ABSTRACT
Purpose
To highlight the scientific progress in immunotherapy of urological cancer by identifying and analyzing the 100 top-cited (T100) articles from the last 15 years.
Methods
Papers in immunotherapy of urological cancer were identified from Clarivate Web of Science Core Collection database. Data of the T100 articles and papers published in recent 2 years, including citations, topic, year of publication, country of origin, institution and authorship, were extracted and analyzed.
Results
Of the T100 articles, the citation number ranged from 7387 to 183 with a mean of 590.66. The USA led the field with 80 T100 articles and 53097 citations. Pro Sharma P from MD Anderson Cancer Center was at the top of list with 8 T100 articles (3 as first author and 6 as corresponding author). Memorial Sloan Kettering Cancer Center ranked first with 26 T100 articles and 22573 citations, followed by Johns Hopkins University with 21 T100 articles and 25095 citations. Forty-nine T100 articles were related to the renal cancer, followed by prostate cancer (29), bladder cancer (13) and urothelial cancer (13). According to the type of immunotherapy, most T100 articles were related to ICI (55 articles) and vaccine (19 articles).
Conclusions
It is the first bibliometric analysis to identify the T100 articles on immunotherapy of urological cancer. The USA made great contribution in the field of immunotherapy related to urological cancer. Renal, bladder and prostate cancers were the major organs treated by immunotherapy especially by ICIs and vaccines. The multiple aspects of ICIs research in renal and bladder cancer and the neoantigen-based vaccine therapy will be hotspots for future research.
Background
Immunotherapy is considered a promising approach to enhance and harness the abilities of the human immune system to identify and eliminate cancer cells. Within the last few decades, various immune-based therapeutic agents have been attempted for the treatment of urological cancers. Bacillus Calmette-Guérin (BCG) therapy was the first immunotherapy to treat superficial bladder cancer reported by Morales et al. in 1976.Citation1 Later, cytokines such as interleukin 2 (IL2) and interferon alpha (IFNα) were used for the treatment of advanced renal cancer.Citation2,Citation3 To achieve better therapeutic effects, the adoption of new anticancer therapies, such as immune checkpoint inhibitors (ICIs), adaptive cell transfer therapy (ACT) and vaccines, is being developed and has proven to be beneficial to some patients. In particular, ICIs have dramatically changed the therapeutic landscape for patients with advanced cancers, including kidney and bladder cancer.Citation4,Citation5 In summary, a diverse range of immunotherapeutic approaches has emerged in an endless stream in recent decades with in-depth investigations into the mechanisms at the basis of immune escape or immune suppression.
As an important part of bibliometric analysis, citation analysis is a widely used method to evaluate the academic influence of a paper.Citation6,Citation7 The top-cited papers were usually considered the most important or most impactful research within the field.Citation7,Citation8 Bibliometric analysis, including citation analysis, has been widely used to evaluate the current status of the research field, such as urological surgery,Citation9 metastatic castration-resistant prostate cancer,Citation10 robotic exoskeletons,Citation11 analytic network process.
A bibliometric analysis will first be performed to identify the top 100 most-cited articles (T100) and articles published in the last 2 years on immunotherapy for urological cancer. The purpose of this study is to identify the detailed characteristics of the T100 articles, provide an overview of the whole study area and attempt to predict some future trends on the basis of current research.
Methods
The Clarivate Web of Science Core Collection (Science Citation Index Expanded) database, one of the most important scientific literature databases, was searched to identify related papers in the field of immunotherapy related to urological cancer on January 17, 2021. A complicated search strategy was adopted to include all the relevant papers. Papers published between 2005 and 2020 related to immunotherapy, especially immune checkpoint inhibitors (ICIs), adoptive cell transfer therapy (ACT), vaccines, bacillus Calmette-Guerin (BCG) and cytokines in urological cancers, were selected and ranked according to the citation number. Only peer-reviewed article types and reviews were included, while practice guidelines were excluded. All 100 cited articles (T100) were carefully reviewed by two independent urologists in several rounds. T100 articles were further reviewed and analyzed according to the following information: citations, topic, year of publication, country of origin, institution and authorship. Papers from England, Wales and Scotland were grouped under the heading United Kingdom (UK).
