to lead to an underestimation of vaccine effectiveness. Conversely, 47% of all admissions that could be linked to a SARS-CoV-2 PCR positive test were not included in this analysis, as COVID-19 was not listed as the primary reason for admission, these too could be miscoded.
Due to the rapid roll out of the vaccine program and high coverage, the individuals who remain unvaccinated are likely to have different characteristics or be less engaged with healthcare services compared to those who have received the vaccine, and if there is lower access to health care in the unvaccinated, case ascertainment is also likely to be lower, resulting in under-estimation of VE. We have aimed to limit the impact of healthcare access bias by adjusting for number of GP consultations and previous vaccination history for this population. However, there is currently disruption in healthcare-seeking patterns, it is not known whether propensity to consult with a GP will be as good at predicting likelihood of accessing health care in the current context.
Propensity scores are commonly used in observational studies where ‘gold standard’ randomization of exposed and unexposed individuals is not possible.Citation28 Assigning propensity weights based on odds of being vaccinated with one dose as at 17 June 2021, when coverage was high produced large weights, which can cause estimation problems.Citation29 To account for this, weights can be trimmed to remove extreme values, however, this method lacks a clear framework and can be variably applied.Citation30 A sensitivity analysis in this study produced a range of estimates (data not shown), when applying different IPW trimming methods; therefore, these extreme estimates produced from the IPW model are potentially over adjusting and should be interpreted with caution, Acknowledging this, the adjusted model without propensity weighting is potentially underestimating VE.
The main limitation to this study was being unable to obtain further information on severity of illness. Having symptom information available to determine VE against asymptomatic and symptomatic infection would be beneficial. The proportion of missing data for the GP-derived variables used to control for propensity to consult (approximately 20%) may effect VE estimates, although sensitivity analysis shows this is minimal. Due to small population sizes, broad ethnic grouping was used in this analysis. As ethnicity is strongly associated with vaccination uptake, being able to use more refined categories to identify intra-group variation may have been beneficial.
These analyses included a diverse population and therefore further analysis for sub-groups, such as healthcare workers who have higher exposure or care home residents who may have higher transmission rates, is under way. In this study, the exclusion of care home residents may cause a bias to lower death rate in older age groups who are infected.
In conclusion, vaccine uptake has been high amongst adults in Wales and VE estimates are encouraging, with two doses providing considerable protection against severe infection. Continued roll-out of the vaccination program within Wales, and more globally, and ensuring people complete the two dose course, is crucial in our fight against COVID-19. Continued evaluation of effectiveness is important to assess issue such as waning and the impact of new variants.
Authors’ contributions
MP led the conception and design of this work with input from LG, SC, MG, SB, CW and JS. MP performed the analysis, JL created the underlying QCOVID dataset and SB provided the code for the imputation, AA completed the linkage and analysis to create the ethnic group variable. MP drafted the first iteration of the manuscript. All authors critically reviewed the manuscript, provided important intellectual input, approved the final version and agreed to be accountable for their contributions.
Availability of data and materials
The data used in this study are available in the SAIL Databank at Swansea University, Swansea, UK, but as restrictions apply they are not publicly available. All proposals to use SAIL data are subject to review by an independent Information Governance Review Panel (IGRP). Before any data can be accessed, approval must be given by the IGRP. The IGRP gives careful consideration to each project to ensure proper and appropriate use of SAIL data. When access has been granted, it is gained through a privacy protecting safe haven and remote access system referred to as the SAIL Gateway. SAIL has established an application process to be followed by anyone who would like to access data via SAIL at https://www.saildatabank.com/application-process
Supplemental Material
Download MS Excel (26.1 KB)Acknowledgments
This work was led by MP as part of her PhD at Swansea University contributing to the enhanced COVID-19 vaccination surveillance activities led by Public Health Wales Vaccine Preventable Disease Programme (VPDP) and Communicable Disease Surveillance Centre (CDSC). Authors of this report would like to thank to the DHCW WIS, Data Warehouse and IS teams, the WIS Data Quality Group and all those carrying out data entry for vaccinations into WIS and associated QA work.
We wish to acknowledge the collaborative partnership that enabled acquisition and access to the de-identified data, which contributed to this analysis. The collaboration was led by the Swansea University Health Data Research UK team under the direction of the Welsh Government Technical Advisory Cell (TAC) and includes the following groups and organisations: the Secure Anonymised Information Linkage (SAIL) Databank, Administrative Data Research (ADR) Wales, NHS Wales Informatics Service (NWIS), Public Health Wales, NHS Shared Services Partnership and the Welsh Ambulance Service Trust (WAST). All research conducted has been completed under the permission and approval of the SAIL-independent Information Governance Review Panel (IGRP) project number 0911. The team at Swansea University who support and maintain the One Wales collaboration, Con-COV project and associated COVID-19 e-cohorts and data include Ashley Akbari, Gareth Davies, Rowena Griffiths, Jane Lyons, Ronan Lyons, Laura North, and Fatemeh Torabi.
Disclosure statement
MP, AA, SC, RR, RAL and LG sit on the Wales COVID-19 Vaccination Board (CVB) and/or subgroups of the CVB. SC, CW and RAL are members of the Welsh Government COVID-19 Technical Advisory Group.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2031774
Additional information
Funding
References
- World Health Organisation. WHO Coronavirus (COVID-19) dashboard. Geneva. 2021 Sep 12 [accessed 2021 Sep 13]. https://covid19.who.int/ .
