ABSTRACT
Objectives
To evaluate the safety of 13-valent pneumococcal conjugate vaccine (PCV13) after its licensure.
Methods
Review and describe the AEFI reported to national adverse event following immunization surveillance system (NAEFISS) in Zhejiang province from 2017 to 2020. Reporting rates of AEFI were calculated by age, city, severity of AEFI, categories of AEFI, and reaction categories. The data mining algorithm used in this study was reporting odds ratio (ROR). A value of ROR-1.96SE >1 (standard error [SE]) was considered as the positive signal.
Results
NAEFISS received 3332 AEFI cases following PCV13, with a reporting rate of 17.58/10000 doses. Of the reported AEFI, 652 were serious AEFI cases and the reporting rate was 3.44 for serious AEFI. The reporting rate of fever was the highest among all the clinical diagnosis (7.39/10000 doses). The positive signals were obtained for injection site reaction (ROR-1.96SE: 1.55), hypotonic hyporesponsive episode (HHE) (ROR-1.96SE: 1.62) and febrile seizure (ROR-1.96SE: 1.52).
Conclusion
The present results supported previous observations that the PCV13 administered as the four-dose schedule was generally well tolerated in Chinese infants as we did not identify any new/unexpected safety concern from the NAEFISS during a four-year time period.
Introduction
Streptococcus pneumoniae (pneumococcus) is a human pathogen causing wide range of disease including pneumonia, septicemia, meningitis, and otitis media with the burden of disease greatest in infants and the elderly.Citation1 S. Pneumoniae is a leading cause of morbidity and mortality in children worldwide, particularly in infants and young children up to the age of 5 years.Citation2 More than 90 different serotypes of S. pneumoniae have been isolated worldwide. Although there is variability in terms of pathogenicity and prevalence, it is considered that 20 serotypes account for 80% of invasive pneumococcal diseases (IPD) globally.Citation3 The world health organization (WHO) recommends that pneumococcal vaccines should be included in immunizations programs worldwide.Citation4
The pneumococcal polysaccharide vaccine has been available for three decades, but it exhibits poor immunogenicity in children under 2 years of age (the group at highest risk of pneumococcal diseases). It does not elicit immune memory, nor does it reduce the mucosal carriage of S. pneumoniae. These defects were overcome with the introduction of a 7-valent pneumococcal conjugate vaccine (PCV7), which contains the polysaccharides of the serotypes responsible for the majority of pneumococcal diseases in young children at the time it was licensed.Citation5,Citation6 The effectiveness of routine immunization with PCV7 on rates of pneumococcal disease has been considerable. For example, among children under 5 years in Australia, the notification rate for IPD decreased by 68% after introduction of PCV7 into the immunization schedule and a significant reduction in hospitalization rates for pneumonia was also observed.Citation7 Based on the serotype replacement studies post PCV7 introduction, the 13-valent pneumococcal conjugate vaccine (Prevenar 13®,PCV13) was developed to provide broader coverage of disease-causing pneumococcal serotypes.Citation8 It comprises 13 serotype-specific polysaccharides of S. pneumoniae conjugated individually to nontoxic diphtheria CRM197 protein, of which seven are the same to the PCV7 (4, 6B, 9 V, 14, 18C, 19 F, and 23 F) and six are additional serotypes (1, 3, 5, 6A, 7 F, and 19A).
Zhejiang province is located at the east coast line of China, with a large population size of 70 million. The national immunization program has been launched since 1978 with four vaccines and it has integrated 11 vaccines since 2008. Over 20 million vaccinations were administered each year. Of these vaccinations, almost 8 million were self-paid vaccines. PCV13 has been approved for use as a self-paid vaccine, among children ranging in age from 6 weeks to 15 months in China since 2016. The vaccination schedule of PCV13 is four-dose series, including a primary series at 2, 4, 6 months of age and a boost dose at 12–15 months of age. The first dose can be administered as early as 6 weeks of age and the minimal interval between two doses was 4 weeks.
The safety of vaccines after its introduction is of significant public health interest and is critical in maintaining confidence in the vaccination program. Pre-licensure, the safety profile of PCV13 was evaluated in many clinical trials.Citation9–12 Of them, the incidence and severity of both local and systemic adverse events following immunization (AEFI) were very similar between PCV7 and PCV13 recipients, indicating a comparable safety profile for the two vaccines. However, AEFI detected following the introduction of a new vaccine into a population is different to those detected in pre-licensure controlled clinical trials, as these are rarely powered sufficiently to detect the rare AEFI.
