ABSTRACT
Metastatic uveal melanoma (UM) is a poor prognosis malignancy. Immunotherapy is commonly employed, despite the low activity, considering the lack of other effective systemic treatments. In this study, the prognostic and predictive role of soluble immune checkpoints and inflammatory cytokines/chemokines in 22 metastatic UM patients was evaluated. Baseline levels of these molecules were assessed, as well as their changes during anti-PD-1 therapy. The correlation between soluble immune checkpoints/cytokines/chemokines and survival was analyzed. A comparison between circulating immune profile of metastatic cutaneous melanoma (CM), for which immunotherapy is a mainstay of treatment, and UM during anti-PD-1 therapy was also performed. Three immune molecules resulted significantly higher in metastatic UM patients with survival <6 months versus patients with survival ≥6 months: IL-8, HVEM and IDO activity. Considering these three molecules, we obtained a baseline score able to predict patients’ survival. The same three molecules, together with soluble(s) CD137, sGITR and sCD27, resulted significantly lower in patients with survival >30 months. We also observed an increase of sCD137, sCD28, sPD-1, sPD-L2 sLAG3, sCD80 and sTim3 during anti-PD-1 treatment, as well as IDO activity, IP-10 and CCL2. Several of these molecules were significantly higher in UM compared to CM patients during anti-PD-1 therapy. The analysis of circulating immune molecules allows to identify patients with poor prognosis despite immunotherapy and patients with long survival treated with an anti-PD-1 agent. The different serum concentration of these molecules during anti-PD-1 therapy between UM and CM reflects the different efficacy of immune checkpoint inhibitors.
Introduction
Uveal melanoma (UM) represents the most common tumor with origin in the eye and is a rare malignancy, with an incidence of 4.9 cases per million.Citation1 Despite the radical treatment of primary tumor, metastatic spread often occurs.Citation2 The first and most frequent site of metastases is the liver.Citation3 Hepatic involvement, which accounts for about 90% of metastatic disease,Citation4 is usually characterized by multifocal metastases. Therefore, surgical resection of hepatic metastases is not possible for the majority of the patients.Citation5 Survival for patients with metastatic disease is limited. The systemic treatments commonly used are the same tested in clinical trials for cutaneous melanoma (CM)Citation6despite the different clinical and biological features of these tumors.
Chemotherapy and target therapies have been employed with poor results.Citation7–11 To date, immune checkpoint inhibitors (ICIs) are used for the treatment of metastatic uveal melanoma (mUM).Citation6
Ipilimumab showed a modest activity, with a survival ranging from 6.8 to 9 months.Citation12,Citation13 In pre-treated patients, pembrolizumab, nivolumab and atezolizumab demonstrated a progression-free survival (PFS) of about 3 months.Citation14,Citation15 Pembrolizumab showed a limited efficacy with a PFS of 3.8 months in first-line setting, as demonstrated by a prospective observational study.Citation16 The association of nivolumab and ipilimumab allowed an overall survival (OS) of 12.7 months and a PFS of 3 months.Citation17
In contrast to CM, UM is unresponsive to checkpoint inhibitors in the majority of the casesCitation18 and the reasons of the poor response remain speculative. The low activity of the ICIs can be explained by the ability of UM cells to elude immunity, upregulating and expressing immunosuppressive molecules.Citation19,Citation20 Moreover, the eye is considered an immune-privileged site with own immunosuppressive mechanisms. UM cells are able to escape from systemic immune surveillance also in the liver.Citation21 In addition, the low mutational burden of this type of melanoma can be responsible for the poor results obtained with immunotherapy until now.Citation22
Understanding the immune status of UM patients is essential to identify biomarkers useful for selecting patients who could benefit more from immunotherapy.Citation23,Citation24
Interestingly, recent studies indicate that soluble isoforms of immune checkpoint (IC) receptors, released in the serum of patients, are centrally involved in immune regulation and associated with clinical outcomes.Citation25,Citation26 The origin of the soluble receptors has not been completely elucidated. It has been reported that they can be produced by a proteolytic cleavage of membrane bound, by an alternative splicing of mRNA, or released with exosomes or microvesicles.Citation27,Citation28 It was demonstrated that high serum levels of several IC molecules correlate with resistance to immunotherapy in melanoma patients.Citation29
Cytokines and chemokines also play a key role during immunotherapy. For example, an upregulation of 11 cytokines was observed in melanoma patients treated with an anti-PD-1 alone or in combination with an anti-CTLA-4 who developed high grade immune-related adverse events.Citation30
Here, we have studied for the first time the circulating immune profile of UM patients in order to evaluate their immunological status and investigate the role of soluble immune molecules in patients during anti-PD-1 treatment.
Patients and methods
Patients enrollments and samples collection
We considered 22 patients with metastatic UM referred to the Oncology Unit of Fondazione Policlinico Universitario Agostino Gemelli IRCCS. Patients older than 18 years, with measurable unresectable metastatic disease, received pembrolizumab as first-line therapy, administered intravenously at a dose of 2 mg/kg every 3 weeks or 200 mg flat dose every 3 weeks (when the flat dose has been introduced) until disease progression, unacceptable toxicity or consent withdrawn. Toxicity was reported according to the Common Terminology Criteria for Adverse Events (CTCAE v. 5.0). Radiological and clinical assessments were performed according to good clinical practice. Progression-free survival, response rate, clinical benefit, OS and tolerability were evaluated. Responses were assessed in accordance with the RECIST criteria 1.1. PFS was calculated from the first day of treatment to progression or death for any reason. Survival was defined as the interval from the first detection of metastases to death for any cause.