To better understand the current situation and predict future trends, the same search strategy was adopted on November 21, 2021. 3741 papers published in recent 2 years were selected for further keywords analysis, including 45 ESI highly cited papers and 5 ESI hot papers. According to the Essential Science Indicators ™ database, highly cited papers reflect the top 1% of papers by field and publication year. Hot papers are defined as papers cited in the top 0.1% in a current bimonthly period and the papers are selected in each of 22 fields of science and must be published within the last two years.Citation12
Results
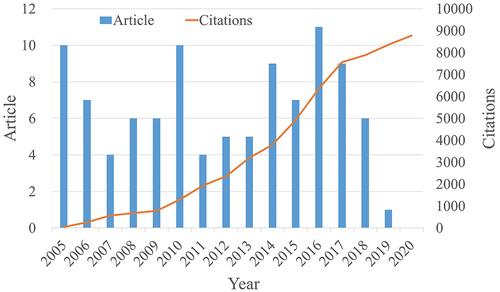
There were 18,471 articles that fit the search criteria from 2005 to 2020. The T100 articles in the field of immunotherapy related to urological cancer were selected by two independent reviewers, as shown in and Supplementary Table. The citation number of the T100 articles ranged from 183 to 7387, with a mean of 590.66. The top-cited paper was written in 2012 by Professor Topalian, SL, describing the safety and activity of anti-PD-1 antibody in cancer. The most recent T100 article was published in 2019 with 274 citations and discussed the tumor mutation burden as an immunotherapy biomarker.
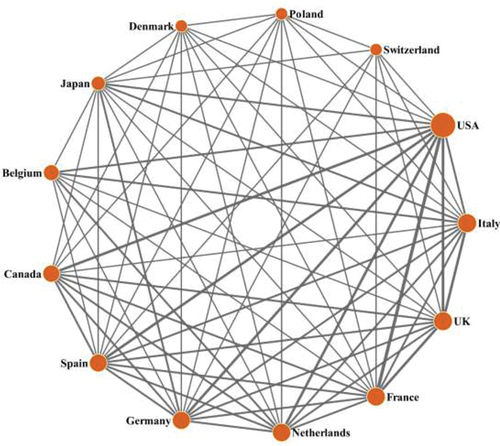
Twenty-eight countries in the world had participated in the T100 articles, 13 of which were related to more than 4 T100 articles, as seen in . The international collaboration network within the 13 countries is shown in . The USA dominated the field with 80 T100 articles and 53,097 citations. Scientists from the USA collaborated with scientists from 25 other countries. The UK ranked second with 18 T100 articles and 13,247 citations (collaborating with scientists from 26 countries), followed by Italy (18 T100 articles and 11,399 citations) and France (17 T100 articles and 13,794 citations). The USA was the leader of the scientific collaboration network and had strong collaborative links with other countries, such as the UK, Canada and France.
Table 1. Countries of origin of the T100 articles
Overall, 1006 researchers from the whole world contributed to the T100 articles. The scientists publishing more than 3 T100 articles as first or corresponding authors are displayed in . Prof. Sharma P from the MD Anderson Cancer Center was at the top of list with 8 T100 articles (3 as first author and 6 as corresponding author) and 5466 citations. Prof. McDermott DF ranked second, with 9 T100 articles (4 as first author and 4 as corresponding author) and 12,918 citations. Prof. Topalian SL from Johns Hopkins University published 9 T100 articles (1 as first author and 2 as corresponding author) with the largest number of citations of 17,330. Notably, 15 scientists publishing 2 T100 articles as first or corresponding authors were not included in the table.