China ministry of health (MOH) has established a nation-wide AEFI surveillance since 2005, with the technical support of WHO and the experience from other countries.Citation13 The national AEFI surveillance system (NAEFISS), which was a passively collected spontaneous database, has been in operation since 2005 and was upgraded in 2012 by adding variables of the case reporting form and improving the logic control of data entry and statistical functions. The reporting sensitivity has improved in Zhejiang province from 9.2/100,000 doses for the time period of 2008–2011 to 56.64/100,000 doses in 2019.Citation14 The passive or post-marketing surveillance systems, such as NAEFISS, play a key role in signal detection of the potential AEFI to guide further investigation if warranted. However, reporting rates of AEFI detected through the passive surveillance systems are likely to be lower than the true rate, due to the bias of under-reporting, but may still flag important AEFI for follow-up.
This study aimed to describe all AEFI and AEFI reporting rates from the NAEFISS data following the PCV13 administration, compared to those reported following other vaccines in the same period.
Methods
National AEFI surveillance system
In March, 2005, with the technical support of WHO, Chinese center for disease control and prevention (CDC) launched the passive surveillance for AEFI. Subsequently, the AEFI surveillance guidelines were issued, supporting by the law on the prevention and treatment of infectious diseases, the pharmaceutical administration law, the regulation on circulation of vaccines and vaccination, with the intent to improve vaccine safety and immunization service quality. Based on this guidelines, the national AEFI surveillance system (NAEFISS), which was an online spontaneous reporting system for AEFI following all of the vaccines marketed in mainland China, was also developed in 2007 and it soon covered all 31 provinces in 2008.Citation13 The NAEFISS aims to detect new, unusual, or rare AEFI, evaluate the safety of newly licensed vaccines, identify potential risk factors for AEFI, monitor increases in known AEFI, determine the possible reporting clusters, and provide a reliable safety monitoring system.
The definitions of AEFI
An AEFI case is defined as a reaction or an event following vaccination that is suspected to be related to the vaccination.Citation14 Any AEFI case should be classified as one of the five categories: (1) vaccine product-related reaction (non-serious reaction and serious reaction); (2) vaccination error; (3) vaccine quality defect-related reaction; (4) coincidental event; (5) anxiety reaction. Except for this, an AEFI case should also be defined as non-serious or serious by the following principle: (1) non-serious, with no intervention necessary or with physician visit or event interfering with daily activities or loss of working hours; (2) serious, with any untoward medical occurrence that results in death, hospitalization, prolongation of hospitalization, persistent, or significant disability/incapacity, life threatening or birth defect.Citation13 The AEFI was also categorized by the type of doses, as AEFI for 1st, 2nd, 3rd, and 4th dose. China has a mechanism to arrange for panels of AEFI experts to conduct the causality assessments. AEFI experts, which are composed of independent experts from clinical medicine, epidemiology, laboratory practices, pharmacy, vaccinology, vaccine regulation, and other relevant fields, are organized to review the reported AEFI and to make the classification mentioned above.
The reporting and investigation procedures
Healthcare facilities, vaccination clinics, CDCs at all administrative levels, vaccine manufacturers as well as the vaccinees (or guardian) have the responsibility to report an AEFI case. The case is gathered by the county-level CDC, which is responsible for completing the AEFI reporting form and submitting it to NAEFISS. Once case information is entered, it can be viewed by all administrative levels of CDCs.Citation13
Any AEFI case is to be investigated, with the exception of the common adverse reactions that have a clear diagnosis (fever, redness, induration, swelling on the injection site). County-level CDC starts the investigations by collecting the relevant data and completing an AEFI investigation form, which is subsequently entered into NAEFISS, associating with the submitted AEFI reporting form. For death, serious AEFI, AEFI cluster, and AEFI of significant public concerns that are suspected to be related to immunization, upon receiving the case report, municipal or provincial CDC must immediately organize an AEFI expert panel for investigation. During the investigation, the following information should be collected and entered into the NAEFISS: personal information on the vaccinated individual, storage and transportation of vaccines, vaccine administrations and the AEFI itself. Signs and symptoms of AEFI are coded using the international classification of diseases (version 10.0, ICD-10),Citation15 a clinically validated, internationally standardized terminology. A single AEFI report may be assigned more than one term and be referred to more than one suspected vaccine. In cases of co-administration of two or more vaccines in an individual, we attributed the reported AEFI to the reporter suspected vaccine according to the following principle:Citation16 (1) the injection site reaction could be determined by the record of vaccination; (2) the systematic reactions could not be determined which vaccine was to be suspected when the co-administration occurred. In that case, we attributed the reported AEFI to all vaccines co-administered.