A group of 11 BRAF wild-type metastatic cutaneous melanoma (mCM) patients treated in first-line setting with an anti-PD-1 agent were also evaluated for the comparison with mUM patients. Patients with stable brain metastases (no neurological symptoms, no radiologic evidence of progression, no steroid requirement) could be considered.
Peripheral blood samples were drawn from all patients into a tube without anticoagulant and left at room temperature to allow blood to clot. Later, samples were centrifugated to collect serum that was stored and frozen at −80°C until use. In a group of metastatic UM patients, samples were collected before treatment with pembrolizumab (T0) and after three cycles (>T0). Samples from a group of CM patients, during first-line anti-PD-1 therapy, were also collected. The study was conducted in accordance with the Helsinki declaration of 1975 and was approved by the local ethics committee. Informed consent was obtained from all the subjects involved in the study.
Detection of soluble molecules in serum
Sera from uveal and CM patients were assayed to evaluate the levels of cytokines and soluble immune checkpoint molecules (sICs) by multiplex immunoassay analysis using the ProcartaPlex Human Inflammation Panel (20 Plex, catalog number EPX200-12185-901; sE-Selectin; GM-CSF; ICAM-1/CD54; IFN alpha; IFN gamma; IL-1 alpha; IL-1 beta; IL-4; IL-6; IL-8; IL-10; IL-12p70; IL-13; IL-17A/CTLA-8; IP-10/CXCL10; MCP-1/CCL2; MIP-1alpha/CCL3; MIP-1 beta/CCL4; sP-Selectin; TNF alpha) (eBioscence) and the Human Immuno-Oncology Checkpoint 14-plex ProcartaPlex Panel 1 (catalog number EPX14A-15803-901; BTLA; GITR; HVEM; IDO; LAG-3: 47; PD-1; PD-L1; PD-L2; TIM-3; CD28; CD80; CD137; CD27; CD152) (eBioscence). Assay was conducted using 50 µl of serum for each sample and adding it in a 96-well plate with a mixture of color-coded magnetic beads coated with antibody that recognize specific analytes. Later, biotinylated detection antibodies that bind analytes of interest were added and then bound to Phycoerythrin-conjugated streptavidin that through its signal intensity allow to detect the analyte concentration. Samples were measured using Luminex 200 platform (BioPlex, Bio-Rad) and data, expressed in pg/ml of protein, were analyzed using Bio-Plex Manager Software.
Trp/kyn ratio analysis
Serum levels of tryptophan (trp) and kynurenine (kyn) were evaluated through modified liquid chromatography – tandem mass spectrometry method.
Samples were deproteinized using 50 μl of TCA 4% aqueous solution and following centrifuged at 14,000 rpm for 15 min. Supernatants were injected into chromatographic system to perform separation using an Agilent Liquid Chromatography System series 1100 (Agilent Technologies, USA), on a biphenyl column (100 × 2.1 mm, Kinetex 2.6 μm Biphenyl, 100 Å, Phenomenex, CA, USA) equipped with a security guard precolumn (Phenomenex, Torrance, CA, USA). Gradient elution was performed with a flow rate of 400 μl/min and mobile phases consisted of 0.1% aqueous formic acid and 100% acetonitrile.
The mass spectrometry method was performed on a 3200 triple quadrupole system (Applied Biosystems, Foster City, CA, USA), equipped with a Turbo Ion Spray source. The detector was set in the positive ion mode. The instrument was set in the Multiple Reaction Monitoring mode. Data were acquired and processed by the Analyst 1.5.1 Software.
Statistical analysis
PFS and OS were calculated using Kaplan–Meier method.
Summary data were expressed as average and standard error of mean.
Two-tailed nonparametric statistical tests were used to compare different groups. Wilcoxon matched-pairs signed rank test was used to analyze mUM group and compare T0 to >T0.
mUM patients were divided into three groups according to survival: fast progressors (FP), slow progressors (SP) and long survivors (LS); one-way Anova test was used to compare these populations. Comparison between mUM and mCM was performed using unpaired Mann–Whitney test. All the results were considered significant when p value was <0.05.
The level of all the studied molecules was described by calculating mean and standard deviation (SD), and median and interquartile range (IQR). The values were compared between patients with a survival <6 months or ≥6 months using the Mann–Whitney test because the data were not normally distributed. The molecules for which the comparison showed a p value < .05 or for which there was a particular clinical interest (considering a p value not higher than 0.2) were investigated with the objective to identify the better cutoff for distinguishing long survivors or no long survivors and a Receiver Operating Characteristic (ROC) analysis was performed. A score was finally defined by assigning a value of 1 to each molecule associated with the worst prognosis cased at the cut-off. Consequently, the overall score was obtained by summing the single molecule score with the higher value corresponding to the worst prognosis.
Finally, a Spearman correlation was performed to correlate the value of the score with the survival time in months.
Results
Patients’ characteristics
A total of 22 UM patients were evaluated. Patients’ characteristics are summarized in . A group of 11 advanced BRAF wild-type CM patients treated with an anti-PD-1 agent (3 patients treated with pembrolizumab, 8 patients with nivolumab) in first-line setting was also considered.