Table 2. The most prolific authors among the T100 articles
A total of 409 worldwide institutions were involved in the T100 articles, of which 18 were involved in more than 7 T100 articles, as seen in . Thirteen of those top institutions are from the USA, and the remaining five are from the UK (2 in total), France,Citation1 SwitzerlandCitation1 and the Netherlands.Citation1 The first-ranked institution, namely, the Memorial Sloan Kettering Cancer Center, is from the USA, with 26 T100 articles and 22,573 citations. Johns Hopkins University ranked second, with 21 T100 articles and 25,095 citations. Harvard University ranked third, with 21 T100 articles and 24,554 citations.
Table 3. The 18 top institutions among the T100 articles
The T100 articles were published in 39 journals, including general journals and journals specific mostly for oncology, immunology and urology. Of those, 16 journals published more than 2 T100 articles, as given in . The Journal of Clinical Oncology published the most T100 articles with a total of 11,523 citations, followed by Clinical Cancer Research with 10 T100 articles and 4356 citations and Lancet Oncology with 10 T100 articles and 3783 citations. The New England Journal of Medicine published 4 T100 articles with the largest number of citations of 18,434.
Table 4. The journal distribution of the T100 articles
According to the topics and organs in the T100 articles, as identified in , 49 T100 articles were related to renal immunotherapy, of which 27 discussed immune checkpoint inhibitors, 9 discussed cytokines, 3 were about vaccine and adoptive cell transfer therapy, and the remaining 5 were related to other immunotherapies. Prostate cancer ranked second, with 29 T100 articles, while 14 articles discussed vaccines and 13 articles were related to ICIs. In the articles related to bladder and urothelial cancer, 15 articles discussed ICIs, and 8 articles discussed BCG. Regardless of the organ, most T100 articles were related to ICIs (55 articles) and vaccines (19 articles).
Table 5. Topic and organ distribution in the T100 articles
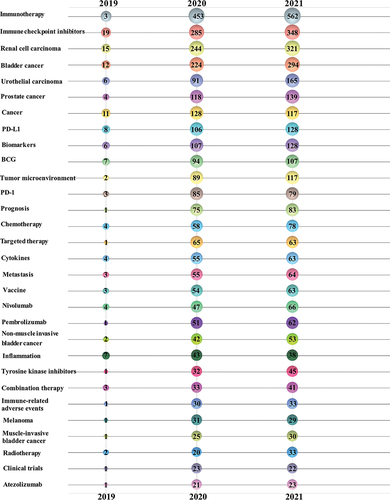
The keywords reflect the core and focus of a paper.Citation10 The author keywords in the 3741 papers published from November 21, 2019 to November 21, 2021 were analyzed. The top 30 author keywords by year were displayed in . The keyword “Immune checkpoint inhibitors” ranked second with appearance of 652, followed by “renal cell carcinoma” (580, third), ”bladder cancer” (530, fourth), ”urothelial carcinoma” (262, fifth), ”prostate cancer” (261, sixth). Besides, “non-muscle invasive bladder cancer” ranked 21th and “muscle invasive bladder cancer” ranked 27th. They were excluded from the keyword “bladder cancer.”There were other therapies including “BCG,” ”chemotherapy,” ”targeted therapy,” ”cytokines,” ”vaccine” and “radiotherapy.”
To better understand the hotspot and predict the future research, the 45 ESI highly cited papers and 5 ESI hot papers were analyzed in depth, as seen in Supplementary Table S2. Of the 45 papers, 36 papers were directly or indirectly related to ICI while only 4 papers were associated with vaccine. Of the 36 papers, 11 papers involved clinical trials while 13 papers involved predictive biomarkers, action and resistance mechanisms, and 10 papers were literature reviews. Of the four papers relevant to vaccine, two papers reported animal experiments, while one involved clinical trial and the rest one was literature review. As most studies investigated renal cancer, bladder cancer and urothelial carcinoma, only 6 articles were relevant to prostate cancer (four about biomarkers and mechanisms, two about animal experiments).