- World Health Organisation. COVID-19 vaccine tracker and landscape. Geneva. 2021 Sep 7 [accessed 2021 Sep 13]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–10. doi:10.1016/S0140-6736(21)00947-8 .
- Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. doi:10.1016/S0140-6736(21)00790-X .
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. doi:10.1016/S0140-6736(21)00677-2 .
- Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–89. doi:10.1056/NEJMc2104974 .
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi:10.1136/bmj.n1088 .
- Nasreen S, He S, Chung H, Brown K, Gubbay JB, Buchan SA, Wilson SE, Sundaram ME, Fell DB, and Chen B, et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv Prepr 2021. doi:10.1101/2021.06.28.21259420 .
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier R, Groves N, Dabera G, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–94. doi:10.1056/NEJMoa2108891 .
- Sheikh A, McMenamin J, Taylor B, Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–62. doi:10.1016/S0140-6736(21)01358-1 .
- Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, Blane B, Bonsall D, Cicconi P, Charlton S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351–62. doi:10.1016/S0140-6736(21)00628-0 .
- Public Health England. COVID-19 – SARS-CoV-2: the green book chapter 14a. London. 2021 [accessed 2021 Sep 13]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/978508/Green_book_chapter_16April2021.pdf .
- Public Health Wales. Rapid COVID-19 surveillance. Cardiff. 2021 Sep 12 [accessed 2021 Sep 13]. https://public.tableau.com/profile/public.health.wales.health.protection#!/vizhome/RapidCOVID-19virology-Public .
- Jones KH, Ford DV, Thompson S, Lyons RA. A profile of the SAIL databank on the UK secure research platform. Int J Popul Data Sci. 2019;4(2):1134. doi:10.23889/ijpds.v4i2.1134 .
- Lyons RA, Jones KH, John G, Brooks CJ, Verplancke JP, Ford DV, Brown G, Leake K. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. 2009;9:3. doi:10.1186/1472-6947-9-3 .
- Ford DV, Jones KH, Verplancke JP, Lyons RA, John G, Brown G, Brooks CJ, Thompson S, Bodger O, Couch T, et al. The SAIL databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009;9:157. doi:10.1186/1472-6963-9-157 .
- Lyons J, Akbari A, Torabi F, Davies GI, North L, Griffiths R, Bailey R, Hollinghusrt J, Fry R, Turner SL, et al. Understanding and responding to COVID-19 in Wales: protocol for a privacy-protecting data platform for enhanced epidemiology and evaluation of interventions. BMJ Open. 2020;10(10):e043010. doi:10.1136/bmjopen-2020-043010 .
- Office for National Statistics. Coronavirus (COVID-19) infection survey: characteristics of people testing positive for COVID-19 in England, 22 February 2021. Newport 2021 Feb 22 [accessed 2021 Nov 11]. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/characteristicsofpeopletestingpositiveforcovid19inengland
- National Health Service. Who is at high risk from coronavirus (clinically extremely vulnerable). London. 2021 Sep 2 [accessed 2021 Sep 13]. https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus-clinically-extremely-vulnerable .
- Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, Hayward A, Hemingway H, Horby P, Mehta N, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi:10.1136/bmj.m3731 .
- Olmos A, Govindasamy, and Priyalatha P. A practical guide for using propensity score weighting in R. Pract Assess Res Eval. 2015;20(13).http://www.math.umd.edu/~slud/s818M-MissingData/PropensityScoreWeightingR.pdf
- Welsh Government. Welsh index of multiple deprivation 2019. Cardiff. 2020 Oct 1 [accessed 2021 Sep 13]. http://gov.wales/statistics-and-research/welsh-index-multiple-deprivation/?lang=en .
- Office for National Statistics. 2011 rural/urban classification. Newport. 2016 Jan 27 [accessed 2021 Sep 13]. https://www.ons.gov.uk/methodology/geography/geographicalproducts/ruralurbanclassifications/2011ruralurbanclassification .
- Nafilyan V, Humberstone B, Mehta N, Diamond I, Coupland C, Lorenzi L, Pawelek P, Schofield R, Morgan J, Brown P, et al. An external validation of THE QCovid risk prediction algorithm for risk of mortality FROM COVID-19 in adults: a NATIONAL Validation cohort study in England. Lancet Digital Health. 2021;3(7):e425–e433. doi:10.1016/S2589-7500(21)00080-7 .
- Government Statistical Service. Harmonised concepts and questions for social data sources: ethnic group. Newport UK: Office for National Statistics; 2015 [accessed 2021 Sep 13]. https://gss.civilservice.gov.uk/wp-content/uploads/2016/03/P3-Ethnic-Group-June-16-1.pdf .
- Welsh Parliament. Coronavirus timeline: welsh and UK governments’ response. Cardiff. 2021 May 27 [accessed 2021 Sep 13]. https://research.senedd.wales/research-articles/coronavirus-timeline-welsh-and-uk-governments-response/ .
- Thomas SJ, Moreira JED, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, and Zerbini C, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. medRxiv Prepr 2021. doi:10.1101/2021.07.28.21261159 .
- Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther. 2017;98:76–90. doi:10.1016/j.brat.2017.01.005 .
- Lanza ST, Moore JE, Butera NM. Drawing causal inferences using propensity scores: a practical guide for community psychologists. Am J Community Psychol. 2013;52(3–4):380–92. doi:10.1007/s10464-013-9604-4 .
- Potter FJ. The effect of weight trimming on nonlinear survey estimates. Proceedings of the Section on Survey Research Methods of American Statistical Association; San Francisco, CA: American Statistical Association; 1993 .