Table 1. Patients’ characteristics
Among the metastatic UM patients, the median age was 67.9 years (range 54–87). Eleven subjects were male and 11 female. Liver metastases were found in 21 patients. None of the patients had BRAF mutation. Ocular enucleation was previously performed for the treatment of primary tumor in 16 patients, while 6 patients never underwent enucleation. Local treatment of liver metastases (metastasectomy) was previously carried out in two patients. One of them developed a non-resectable metastatic disease, while the other patient was free from disease recurrence at the time of data analysis.
All 20 patients with non-resectable metastatic disease underwent anti-PD-1 treatment with pembrolizumab. A median of 8.8 cycles for patients were administered (range 1–52)
A score based on immune molecules can select UM patients with better survival
Twelve mUM patients were divided into two groups according to OS from the time of starting pembrolizumab: patients with survival <6 months and patients with survival ≥6 months. The concentration of circulating immune checkpoints and cytokines/chemokines released in serum before starting pembrolizumab (T0) was analyzed. Indoleamine 2,3-dioxygenase (IDO) activity, evaluated as Kynurenine/tryptophan ratio was also measured.
Serum level of two molecules resulted significantly higher in patients with survival <6 months: HVEM with a median value of 47 pg/ml (IQR 9–111,75) for those with survival <6 months and 6 pg/ml (IQR 6–35) for those with survival ≥6 months (p = .045). IDO activity showed a median value of 0.038 (IQR 0.024–0.043) for patients with survival <6 months and 0.019 (IQR 0.017–0.024) for those with survival ≥6 months (p= .035).
A third molecule, IL-8, was considered for the ROC analysis because of clinical interest. Indeed, it has been previously demonstrated that increased levels of this cytokine are predictive of poor efficacy in patients treated with an ICI.Citation31 In our study, IL-8 showed a median value of 289.92 pg/ml (IQR 48.30–314.38) for patients with survival <6 months and of 7 pg/ml (IQR 2.70–57.15) for those with survival ≥6 months.
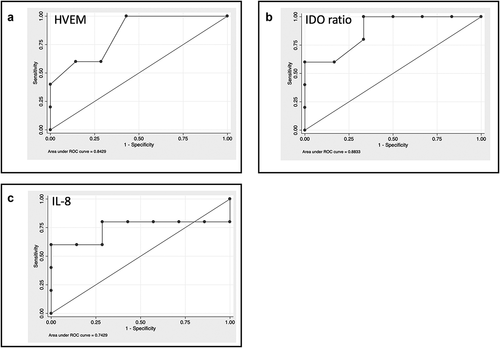
ROC curve analysis identified the better cut-off for the three selected molecules (): HVEM ≥50 pg/ml (sensibility 60%; specificity 83.3%; Accuracy 72.7%; AUC 0.817); IDO activity ≥0.024 (sensibility 100%; specificity 66.7%; Accuracy 81.8%; AUC 0.883); IL-8 ≥ 50 pg/ml (sensibility 80%; specificity 66.7%; Accuracy 72.7%; AUC 0.743).
Figure 1. Baseline levels of cytokines and soluble immune checkpoint inhibitors can select patients with better survival. A-C. ROC curve analysis for the identified molecules: A: HVEM; B IDO ratio; C: IL-8. The values were compared between patients with a survival <6 months or ≥6 months using the Mann–Whitney test.
Assigning a score of 1 to values higher than the cut-off for each of the three identified molecules and by summing single scores, an overall score ranging from 0 to 3 was obtained, where 3 corresponds to the worst prognosis.
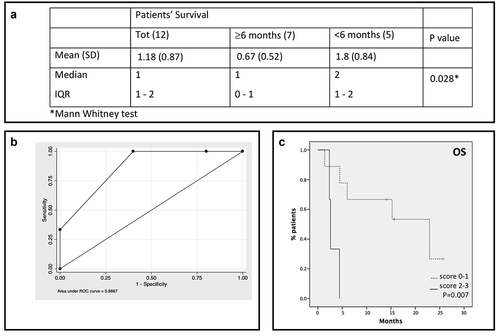
The overall score showed significantly higher value in patients with survival <6 months (p= .028) (). The ROC analysis for the overall score () identified the better cutoff for worst survival prediction, which was 2 (overall score ≥2; sensibility 60%; specificity 100%; Accuracy 81.8%; AUC 0.867). These results suggest that the presence of two or more of the identified molecules with values higher than the critical level is associated with a worst prognosis. Furthermore, higher values of the overall score correlate with lower survival (in months) with a coefficient rho = −0.490. shows the survival of mUM patients according to the score (p= .007).
Figure 2. A score based on cytokines and soluble immune checkpoint inhibitors can predict patients’ survival. A. Overall score for total sample and by patients’ survival. B. ROC curve analysis for the overall score. A score of 1 was assigned to values higher than the cutoff for each of the three identified molecules; an overall score ranging from 0 to 3 was obtained by summing single scores. C. Patients survival based on the score: solid line: patients with score 2–3; dotted line: patients with score 0–1.
Levels of soluble immune molecules are associated with prognosis in mUM patients
Based on the course of the metastatic disease, we identified three different groups of UM patients associated with OS ():
Patients defined as “fast progressors” (FP) who rapidly progressed with a median survival <6 months despite immunotherapy;
Patients defined as “slow progressors” (SP) who remained alive despite disease progression but with a median survival shorter than 30 months;
Patients defined as “long survivors” (LS) with a median survival >30 months. Among them, three patients were undergoing treatment with pembrolizumab at the time of inclusion in the study.