Discussion
Numerous urologists, oncologists and immunologists have made a variety of attempts to develop immunotherapies for urological cancer. BCG was first reported to treat bladder cancer in the 1970s and then became the standard therapy for high- or intermediate-risk NMIBC because it could reduce the risk of NMIBC recurrence.Citation13 Renal cancer was reported to respond to cytokines such as IFNs and interleukin 2 (IL-2) in the 1980s and was treated with cytokine therapy for nearly 20 years until the appearance of targeted therapy in 2005.Citation14 With the understanding of the mechanisms related to failures of the immune system and the development of new technologies such as sequencing technology or omics technologies, various immune approaches have been attempted, while some have shown promise in treating advanced cancers. Most immune agents have been reported to result in only modest survival benefits. The dendritic cell vaccine (sipuleucel-T), targeting PAP for MCRPC, was the first FDA-approved cell-based cancer vaccine in 2010.Citation15 In 2011, ipilimumab, a CTLA-4 blockade, was the first FDA-approved immune checkpoint inhibitor (ICI) to treat melanoma.Citation16 Around the year 2015, there was an increase in ICIs with unprecedented therapeutic efficacy, such as anti-PD1 and anti-PD-L1 antibodies, proving that the appearance of ICIs was a landmark breakthrough in the treatment of a variety of solid tumors, including renal and bladder cancer.Citation17,Citation18 Other immunotherapeutic approaches, such as vaccines, ACT and oncolytic virotherapy, also seem to play a critical role in the future treatment of cancer. Several agents (e.g., BCG) have become the standard treatment from the beginning, while some (IL-2) have even been widely used for decades and have been excluded from mainstream programs today.Citation2,Citation19 Immunotherapy of urological cancer has changed dramatically, so a retrospective and bibliometric study is required to reveal an overview of the whole study area and attempt to predict some future trends on the basis of current research.
Similar to other studies, the USA is also the leader in the area of immunotherapy of urological cancer in terms of T100 articles, citation numbers, institutions and scientists.Citation20,Citation21 Several factors could have contributed to this result: impacts of science and technology (S&T) policy,Citation9,Citation22 abundant financial support from the national government as well as private industries and foundations, global talent attraction,Citation23 the innovative spirit of the USA,Citation24 the emphasis on applied research and industrial development, close domestic and international collaboration and so on. While cancer is a global burden, new economic powers such as China and India should make more efforts to help solve this problem with respect to financial support, more active public health policy on cancer and more research programs. More importantly, cross-disciplinary communication and cooperation, especially international cooperation, should be promoted with great advantages.Citation25,Citation26 Most scientists, such as Prof. Sharma P (MD Anderson Cancer Center) and McDermott DF (Beth Israel Deaconess Medical Center), published T100 articles from top institutions, and they were forerunners and authoritative experts in their fields and made great contributions to global cancer treatment. They were also fit for international cooperation and communication, but further studies are needed.
Considering the outline of immunotherapy in urological cancer during the past 15 years, the treatment effect and type of immunotherapy differed within organs. In the T100 articles, urological cancer has been reported to respond to several kinds of immunotherapy (i.e., ICIs, vaccines and ACT therapies) with different response rates due to their immunogenic cancer cells. ICIs have been used to treat advanced renal cancer, bladder cancer and urothelial cancer, with impressive response rates and manageable toxicity profiles.Citation4,Citation5 In terms of prostate cancer, only a few limited patients have shown a response to ICIs.Citation27,Citation28 Researchers have found that some signaling pathways are related to immunosuppression, so ICIs in combination with targeted therapy (tyrosine kinase inhibitors such as sunitinib) could also enhance the antitumor effect.Citation14,Citation29 Now, ICIs-based therapy has been the standard of care as first-line therapy for patients with advanced renal cancer. Judging from the papers published in the last 2 years and the 45 ESI highly cited papers, ICIs will still be the hotspot in the next few years. The first present research focused on the application in different stages of renal and bladder cancer. The clinical trials have proven Pembrolizumab’s positive treatment effects as adjuvant therapy in patients with renal cell carcinoma,Citation30 alternative therapy in patients with BCG-unresponsive non-muscle-invasive bladder cancer,Citation31 first line therapy in patients with advanced advanced clear cell renal cell carcinoma and non-clear cell renal cell carcinoma.Citation32,Citation33 