Data extraction
AEFI cases following PCV13 were reported from 01 January 2017 to December 31, 2020. The AEFI data were extracted from the NAEFISS in March 2021, when all the revision or modification of each report had been done and the data fixed. The number of various vaccines doses in Zhejiang province during the same period was obtained from the online individual immunization information system of Zhejiang province, which was established in 2005.Citation17
Data analysis
Descriptive statistics were used to describe the epidemiology of AEFI cases and a database was organized as an Excel file (Microsoft Office Excel 2020). The reporting trends during the study period was displayed through a monthly reporting rate chart. The AEFI reporting rates of PCV13 were presented by the variables including gender, dose number, the AEFI onset interval (from vaccination date [day 0] to onset of first symptoms), AEFI categories, severity, type of reporter and the clinical diagnosis. The reporting rate where PCV13 was administered alone or concomitantly with other vaccines and the reporting municipal were also described. This was achieved by dividing the total number of AEFI cases for each variable level by the total number of vaccine doses administered during the study period. These reporting rates between these variables were compared through chi-square test at a two-tail significance of 0.05.
Disproportionality analysis was applied by using the algorithm of reporting odds ratio (ROR).Citation18 The ROR is defined as the ratio of the odds of reporting of one specific AEFI versus all other AEFIs for a given vaccine compared to the reporting odds for all other vaccines present in the same database. The value of ROR-1.96SE [(standard error (SE)] > 1 is defined as the threshold. We assumed as a positive signal if the actual observed value is above the threshold. The positive signal meant that the reporting rate of PCV13 was higher than the average level of the other vaccines used in Zhejiang province during the study period. The higher the value, the stronger the disproportion appears to be.
Results
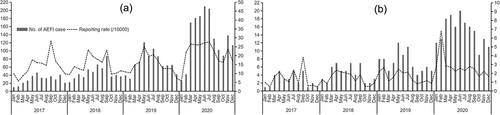
During the study period, the number of vaccine doses of PCV13 included in the study was 1,895,548 and the number of received AEFI cases associated with PCV13 was 3332, with a crude reporting rate of 17.58/10000 doses. The number of serious AEFI cases was 652 and the crude reporting rate was 3.44 for the serious AEFI following PCV13. The monthly reporting rate was highest in Sep 2017 for the total AEFI cases and was highest in Feb 2020 for the serious AEFI cases ().
Figure 1. The reporting rate of AEFI following 13-valent pneumococcal conjugate vaccine from 2017 to 2020, by month of onset (A refer to the total AEFI and B refer to the serious AEFI).
Of the reported AEFI cases, 50.27% were male, with a reporting rate of 17.57/10000 doses. The reporting rate of AEFI following the 4th doses of PCV13 (20.12/10000 doses) was significantly higher than the other doses. The significantly highest rate of AEFI categories was the minor vaccine product-related reaction (13.04/10000). The majority of AEFI cases were non-serious, with a reporting rate of 14.14/10000 doses. The primary source of AEFI reports was healthcare provider, with over 90% of the reports were from healthcare provider. Most of the AEFI cases occurred in 4–7 days after vaccination, with the significantly highest reporting rate of 12.28/10000 doses for AEFI occurred within the interval of 4–7 days after PCV13 vaccination. The reporting rate of AEFI when the PCV13 was administered alone 17.30/10000 doses ().
Table 1. Characteristics and reporting rate of AEFI cases following 13-valent pneumococcal conjugate vaccine from 2017 to 2020
presents the surveillance results among cities in Zhejiang province. For the general AEFI cases, the sensitivity was highest in Quzhou city (21.97/10000 doses) and lowest in Lishui city (12.05/10000 doses). For the serious AEFI cases, the highest reporting rate was observed in Taizhou city (4.82/10000 doses) while the lowest reporting rate was observed in Jiaxing city (1.05/10000 doses).