Figure 3. Profiling of soluble immune molecules in UM patients stratified according to the course of metastatic disease. A. UM patients were classified in Fast progressor (FP), slow progressor (LP) and long survivor (LS). In the histograms, the serum levels of each protein were reported as average value ± SEM: B. Levels of IL-8 (FP: 212.31 pg/ml ± 114.3; SP: 12.33 ± 4.017; LS: 6.333 ± 2.348), sHVEM (FP: 325.6 pg/ml ± 282; SP: 94.91 pg/ml ± 41.98; LS: 6.8 ± 1.562) and IDO activity (FP: 0.047 ± 0.021; SP: 0.02 ± 0.007; LS: 0.023 ± 0.006). C. sCD137 (FP: 361.8 pg/ml ± 123.1; SP: 140.1 pg/ml ± 16.77; LS: 65.5 pg/ml ± 20.75), sGITR (FP: 70.2 pg/ml ± 29.81; SP: 25.33 ± 6.644; LS: 11.4 ± 0.4) and sCD27 (FP: 10258 ± 3758; SP: 6027 ± 870.5; LS: 2610 ± 460.5). ANOVA test was used to compare three groups. Student’s unpaired t-test for two groups. p < .05 was considered statistically significant.
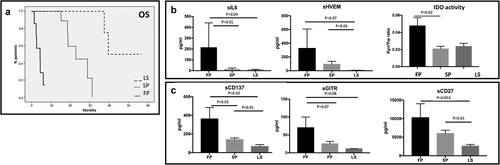
As expected, among the molecules analyzed, IL-8 resulted higher in serum of FP patients compared to LP (p= .01) and LS (p= .04) patients. Similarly, high levels of IDO activity were detected in fast progressive patients (p= .02), whereas LS and SP patients showed comparable amounts. Serum HVEM also highlighted a major concentration in FP vs LS (p= .07) and a significantly higher value in SP patients vs LS (p= .04) ().
Other immune checkpoint molecules such as sCD137, sGITR and sCD27 resulted significantly changed among the three groups of patients (p< .05) (). Patients with fast-progressive disease had higher concentration of sICs compared to SP and LS patients (sCD137: FP vs SP p = .03; FP vs LS p = .02; sGITR: FP vs SP p = .04; sCD27: FP vs LS p = .05). Similarly, patients with slow progressive disease showed higher levels of sCD137 (p = .01), sGITR (p = .01) and sCD27 (p = .01) compared to long survivors. These results suggest that the release in serum of these immune molecules could be related to the severity of the disease.
Modulation of immune molecules during anti-PD-1 treatment in metastatic uveal melanoma patients
In order to evaluate the impact of anti-PD-1 treatment on the release of immune molecules, the serum of UM patients was also collected and analyzed after three cycles of therapy (>T0) (except for three patients who rapidly progressed). The concentration of numerous sICs resulted significantly modulated during anti-PD-1 treatment (). In particular, analyzing the levels of sCD137 and sCD28 at T0 and >T0 (), the molecules resulted significantly enhanced by 3.25-fold (p = .007) and 2.4-fold (p = .01), respectively. Similarly, the concentration of the soluble form of inhibitory receptors belonging to the immunoglobulin family significantly increased during anti-PD-1 therapy, including sPD-1 (1.96-fold, p = .01), sLAG3 (1.6-fold, p = .007) and sTim3 (1.54-fold, p = .03) (). In addition, also the levels of sPD-L2 (1.38-fold, p = .04) and sCD80 (1.3-fold, p = .01), the soluble forms of PD-1 and CTLA-4 ligands, respectively, resulted augmented at >T0 compared to baseline (T0) ().
Figure 4. Modulation of soluble immune molecules in mUM patients during anti-PD-1 treatment. Levels of soluble immune checkpoint proteins and inflammatory cytokines/chemokines were measured in sera of metastatic uveal melanoma patients at baseline (T0) and during anti-PD-1 treatment (>T0). Proteins were analyzed by Luminex multiplex beads and results are reported as concentration (pg/ml). A-C. sCD137, sCD28, sPD-1, sLAG3, sTim3, sPD-L2 and sCD80; D. chemokines IP-10 and CCL2. E. IDO activity measured as KYN/trp ratio. The kyn/trp ratio was reported as average value ± SEM (T0: 0.033 ± 0.015; >T0: 0.063 ± 0.032). Wilcoxon matched-pairs signed rank test was used and a p value <.05 was considered statistically significant.
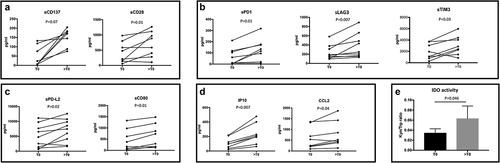
Pro and anti-inflammatory cytokines and chemokines were also evaluated. The most remarkable results were the significant increase of IP-10 (2.16-fold, p = .007) and CCL2 (1.29-fold, p = .004) after the beginning of anti-PD-1 therapy (>T0) (). Furthermore, also IDO activity resulted increased (p = .046) during anti-PD-1 treatment ().
Metastatic uveal melanoma vs metastatic cutaneous melanoma: differences in the release of soluble immune molecules during anti-PD-1 treatment
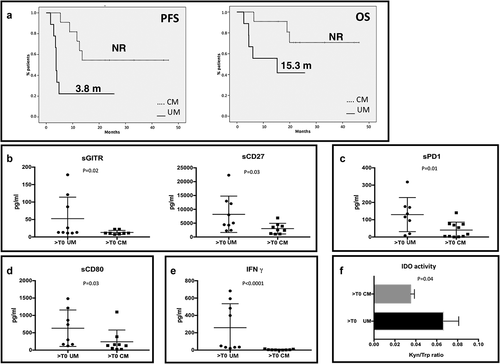
Since UM shares the same treatment with metastatic CM but shows differences in clinical benefit, we compared these settings of patients during first-line anti-PD-1 treatment in order to evaluate possible discrepancies in the regulation of the immune system.