However, adjuvant atezolizumab in muscle-invasive urothelial carcinoma did not met its primary endpoint. Citation34 Thus, the application of ICIs need further investigation to expand and we could expect the changes of the standard care in the following few years. The combination of ICIs and other therapies have also attracted some attention. The possible combined approaches include target therapy, chemotherapy, radionuclide therapy,Citation35 cytokine,Citation36 vaccineCitation37 and so on. With the deeper understanding of the response and resistance mechanisms, academic circles will still pay much attention to discover more effective predictive biomarkers, to design more rational and effective therapeutic combination strategies, to improve the management of immune-related adverse events.Citation38 In addition, new ICIs targeting TIM-3, LAG-3, B7-H3, B7-H4, VISTA, CEACAM1, BTLA, CD200-CD200R, Siglec15 and TGIT are being developed and might be different from the current ICIs of urological cancers.Citation5,Citation39
Cancer vaccines are the research priorities for the development of immunotherapy. However, most vaccines for cancer are promising but fraught with difficulty, except for two prophylactic vaccines targeting papillomavirus and hepatitis B virus and one therapeutic cancer vaccine sipuleucel-T. Sipuleucel-T was an immune cell-based vaccine that prolonged overall survival among men with metastatic castration-resistant prostate cancer and has been part of the standard therapy for advanced prostate cancer.Citation40,Citation41 Most of the vaccines in clinical trials had a modest efficacy because of the lack of tumor specificity and poor immunogenicity. Attributed to improvements in high-throughput technologies and prediction algorithms, neoantigen identification (mostly tumor-specific antigens, TSAs) makes personalized vaccines promising, as TSAs are tumor-specific and have the lowest risks of autoimmunity.Citation42 Given that ICIs had great effect on advanced cancer, personalized neoantigen-based vaccine therapy in combination with ICIs in patients with advanced bladder cancer and other solid cancer, had demonstrated encouraging results.Citation37 Besides, more clinical trials of neoantigen-based cancer vaccines are ongoing for bladder and renal cancer.Citation43 Another aspect of vaccine study is the research of vaccine delivery systems about how to increase the delivery efficiency. Novel nanoparticle-based delivery systems such as lipid based, polymer based, inorganics based, and bio-inspired delivery systems have been explored as potential delivery tools.Citation44 In conclusion, neoantigen-based cancer vaccines will be a promising approach in the future. More work needs to be done to identify the different vaccine platforms and combination therapies to break tumor tolerance and induce long-lasting immunity.
Although vaccine therapies and ICIs have clinical effects in selected patients, new therapies, such as BiTEs, CAR-T cells and oncolytic virus therapy, especially armed with functional transgenes, will offer intriguing and promising avenues for advanced cancers.Citation27,Citation45 With a greater understanding of the mechanisms related to the recovery of specific immunosuppressive pathways, identifying suitable targets for different target mechanisms, making personalized and combined immunotherapy to improve the treatment effects and reduce the side effects possible, will be a promising approach for advanced cancer treatment.
The study also has some limitations. Firstly, the Web of Science Core Collection (WoS) covered fewer journals than Scopus and might have missed some articles and underestimated the number of citations;Citation46 Secondly, some recently published but important papers were not included with fewer citations. Thirdly, immunotherapy is far-ranging, and some related papers were excluded due to selection bias, even though urology doctors were carefully selected.
Conclusions
This is the first bibliometric analysis to identify the characteristics of T100 articles on immunotherapy for urological cancer and to reveal an overview of the whole study area and attempt to predict some future trends on the basis of current research. The USA made great contribution in the field of immunotherapy related to urological cancer. Renal, bladder and prostate cancers were the major organs treated by immunotherapy, especially by ICIs and vaccines. With deeper research on tumor immunology, the applications, predictive biomarkers, response or resistance mechanisms, combination strategies of ICIs and neoantigen-based vaccine therapy will be promising focuses of immunotherapy research in the next few years.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Authors’ contributions
LG He: Project development, manuscript writing
XL Wang: Project development, Data collection,Manuscript writing
CJ Li: Data collection
YH Wan: Data analysis
H Fang: Project development, Data analysis, manuscript writing
LG He and XL Wang contributed equally to this work and should be considered co-first author. All authors were involved in final approval of the version to be published.