Table 2. Serious AEFI and non-serious AEFI cases following 13-valent pneumococcal conjugate vaccine from 2017 to 2020
After a review of the clinical diagnoses of the 488 serious vaccine product-related reactions, the majority were general allergic reaction (217 cases), followed by rash/urticaria (186 cases). Of the 2472 minor vaccine product-related reactions, fever was the most common AEFI (1393 reports), followed by injection site reaction (1034 reports). The reporting rate of fever was the highest among all the clinical diagnosis (7.39/10000 doses), followed by injection site reaction (5.49/10000 doses), general allergic reaction (1.39/10000 doses), and rash/urticaria (1.35/10000 doses). The positive signals were obtained for injection site reaction (ROR-1.96SE: 1.55), hypotonic hyporesponsive episode (HHE) (ROR-1.96SE: 1.62) and febrile seizure (ROR-1.96SE: 1.52) ().
Table 3. Clinical diagnosis of AEFI cases following 13-valent pneumococcal conjugate vaccine from 2017 to 2020
Discussion
This study presented an evaluation of AEFI reported to a passive surveillance system in Zhejiang province, soon after the PCV13 vaccine was approved by the China Food and Drug Administration. As the first post-licensure evaluation of safety of PCV13 from China, our results added to the existing evidence on safety profile from the pre-licensure clinical trials. Our review of NAEFISS reports following PCV13 did not identify any new or unexpected safety concerns. Most AEFI cases reported following PCV13 were either consistent with well-recognized vaccine side effects or with common symptoms of illness expected in infants that might be unrelated to vaccination.Citation19 Another supportive evidence for the safety profile of PCV13 was that over 70% of AEFI cases were mild, self-limited conditions (e.g., fever, injection site reaction) and the reporting rates of AEFI were similar to those from other previous reports based on the passive surveillance systems of AEFI (22.8/10000 doses for the total AEFI reporting rate).Citation12 There were no specific rare or serious AEFI signals detected in our study that required further investigation. We assumed that PCV13 appeared to be a safe, well-tolerated vaccine when it was introduced into the community.
The highest monthly reporting rate was observed in 2017 but the highest monthly reporting rate for serious AEFI was not found in the same period. One possible explanation for this difference was the heightened reporting sensitivity post introduction of a new vaccine. However, the reporting sensitivity of serious AEFI would not be changed as it was always confirmed through medical consultation or test.Citation20 In the first year, reporters could have been more likely to report more AEFI cases due to the awareness of PCV13 as a new vaccine. Similar rises in reporting rates following vaccine introduction had been observed in the literature, a phenomenon known as the Weber effect.Citation21 It described an increase in the reporting rate of adverse event occurred in the time period shortly after the drug marketing followed by a stabilization and this had been demonstrated with a variety of drugs, including vaccines. Our findings might be used a reference for the future introduction of new vaccine. An uncertain signal generation would emerge when we starts a large-scale use of a new vaccine. Another indication is that not only the total AEFI reporting rate but also the serious AEFI reporting rate should be worthy of attention when we evaluate the safety of a new vaccine.
We did not identify an uncertain signal generation could emerge in reporting rate of AEFI associated with PCV13 between male and female, which was similar to the previous reports on AEFI surveillance.Citation22 Compared with the initial dose, we found the later dose of PCV13 would be more likely to induce the adverse reactions, which was consistent with the findings of AEFI surveillance work from other areas and on other vaccines.Citation23–25 One possible explanation was that the subsequent dose was more likely to induce or stimulate the allergic reactions due to the body sensitization by the initial dose. Minor vaccine product-related reaction, such as fever and injection site reactions, were the most frequently reported AEFI. It was the same as the other findings on AEFI surveillance reports, in which the minor reactions or the non-serious reactions were the most prevalent forms of reactogenicity experienced after vaccination.
We found that most of the AEFI cases were detected by the healthcare providers, which was different with some previous reports that most of the AEFI cases were reported by caregivers.Citation14,Citation16 The possible explanation was that PCV13 was a new vaccine and the reporting sensitivity was higher than other vaccines due to more attention had been attracted. The advantage of healthcare provider as the reporter was that the reporting bias would be avoided since caregivers would not be sensitive enough or even ignored to some mild AEFI.Citation26 Another advantage was that healthcare provider could provide more sufficient clinical information and help the following case review.