As expected, first-line anti-PD-1 therapy allowed different clinical outcomes in mUM and mCM. Median PFS for UM melanoma patients was 3.8 months, while median PFS for CM patients was not reached at the time of data analysis (). Median OS for UM patients was 15.3 months, not reached for CM (). Although all patients were treated with an anti-PD-1 agent, the levels of several soluble immune molecules resulted remarkably different between the two groups during the treatment. The concentration of sGITR and sCD27 () were significantly higher in UM patients (p = .02 and p = .03, respectively). sPD-1 was the only soluble form of immune checkpoint receptors whose concentration was statistically different between the two types of cancer: higher in UM patients compared to CM (p = .01) (). Among the soluble form of ligands, also the levels of sCD80 resulted increased in UM (p = .03) (). Moreover, we observed that UM and CM release differently IFNγ, whose concentration was higher in the serum UM patients (p < .001) (). As IFNγ is a potent inducer of IDO expression, we also compared IDO activity between the two tumors and the results confirmed the higher presence of this enzyme in UM patients (p = .04) ().
Figure 5. Immune profile of mUM patients compared to mCM. A. PFS and OS of patients treated with an anti-PD-1 as first line treatment for metastatic disease. Solid line: uveal melanoma. Dotted line: cutaneous melanoma. B-E. mUM patients were compared to CM patients, both treated with anti-PD-1 therapy. In the scatter plot the longest bars represent the average value, the shortest ones indicate the error bar (± SEM). B. sGITR (mUM: 52.49 pg/ml ± 20.56; mCM: 12.88 ± 2.065) and sCD27 (mUM: 8204 ± 2190, mCM: 3025 ± 641.4); C. sPD-1 (mUM: 129.6 ± 34.79; mCM: 40.18 ± 13.84; D. levels of sCD80 (mUM: 630.9 ± 185.6; mCM: 240.1 ± 113.1); E. IFNγ (mUM: 258.1 ± 92.4; mCM: 4.778 ± 1.665). F. The histogram shows the IDO activity (Kyn/trp ratio) in mUM and CM (UM: 0.069 ± 0.01; CM: 0.035 ± 0.0036).
No significant modulation was observed for the other molecules tested (data not shown).
Discussion
UM and CM share systemic treatments despite their different clinical and biological behaviors. Indeed, anti-PD-1 therapy is largely used also for metastatic UM but it allows a limited benefit in this disease.Citation16 The poor efficacy can be related to the low mutational burden with few nonsynonymous mutations and no ultraviolet-induced mutational damage.Citation6,Citation22
Therefore, a current issue is to understand if there is a rationale for immunotherapy in metastatic UM.Citation24 A deeper knowledge of immunological features of this disease can help to answer the questions: 1) if alternative strategies involving pathways different from PD-1 and CTLA-4 can be more promising; 2) if a selection of the patients based on immunological factors can allow a better outcome also for patients treated with anti-PD-1 agents; 3) if a combination therapy including radiotherapy + immunotherapy can have a role for the treatment of UM through the immunogenicity induced by radiation therapy.Citation32
Our study aimed to investigate the circulating immune profile of metastatic UM in order to find an answer to these issues. Our data demonstrated that after three cycles of pembrolizumab, the concentration of several soluble immune checkpoint molecules is significantly changed compared to T0. In particular, the levels of sCD137 and sCD28 during anti-PD-1 treatment resulted significantly enhanced. CD137 and CD28 are known as costimulatory receptors but previous studies have shown that their soluble forms have an inhibitory role in immune response.Citation33,Citation34 Similarly, the concentration of the soluble form of inhibitory receptors including sPD-1, sLAG3 and sTim3 significantly increased during anti-PD-1 therapy. Trials investigating the addition of an anti-LAG3 to an anti-PD-1 agent as first-line therapy for mUM are ongoing.Citation35
The chemokines IP-10 and CCL2 also increased after the beginning of anti-PD-1 therapy (>T0). Considering that these chemokines, as well as the soluble immune checkpoint molecules, are associated with poor prognosis in many tumors,Citation36,Citation37 these results suggest that immunosuppression appears to be predominant in this setting of patients despite anti-PD-1 therapy.
Furthermore, the IDO activity increased during the systemic treatment. IDO acts as immune checkpoint involved in peripheral immune tolerance due to its ability to inhibit T-cell proliferation by depleting them from tryptophan to sensitize T-cells to apoptosis,Citation38–43 reducing immune activation. Moreover, it was demonstrated that IDO can predict primary resistance to anti-PD-1 treatment in solid tumors, such as non-small cell lung cancer.Citation44,Citation45 This data supports the observation that an immunosuppressive profile can be found in patients with metastatic UM under anti-PD-1 treatment.
In this study, we also investigated the predictive role of survival of sICs, cytokines and chemokines at baseline in patients treated with pembrolizumab.