Supplemental Material
Download Rich Text Format File (207.8 KB)Supplemental Material
Download Rich Text Format File (311.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2035552
Additional information
Funding
References
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–8. doi:10.1016/s0022-5347(17)58737-6.
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–58. doi:10.4049/jimmunol.1490019.
- Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005:CD001425. doi:10.1002/14651858.CD001425.pub2.
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi:10.1200/JCO.2014.59.4358.
- Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33:23–35. doi:10.1016/j.coi.2015.01.006.
- Cui L. Rating health web sites using the principles of citation analysis: a bibliometric approach. J Med Internet Res. 1999;1:E4. doi:10.2196/jmir.1.1.e4.
- Jia Z, Ding F, Wu Y, He Q, Ruan D. The 50 most-cited articles in orthopaedic surgery from mainland China. Clin Orthop Relat Res. 2015;473(7):2423–30. doi:10.1007/s11999-015-4132-1.
- Gisvold SE. Citation analysis and journal impact factors–is the tail wagging the dog? Acta Anaesthesiol Scand. 1999;43:971–73. doi:10.1034/j.1399-6576.1999.431001.x.
- He L, Fang H, Wang X, Wang Y, Ge H, Li C, Chen C, Wan Y, He H . The 100 most-cited articles in urological surgery: a bibliometric analysis. Int J Surg. 2020;75:74–79. doi:10.1016/j.ijsu.2019.12.030.
- He L, Fang H, Chen C, Wu Y, Wang Y, Ge H, Wang L, Wan Y, He H. Metastatic castration-resistant prostate cancer: academic insights and perspectives through bibliometric analysis. Medicine (Baltimore). 2020;99:e19760. doi:10.1097/MD.0000000000019760.
- Bao G, Pan L, Fang H, Wu X, Yu H, Cai S, Yu B, Wan Y. Academic review and perspectives on robotic exoskeletons. IEEE Trans Neural Syst Rehabil Eng. 2019;27(11):2294–304. doi:10.1109/TNSRE.2019.2944655.
- Essential Science Indicators.
- Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FCD, Oosterlinck W, Prescott S, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette-Guerin. Eur Urol. 2016;69:60–69. doi:10.1016/j.eururo.2015.06.045.
- Hasanov E, Gao J, Tannir NM. The immunotherapy revolution in kidney cancer treatment: scientific rationale and first-generation results. Cancer J. 2020;26:419–31. doi:10.1097/PPO.0000000000000471.
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–26. doi:10.1158/1078-0432.CCR-10-3126.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi:10.1056/NEJMoa1003466.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi:10.1056/NEJMoa1200690.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi:10.1056/NEJMoa1200694.
- Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus Calmette-Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174(1):86–91. discussion 91-82. doi:10.1097/01.ju.0000162059.64886.1c.
- Connelly TM, Malik Z, Sehgal R, Byrnes G, Coffey JC, Peirce C. The 100 most influential manuscripts in robotic surgery: a bibliometric analysis. J Robot Surg. 2019;14:155–65. doi:10.1007/s11701-019-00956-9.
- Kim HJ, Yoon DY, Kim ES, Lee K, Bae JS, Lee J-H. The 100 most-cited articles in neuroimaging: a bibliometric analysis. Neuroimage. 2016;139:149–56. doi:10.1016/j.neuroimage.2016.06.029.
- Mehta G, Hopf H, Krief A, Matlin SA. Realigning science, society and policy in uncertain times. R Soc Open Sci. 2020;7(5):200554. doi:10.1098/rsos.200554.