Our result of the incubation between vaccination and AEFI was similar to that from other reports from North America, Europe, and Asia, in which the recorded local and systemic adverse events experienced by the infants and children occurred in the 4- to 7-day period after each dose of vaccine.Citation25 The reasonable explanation was that the interval between vaccination and the most common AEFI (fever or injection site reactions) were induced by local inflammation or central thermoregulation disorder. These reactions were shortly occurred after vaccination.Citation27,Citation28 Most of the AEFI associated with PCV13 was administered alone in this report. The possible reason for this was that the package insert of PCV13 recommended that PCV13 better not to be co-administered with other vaccines.
The injection site reactions were found as a positive signal, which was consistent with previous reports from other areas and with the data from PCV13 clinical studies.Citation19,Citation29 These data demonstrated an increase in injection site reactions, such as tenderness, swelling, and redness, especially for the subsequent doses. To our knowledge, the injection site reactions were the results from the stimulation of nociceptive sensory neurons at the time of vaccine administration or inflammatory process in the damaged tissue afterward. The PCV13 has the aluminum adjuvant, which had been reported it could induce the redness and induration. Another possible reason was the syringes and needles that were used in the vaccination, but it needed to be further investigated. In this study, HHE was reported as another positive signal, which was also in line with the post-licensure data of PCV13.Citation30 HHE reactions were traditionally associated with diphtheria, tetanus, Haemophilus influenzae type B, and hepatitis B vaccines, but have also been reported uncommonly following other vaccines.Citation31 The wide variation in incidence of HHE following vaccination (0.4–25/10000)Citation32,Citation33 probably reflected the various case definitions and case ascertainments rather than the inherent properties of different vaccines. The pathogenesis of HHE was still unknown and had been poorly studied given the constraints of investigating a condition that the results in transient symptoms. The pathogenesis of HHE was likely to be multifactorial and might result from the factors both idiosyncratic to the child and inherent in the vaccine.
Febrile convulsion was a pre-identified solicited adverse reaction of significant interest. As similar to the previous report,Citation34 we observed the febrile convulsion as a positive signal of AEFI following PCV13. One vaccine safety datalink study had found that trivalent inactivated influenza vaccine and PCV13 were each associated with an increased risk of febrile convulsion independent of concomitant receipt of the other vaccine in children ages 6–59 months; however, another study reported there was no increased risk of febrile seizures identified in any age group.Citation35,Citation36 More studies were needed to further examine this issue. The most possible reason for febrile convulsion following PCV13 was that the body temperature would elevate following vaccination,Citation37 which could induced the febrile convulsion as the most common type of non-epileptic seizure observed following immunization. Febrile convulsion soon after immunization are mostly triggered by fever induced by the vaccine or not vaccine related.Citation38
Our results came from a passive surveillance system of AEFI, which has several inherent limitations. First, passive surveillance system relied on self-initiated notification and would induce the under-reporting, as well as being subject to inconsistency in data quality and completeness. Second, the sensitivity of passive surveillance system would be influenced by factors like media attention or heightened awareness of a new vaccine, resulting the reporting bias following commencement of a new vaccine. Despite of these limitations, there was currently no other way to get the routine surveillance data on AEFI to evaluate vaccine safety. Any detection of potential safety signals of rare or previously unknown AEFI, could then be further investigated.
Conclusion
The present findings supported previous observations that the PCV13 administered as the four-dose schedule was generally well tolerated in Chinese infants as we did not identify any new/unexpected safety concern from the NAEFISS during a four-year time period. This evaluation would serve as a reference for discussing the benefits and risks of PCV13 vaccination strategy.
Ethics approval and consent to participate
This study was approved by the ethical review board of Zhejiang provincial CDC. All the data were anonymous when we exported them from ZJIIS and kept confidential without individual identifiers.
Author contributions
Y.H. and XJ. P conceived and designed the experiments; H.L. and Y.C. performed the experiments; HK. L. and Y.W. analyzed the data; LZ.S. and FX. C. contributed reagents/materials/analysis tools; XJ. P and Y.H. wrote the paper.