Among all the factors evaluated, we found that HVEM, IDO activity and IL-8 were correlated with survival, with higher values in patients with poor survival (<6 months). Similar to IDO, HVEM seems to promote Treg functions.Citation46 Thus, in mUM an immunosuppressive environment detectable in patients’ blood samples is associated with poor prognosis despite anti-PD-1 treatment. IL-8 is a proinflammatory cytokine: high plasmatic levels of IL-8 are associated with decreased efficacy of checkpoint inhibitors in an inflamed tumor.Citation31,Citation47 Considering IDO, HVEM, and IL-8, we obtained a score based on their serum basal levels, able to predict patients’ survival. A prospective validation in a larger patients’ population is advisable to avoid ineffective treatments.
Considering all the mUM patients included in the study, we found three different course of disease with specific profiles of circulating immune checkpoints and cytokines. All the patients with mUM were divided into three groups considering the survival: fast progressors (FP), slow progressors (SP) and long survivors (LS). IL-8, IDO activity and HVEM are also able to distinguish the different course of the disease. Indeed, their concentration was higher in patients with dismal survival. Moreover, serum levels of CD137, GITR, and CD27 resulted significantly different among the three groups of the patients, with fast-progressive disease group that had higher concentration of sICs compared to SP and LS.
In contrast to CM, UM is usually poorly responsive to checkpoint inhibitors.Citation18 In order to define immunological differences between metastatic UM and mCM during anti-PD-1 therapy, we evaluated the release of sICs, cytokines/chemokines and IDO activity in CM patients and the results were compared with UM patients. Although all the patients were treated with an anti-PD-1 agent, the levels of several soluble immune molecules were remarkably different between the two groups during the treatment. In fact, the concentration of sGITR and sCD27 were significantly higher in UM patients. It has been demonstrated that sGITR promotes Helios expression and enhances the function of regulatory T cells.Citation48 On the other hand, the immunological function of sCD27 has not yet been clarified. Among the soluble forms of inhibitory receptors, only sPD-1 concentration was statistically different between the two types of cancer: higher in UM patients compared to CM. Regarding the soluble form of ligands, the levels of sCD80 resulted increased in UM. It is well known that CD80 on APC cells is required for rejection of immunogenic tumor in animal models.Citation49 Recently, it has been demonstrated that CD80 on APC can bind PD-L1 avoiding PD-1/PD-L1 interaction and consequently blocking an inhibitory signal for T cell activation.Citation50 High serum level of CD80 could reflect the shedding of CD80 and contribute to maintain an immunosuppressive microenvironment.
Furthermore, we observed a higher concentration of both IFNγ in the serum of UM patients. IFNγ is a potent inducer of IDO expression, and indeed a higher presence of this enzyme in UM patients was confirmed.
The different expression of circulating factors in the serum of patients with metastatic UM and mCM during anti-PD-1 therapy can offer a possible explanation of the different clinical efficacy obtained with checkpoint inhibitors in these melanoma subtypes. Indeed, our findings show immunosuppressive features of UM compared to CM.
The rarity of the disease influenced the number of patients enrolled. The small sample size and the unavailability of a baseline sample for all the patients with UM represent limitations of the study. Further analyses with the largest number of patients are necessary to confirm our observations.
Conclusions
This study provides preliminary data on a limited population of patients with a rare disease as UM. This is the first study to evaluate the correlation between sICs, cytokines and chemokines with clinical outcomes in metastatic UM.
The unsatisfactory response to anti-PD-1 therapy in UM may be justified by poor activation of the immune system. However, some patients with metastatic UM had a long survival. These patients could be identified by a score based on the circulating immune molecules such as HVEM, IDO, and IL-8. The immune response could influence the course of advanced disease and some of the studied immunological molecules could also offer new therapeutic targets.
Moreover, the comparison of circulating immune profile during anti-PD-1 therapy between UM and CM could reflect the different efficacy of ICIs in these diseases.
Supplemental Material
Download MS Word (95.9 KB)Acknowledgments
EB is currently supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) under In-vestigator Grant (IG) No. IG20583 and by Institutional funds of Università Cattolica del Sacro Cuore (UCSC-project D1-2018/2019). GT is supported by AIRC, IG18599, AIRC 5 × 1000 21052. GS is currently supported by Institutional funds of Università Cattolica del Sacro Cuore (UCSC-project D1 2018/2019). MN reports research grant from Incyte and IPSEN.
Disclosure statement
The authors declare that they have no competing interests. ER had a role as consultant for MSD and Novartis. EB reported speakers’ and travel fees from MSD, Astra-Zeneca, Celgene, Pfizer, Hel-sinn, Eli-Lilly, BMS, Novartis, and Roche; consultant’s fees from Roche and Pfizer; and institution-al research grants from AstraZeneca and Roche. PM has/had a role as consultant/advisory for BMS, Roche Genentech, MSD, Novartis, Amgen, Merk Serono, Pierre Fabre and Incyte. GT has a role as consultant for BMS and MSD.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2034377.
Additional information
Funding
References
- McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the US. Cancer. 2005;103:1000–07. doi:https://doi.org/10.1002/cncr.20866.
- Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–59. doi:https://doi.org/10.1167/iovs.03-0538.
- Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23:8076–80. doi:https://doi.org/10.1200/JCO.2005.02.6534.
- Collaborative Ocular Melanoma Study Group, Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi I, Jampol LM, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: collaborative ocular melanoma study group report no. 26. Arch Ophthalmol. 2005;123:1639–43. doi:https://doi.org/10.1001/archopht.123.12.1639.
- Agarwala SS, Eggermont AM, O’Day S, Zager JS. Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer. 2014;120:781–89. doi:https://doi.org/10.1002/cncr.28480.