- Hofman K, Kramer B. Human resources for research: building bridges through the Diaspora. Glob Health Action. 2015;8:29559. doi:10.3402/gha.v8.29559.
- Califf RM. The US food and drug administration and cardiovascular medicine: reflections and observations. Circulation. 2016;134:501–03. doi:10.1161/CIRCULATIONAHA.116.022137.
- Frich JC, Brewster AL, Cherlin EJ, Bradley EH. Leadership development programs for physicians: a systematic review. J Gen Intern Med. 2015;30(5):656–74. doi:10.1007/s11606-014-3141-1.
- Anderson G, Metcalfe A. Calling for international collaborative research in nursing, genetics and genomics: a discussion paper. Int J Nurs Stud. 2008;45:323–28. doi:10.1016/j.ijnurstu.2006.08.013.
- Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–93. doi:10.1038/nri2817.
- Fay EK, Graff JN. Immunotherapy in prostate cancer. Cancers (Basel). 2020:12. doi:10.3390/cancers12071752.
- Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–82. doi:10.1158/1078-0432.CCR-07-5212.
- Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y-H, Hajek J, Symeonides SN, Lee JL, Sarwar N, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–94. doi:10.1056/NEJMoa2106391.
- Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF, Grivas P, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919–30. doi:10.1016/S1470-2045(21)00147-9.
- McDermott DF, Lee JL, Bjarnason GA, Larkin JMG, Gafanov RA, Kochenderfer MD, Jensen NV, Donskov F, Malik J, Poprach A, et al. Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol. 2021;39:1020–28. doi:10.1200/JCO.20.02363.
- McDermott DF, Lee JL, Ziobro M, Suarez C, Langiewicz P, Matveev VB, Wiechno P, Gafanov RA, Tomczak P, Pouliot F, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021;39:1029–39. doi:10.1200/JCO.20.02365.
- Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz M, Degaonkar V, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(4):525–37. doi:10.1016/S1470-2045(21)00004-8.
- Czernin J, Current K, Mona CE, Nyiranshuti L, Hikmat F, Radu CG, Lückerath K. Immune-checkpoint blockade enhances 225 Ac-PSMA617 efficacy in a mouse model of prostate cancer. J Nucl Med. 2021;62(2):228–31. doi:10.2967/jnumed.120.246041.
- Sharma M, Khong H, Fa’ak F, Bentebibel S-E, Janssen LME, Chesson BC, Creasy CA, Forget M-A, Kahn LMS, Pazdrak B, et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat Commun. 2020;11(1):661. doi:10.1038/s41467-020-14471-1.
- Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183(2):347–362 e324. doi:10.1016/j.cell.2020.08.053.
- Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–57. doi:10.1158/2159-8290.CD-20-1680.
- Hu J, Yu A, Othmane B, Qiu D, Li H, Li C, Liu P, Ren W, Chen M, Gong G, et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics. 2021;11(7):3089–108. doi:10.7150/thno.53649.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi:10.1056/NEJMoa1001294.
- Cornford P, van den Bergh RCN, Briers E, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–82. doi:10.1016/j.eururo.2020.09.046.
- Pan RY, Chung WH, Chu MT, Chen S-J, Chen H-C, Zheng L, Hung S-I. Recent development and clinical application of cancer vaccine: targeting neoantigens. J Immunol Res. 2018;2018:4325874. doi:10.1155/2018/4325874.
- Chu Y, Liu Q, Wei J, Liu B. Personalized cancer neoantigen vaccines come of age. Theranostics. 2018;8(15):4238–46. doi:10.7150/thno.24387.
- Liang J, Zhao X. Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development. Cancer Biol Med. 2021;18(2):352–71. doi:10.20892/j.2095-3941.2021.0004.
- Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at Dawn. Cancer Sci. 2016;107:1373–79. doi:10.1111/cas.13027.
- Ramos MB, Koterba E, Rosi Junior J, Teixeira MJ, Figueiredo EG. A bibliometric analysis of the most cited articles in neurocritical care research. Neurocrit Care. 2019;31:365–72. doi:10.1007/s12028-019-00731-6.