Acknowledgments
We are grateful to all physicians in all health centers and different administrative CDCs in Zhejiang province, for reporting, collecting, and collating the AEFI records.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rodgers G, Klugman K. The future of pneumococcal disease prevention. Vaccine. 2011;29:43–7. doi:10.1016/j.vaccine.2011.07.047.
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6.
- Pittet LF, Posfay-Barbe KM. Pneumococcal vaccines for children: a global public health priority. Clin Microbiol Infect. 2012;18(Suppl 5):25–36. doi:10.1111/j.1469-0691.2012.03938.x.
- World Health Organization. Pneumococcal vaccines-WHO position paper 2012. Weekly Epidemiol Record 2012;87:129–44.
- Hausdorff WP. The roles of pneumococcal serotypes 1 and 5 in paediatric invasive disease. Vaccine. 2007;25(13):2406–12. doi:10.1016/j.vaccine.2006.09.009.
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5(2):83–93. doi:10.1016/S1473-3099(05)70083-9.
- Barry C, Krause VL, Cook HM, Menzies RI. Invasive pneumococcal disease in Australia, 2007 and 2008. Commun Dis Intelligence Annu Rep. 2012;36:151–65.
- Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children-use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine-recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1–18.
- Chilson E, Scott DA, Schmoele-Thoma B, Watson W, Moran MM, Isturiz R. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in patients with immunocompromising conditions: a review of available evidence. Hum Vaccin Immunother. 2020;16(11):2758–72. doi:10.1080/21645515.2020.1735224.
- Huang L-M, Lin T-Y, Juergens C. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine given with routine pediatric vaccines in Taiwan. Vaccine. 2012;30(12):2054–59. doi:10.1016/j.vaccine.2011.12.054.
- Zhang T, Zhang J, Shao X, Feng S, Xu X, Zheng B, Liu C, Dai Z, Jiang Q, Gessner BD, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against community acquired pneumonia among children in China, an observational cohort study. Vaccine. 2021;39(33):4620–27. doi:10.1016/j.vaccine.2021.06.075.
- Soo Kim D, Hee Shin S, Jong Lee H, Jin Hong Y, Young Lee S, Min Choi K, Chi Eun O, Hwan Kim K, Juergens C, Patterson S, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given to Korean children receiving routine pediatric vaccines. Pediatr Infect Dis J. 2013;32(3):266–73. doi:10.1097/INF.0b013e3182748bb6.
- Liu D, Wendi W, Keli L, Disha X, Jiakai Y, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33:4041–46. doi:10.1016/j.vaccine.2015.04.060.
- Pan X, Huakun L, Chen F, Wang Y, Liang H, Shen L, Chen Y, Hu Y. Analysis of adverse events following immunization in Zhejiang, China, 2019: a retrospective cross-sectional study based on the passive surveillance system. Hum Vaccin Immunother. 2021;17(10):3823–30. doi:10.1080/21645515.2021.1939621.
- Rust J. Updating the international classification of diseases and related health problems, tenth revision (ICD-10). Health Inf Manag. 2010;39:40.
- Hu Y, Li Q, Lin L, Chen E, Chen Y, Qi X. Surveillance for adverse events following immunization from 2008 to 2011 in Zhejiang Province, China. Clin Vaccine Immunol. 2013;20:211–17. doi:10.1128/CVI.00541-12.
- Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L. Using the immunization information system to determine vaccination coverage rates among children aged 1–7 years: a report from Zhejiang Province, China. Int J Environ Res Public Health. 2014;11:2713–28. doi:10.3390/ijerph110302713.
- Kim S, Park K, Kim MS, Yang BR, Choi HJ, Park BJ. Data-mining for detecting signals of adverse drug reactions of fluoxetine using the Korea Adverse Event Reporting System (KAERS) database. Psychiatry Res. 2017;256:237–42. doi:10.1016/j.psychres.2017.06.038.
- Destefano F, Pfeifer D, Nohynek H. Safety profile of pneumococcal conjugate vaccines: systematic review of pre- and post-licensure data. Bull World Health Organ. 2008;86(5):373–80. doi:10.2471/BLT.07.048025.
- Mahajan D, Cook J, Dey A, Macartney K, Menzies RI. Annual report: surveillance of adverse events following immunisation in Australia, 2011. Commun Dis Intel. 2012;36:315–32.
- Weber JCP. Epidemiology of adverse reactions to non-steroidal anti-inflammatory drugs. Adv Inflam Res. 1984;6:1–7.