- Rossi E, Schinzari G, Maiorano BA, Indellicati G, Di Stefani A, Pagliara MM, Fragomeni SM, De Luca EV, Sammarco MG, Garganese G, et al. Efficacy of immune checkpoint inhibitors in different types of melanoma. Hum Vaccin Immunother. 2020;1–10. doi:https://doi.org/10.1080/21645515.2020.1771986.
- Flaherty LE, Unger JM, Liu PY, Mertens WC, Sondak VK. Metastatic melanoma from intraocular primary tumors: the Southwest oncology group experience in phase II advanced melanoma clinical trials. Am J Clin Oncol. 1998;21:568–72. doi:https://doi.org/10.1097/00000421-199812000-00008.
- Nathan F, Sato T, and Hart E. Response to combination chemotherapy of liver metastasis from choroidal melanoma compared with cutaneous melanoma (abstract). Proc Am Soc Clin Onc . 1994;13.
- Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, Papadopoulos N, Benjamin RS. Treatment of uveal melanoma metastatic to the liver; a review of the MD Anderson cancer center experience and prognostic factors. Cancer. 1995;76:1665–70. doi:https://doi.org/10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j.
- Schinzari G, Rossi E, Cassano A, Dadduzio V, Quirino M, Pagliara M, Blasi MA, Barone C. Cisplatin, dacarbazine and vinblastine as first line chemotherapy for liver metastatic uveal melanoma in the era of immunotherapy: a single institution phase II study. Melanoma Res. 2017;27:591–95. doi:https://doi.org/10.1097/CMR.0000000000000401.
- Carvajal RD, Piperno-Neumann S, Kapiteijn E, Chapman PB, Frank S, Joshua AM, Piulats JM, Wolter P, Cocquyt V, Chmielowski B, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicenter, randomized trial (SUMIT). J Clin Oncol. 2018;36:1232–39. doi:https://doi.org/10.1200/JCO.2017.74.1090.
- Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, Boitano M, Calabrò L, Rossi CD, Giacomo AM, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61(1):41–48. doi:https://doi.org/10.1007/s00262-011-1089-0.
- Zimmer L, Vaubel J, Mohr P, Hauschild A, Utikal J, Simon J, Garbe C, Herbst R, Enk A, Kämpgen E, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One. 2015;10:e0118564. doi:https://doi.org/10.1371/journal.pone.0118564.
- Karydis I, Chan PY, Wheater M, Arriola E, Szlosarek PW, Ottensmeier CH. Clinical activity and safety of pembrolizumab in ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology. 2016;5:e1143997. doi:https://doi.org/10.1080/2162402X.2016.1143997.
- Algazi PA, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JP. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–53. doi:https://doi.org/10.1002/cncr.30258.
- Rossi E, Pagliara MM, Orteschi D, Dosa T, Sammarco MG, Caputo CG, Petrone G, Rindi G, Zollino M, Blasi MA, et al. Pembrolizumab as first-line treatment for metastatic uveal melanoma. Cancer Immunol Immunother. 2019;68:1179–85. doi:https://doi.org/10.1007/s00262-019-02352-6.
- Piulats JM, Espinosa E, de la Cruz Merino L, Varela M, Alonso Carrión L, Martín-Algarra S, López Castro R, Curiel T, Rodríguez-Abreu D, Redrado M, et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J Clin Oncol. 2021;39:586–98. doi:https://doi.org/10.1200/JCO.20.00550.
- Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, Snyder H, Feun LG, Livingstone AS, Harbour JW. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat Commun. 2020;11:496. doi:https://doi.org/10.1038/s41467-019-14256-1.
- Niederkorn JY. Immune escape mechanisms of intraocular tumors. Progr Retin Eye Res. 2009;28:329–47. doi:https://doi.org/10.1016/j.preteyeres.2009.06.002.
- Terai M, Londin E, Rochani A, Link E, Lam B, Kaushal G, Bhushan A, Orloff M, Sato T. Expression of tryptophan 2,3-Dioxygenase in metastatic uveal melanoma. Cancers (Basel). 2020;12:405. doi:https://doi.org/10.3390/cancers12020405.
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi:https://doi.org/10.1038/ni.2691.
- Furney SJ, Pedersen M, Gentien D, Dumont AG, Rapinat A, Desjardins L, Turajlic S, Piperno-Neumann S, de la Grange P, Roman-Roman, et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;3:1122–29. doi:https://doi.org/10.1158/2159-8290.CD-13-0330.
- Rodrigues M, Mobuchon L, Houy A, Fiévet A, Gardrat S, Barnhill RL, Popova T, Servois V, Rampanou A, Mouton A, et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat. Commun. 2018;9:1866. doi:https://doi.org/10.1038/s41467-018-04322-5.
- Rossi E, Schinzari G, Zizzari IG, Maiorano BA, Pagliara MM, Sammarco MG, Fiorentino V, Petrone G, Cassano A, Rindi G, et al. Immunological backbone of uveal melanoma: is there a rationale for immunotherapy? Cancers (Basel). 2019;11:1055. doi:https://doi.org/10.3390/cancers11081055.
- Zizzari IG, Napoletano C, Di Filippo A, Botticelli A, Gelibter A, Calabrò F, Rossi E, Schinzari G, Urbano F, Pomati G, et al. Exploratory pilot study of circulating biomarkers in metastatic renal cell carcinoma. Cancers (Basel). 2020;12:2620. doi:https://doi.org/10.3390/cancers12092620.