- Fu Tseng H Sy LS, Amy Liu I-L, Lei Qian, SM Marcy, Weintraub E, Yih K, Baxter R, Glanz JM, Donahue J., et al. Post-licensure surveillance for pre-specified adverse events following the 13-valent pneumococcal conjugate vaccine in children. Vaccine. 2013;31(22):2578–83. doi:10.1016/j.vaccine.2013.03.040.
- White C, Halperin SA, Scheifele DW. Pediatric combined formulation DTaP-IPV/Hib vaccine. Expert Rev Vaccines. 2009;8:831–40. doi:10.1586/erv.09.59.
- Littlejohn ES, Clothier HJ, Perrett KP, Danchin M. Surveillance of adverse events following the introduction of 13-valent pneumococcal conjugate vaccine in infants, and comparison with adverse events following 7-valent pneumococcal conjugate vaccine, in Victoria, Australia. Hum Vaccin Immunother. 2015;11:1828–35.
- Plosker GL. 13-valent pneumococcal conjugate vaccine: a review of its use in infants, children, and adolescents. Paediatr Drugs. 2013;15(5):403–23. doi:10.1007/s40272-013-0047-z.
- Isaacs D, Lawrence G, Boyd I, Ronaldson K, McEwen J. Reporting of adverse events following immunization in Australia. J Paediatr Child Health. 2005;41(4):163–66. doi:10.1111/j.1440-1754.2005.00580.x.
- Michael Marcy S, Kohl KS, Dagan R, Nalin D, Blum M, Connell Jones M, Hansen J, Labadie J, Lee L, Martin BL, et al. Fever as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5–6):551–56. doi:10.1016/j.vaccine.2003.09.007.
- Gidudua JF, Walcob GA, Taddioc A, Zempskyd WT, Halperine SA, Calugara A, Gibbsf NA, Hennigg R, Jovancevich M, Netterlidi E, et al. Immunization site pain: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2012;30(30):4558–77.
- Clarke E, Bashorun A, Adigweme I, Badjie Hydara M, Umesi A, Futa A, Ochoge M, Obayemi D, Edem B, Saidy-Jah E, et al. Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2021;21(6):834–46. doi:10.1016/S1473-3099(20)30735-0.
- DuVernoy TS, Braun MM. Hypotonic-hyporesponsive episodes reported to the Vaccine Adverse Event Reporting System (VAERS), 1996-1998. Paediatrics. 2000;106:E52.
- Heijbel H, Ciofi Degli Atti MC, and Harzer E, Liese J,Tozzi AE. Hypotonic hyporesponsive episodes in eight pertussis vaccine studies. Dev Biol Standard. 1997;89:101–03.
- Gold MS, Kempe A, Osbourn M. A comparison of serious adverse reactions to whole cell and acellular pertussis vaccines in South Australia. Med J Aust. 1999;171:331–32. doi:10.5694/j.1326-5377.1999.tb123677.x.
- Miller E. Overview of recent trials of acellular pertussis vaccine. Biologicals. 1999;27:79–86. doi:10.1006/biol.1999.0184.
- Bonhoeffer J, Menkes J, Gold MS, de Souza-brito G, Fisher MC, Halsey N, Vermeer P; The Brighton Collaboration Seizure Working Group. Generalized convulsive seizure as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine. 2004;22(5–6):557–62. doi:10.1016/j.vaccine.2003.09.008.
- Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine. 2012;30(11):2024–31. doi:10.1016/j.vaccine.2012.01.027.
- Yeh SH, Gurtman A, Hurley DC, Block SL, Schwartz RH, Patterson S, Jansen KU, Love J, Gruber WC, Emini EA, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010;126(3):e493–505. doi:10.1542/peds.2009-3027.
- Vanderkooi OG, Scheifele DW, Girgenti D, Halperin SA, Patterson SD, Gruber WC, Emini EA, Scott DA, Kellner JD; Canadian PCV13 Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers given with routine pediatric vaccinations in Canada. Pediatr Infect Dis J. 2012;31(1):72–77. doi:10.1097/INF.0b013e318233049d.
- Hirtz DG, Nelson KB, Ellenberg JH. Seizures following childhood immunization. J Pediatr. 1983;102:14–18. doi:10.1016/S0022-3476(83)80278-9.