- Zizzari IG, Di Filippo A, Scirocchi F, Di Pietro FR, Rahimi H, Ugolini A, Scagnoli S, Vernocchi P, Del Chierico F, Putignani L, et al. Soluble immune checkpoints, gut metabolites and performance status as parameters of response to nivolumab treatment in NSCLC patients. J Pers Med. 2020;10:208. doi:https://doi.org/10.3390/jpm10040208.
- Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209–15. doi:https://doi.org/10.1038/s41577-019-0264-y.
- Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. 2018;6:132. doi:https://doi.org/10.1186/s40425-018-0449-0.
- Machiraju D, Wiecken M, Lang N, Hülsmeyer I, Roth J, Schank TE, Eurich R, Halama N, Enk A, Hassel JC. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology. 2021 May 25;10(1):1926762. doi:https://doi.org/10.1080/2162402X.2021.1926762.
- Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, Breen EJ, Yang JYH, Ghazanfar S, Kefford RF, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving Anti-PD-1-based immunotherapy. Clin Cancer Res. 2019;25:1557–63. doi:https://doi.org/10.1158/1078-0432.CCR-18-2795.
- Bakouny Z, Choueiri TK. IL-8 and cancer prognosis on immunotherapy. Nat Med. 2020 May;26:650–51. doi:https://doi.org/10.1038/s41591-020-0873-9.
- Tagliaferri L, Lancellotta V, Fionda B, Mangoni M, Casà C, Di Stefani A, Pagliara MM, D’Aviero A, Schinzari G, Chiesa S, et al. Immunotherapy and radiotherapy in melanoma: a multidisciplinary comprehensive review. Hum Vaccin Immunother. 2021:1–8. doi:https://doi.org/10.1080/21645515.2021.1903827.
- Luu K, Shao Z, Schwarz H. The relevance of soluble CD137 in the regulation of immune responses and for immunotherapeutic intervention. J Leukoc Biol. 2020;107:731–38. doi:https://doi.org/10.1002/JLB.2MR1119-224R.
- Hebbar M, Jeannin P, Magistrelli G, Hatron PY, Hachulla E, Devulder B, Bonnefoy JY, Delneste Y. Detection of circulating soluble CD28 in patients with systemic lupus erythematosus, primary Sjogren’s syndrome and systemic sclerosis. Clin Exp Immunol. 2004;136:388–92. doi:https://doi.org/10.1111/j.1365-2249.2004.02427.x.
- Lutzky J, Feun LG, Magallanes N, Kwon D, Harbour JW. NCT04552223: a phase II study of nivolumab plus BMS-986016 (relatlimab) in patients with metastatic uveal melanoma (UM) (CA224-094). Abs TPS9590 2021 ASCO Annual Meeting. Vol. JCO 30, 15 suppl. doi: https://doi.org/10.1200/JCO.2021.39.15_suppl.TPS9590
- Lunardi S, Lim SY, Muschel RJ, Brunner TB. IP-10/CXCL10 attracts regulatory T cells: implication for pancreatic cancer. Oncoimmunology. 2015;4:e1027473. doi:https://doi.org/10.1080/2162402X.2015.1027473.
- Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–72. doi:https://doi.org/10.1038/nri.2017.49.
- Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–73. doi:https://doi.org/10.1016/s0167-5699(99)01520-0.
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi:https://doi.org/10.1084/jem.189.9.1363.
- Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–99. doi:https://doi.org/10.4049/jimmunol.164.7.3596.
- Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–77. doi:https://doi.org/10.1182/blood-2006-07-036863.
- Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–404. doi:https://doi.org/10.4049/jimmunol.181.8.5396.
- Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–63. doi:https://doi.org/10.1182/blood-2008-11-191197.
- Botticelli A, Cerbelli B, Lionetto L, Zizzari I, Salati M, Pisano A, Federica M, Simmaco M, Nuti M, Marchetti P. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J Transl Med. 2018;16:219. doi:https://doi.org/10.1186/s12967-018-1595-3.
- Botticelli A, Mezi S, Pomati G, Cerbelli B, Cerbelli E, Roberto M, Giusti R, Cortellini A, Lionetto L, Scagnoli S, et al. Tryptophan catabolism as immune mechanism of primary resistance to anti-PD-1. Front Immunol. 2020;11:1243. doi:https://doi.org/10.3389/fimmu.2020.01243.
- Jones A, Bourque J, Kuehm L, Opejin A, Teague RM, Gross C, Hawiger D. Immunomodulatory functions of BTLA and HVEM govern induction of extrathymic regulatory T cells and tolerance by dendritic cells. Immunity. 2016;45:1066–77. doi:https://doi.org/10.1016/j.immuni.2016.10.008.
- Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, Rishipathak D, Williams P, Kadel EE 3rd, Koeppen H, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26:693–98. doi:https://doi.org/10.1038/s41591-020-0860-1.
- Li Y, Yang S, Li Z, Meng H, Jin W, Yang H, Yin W. Soluble glucocorticoid-induced tumor necrosis factor receptor regulates Helios expression in myasthenia gravis. J Transl Med. 2019;17:168. doi:https://doi.org/10.1186/s12967-019-1916-1.
- McHugh RS, Ahmed SN, Wang YC, Sell KW, Selvaraj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80). Proc Natl Acad Sci U S A. 1995;92:8059–63. doi:https://doi.org/10.1073/pnas.92.17.8059.
- Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, Okazaki T. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–66. doi:https://doi.org/10.1126/science.aav7062.
