ABSTRACT
It is unknown how long the immunity following COVID-19 vaccination lasts. The current systematic review provides a perspective on the persistence of various antibodies for available vaccines.Both the BNT162b2 and the mRNA-1273 induce the production of IgA antibodies, reflecting the possible prevention of the asymptomatic spread. The mRNA-1273 vaccineʻs antibodies were detectable until 6 months, followed by the AZD1222, 3 months, the Ad26.COV2.S and the BNT162b2 vaccines within 2 months.The BNT162b2 produced anti-spike IgGs 11 days after the first dose and peaked at day 21, whereas the AZD1222 induced a neutralizing effect 22 days after the first dose.These vaccines induce T-cell mediated immune responses too. Each one of the AZD1222, Ad26.COV2.S, mRNA-1273 mediates T-cell response immunity at days 14-22, 15, and 43 after the first dose, respectively. Whereas for the BNT162b1 and BNT162b2 vaccines, T-cell immunity is induced 7 days and 12 weeks after the booster dose, respectively.
1. Introduction
The current COVID-19 pandemic has affected millions of people across the globe. According to the World Health Organization (WHO), nearly 5.1 million deaths have been reported on November 19th,2021.Citation1 Therefore, there is an essential need for vaccination campaigns to reduce COVID-19 mortality and its detrimental impacts on society. Most countries have started vaccination programs as a promising way for their citizens to limit the damages of COVID-19. According to the WHO plan, countries relying on COVID-19 Vaccines Global Access (COVAX) alone may hardly vaccinate more than 20% of their population.Citation2,Citation3 Indeed, an ideal vaccine should elicit long-term protection.Citation4
Furthermore, early diagnosis of COVID-19 is vital for controlling and managing the pandemic. Some of the COVID-19 diagnosis techniques, based on antigen detection, require nasopharynx sampling; alternative sampling methods like saliva have been used in various studies. Antibody detection methods are also common, which target the viral spike protein or the nucleocapsid. The detection technologies of antibodies against these two SARS-CoV-2 antigens also differ. Enzyme-linked immunosorbent assays (ELISA) and chemiluminescence immunoassays (CLIA) are commonly used as laboratory assays. Also, other techniques such as lateral flow immunoassays, fluorescence-label techniques, or colloidal gold are most widely performed. While the non-quantitative serological testing can be used for epidemiological surveys to detect the attack rate of the disease, the quantitative or the semi-quantitative methods are used for the prediction of the severity of the disease. The highest accuracy of the serological testing is reached between 3 and 4 weeks after the onset of the initial signs and symptoms with checking the total immunoglobulins or the IgG levels. Each one of the IgM and IgA levels are related to less accuracy.Citation5
The immune response to the SARS-CoV-2 virus causes a variety of clinical manifestations. While adaptive immune responses play a significant role against this virus, the innate immune cells somehow lead to disease progression. Macrophages, the major players of innate immunity, are associated with significant production of Interleukin-6; therefore, leading to excessive inflammation in COVID-19. In the adaptive immune response, while the T-cell mediated immune response is inhibited as the downregulation of MHC class I and II molecules occurs, the humoral immune system plays an essential role in controlling the COVID-19 disease. Although IgM and IgG antibodies appear to have similar dynamics, IgA response is more robust in comparison to IgM response.Citation6
There is a strong relation between cell-mediated immunity, the severity of infection and survival; while the severe COVID-19 infected individuals had increased levels of anti-receptor binding domain (RBD) antibodies the high potent neutralizing antibodies served as a predictor of survival.
COVID-19 immunity may not remain in individuals who previously had the infection. Mild COVID-19 cases usually reach antibody responses after 4 months, although most patients manifest this response during day 10 to day 21 post-infection. Neutralizing antibodies levels begin to decrease about 2 months after the acute phase of disease.Citation7 On the other hand, some studies demonstrated that natural immunity obtained against SARS-CoV-2 does not wane until 10 months post-infection, and the risk of reinfection is shallow 7 months post-infection, considering the pivotal role of natural immunity in controlling the disease.Citation8
Due to the fast emergence of different COVID-19 vaccines with varying mechanisms of action, recognition of the immune response profile following each vaccine becomes more and more challenging, yet very important.
Insight on the onset and the duration of antibody response following each vaccine helps control the spread of the disease, institute timely isolation strategies, and improve the epidemiological outcomes of this pandemic.
The production of vaccine-induced antibodies causes the body to give an anamnestic immune response in exposure to SARS-COV-2.Citation9
Many studies sought to demonstrate the efficacy and the quality of immunogenicity of COVID-19 vaccines. However, neutralizing antibodies can exist for more extended periods and therefore help reduce the transmission and mortality of COVID-19 longer.Citation10 Although most studies have shown acceptable short-term efficacy of vaccines, information on long-term immunogenicity is still limited.
Furthermore, It is a considerable challenge to assess the effective duration of vaccine-induced immunity.Citation11,Citation12 More research is needed to investigate the long-term efficacy and safety of the vaccines and the impact of different factors on the longevity of the immune response.Citation10 This systematic review provided a summary of immune response profiles, type of antibody response, the onset of humoral immunity, and its duration following each currently approved COVID-19 vaccine.
2. Methods
We performed this systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Before starting the search in the databases to clarify the review process and avoid unintentional duplications, we registered the proposal of this systematic review in PROSPERO [CRD42021262005].
2.1. Search strategy
Three investigators independently looked for research papers related to the longevity of the immune response following COVID-19 vaccines. Articles in PubMed and Scopus were selected using, for example, the following search terms: “COVID-19 Vaccines”, “SARS-CoV-2 vaccine”, “2019-nCoV vaccine”, “2019-nCoV vaccines”, “immunity,” “immune process,” “immune Response,” immunity and “COVID 19 vaccine”, “immune Response” AND “COVID 19 vaccine”.
A similar search was performed in the Google Scholar search engine to identify preprints and unpublished articles. No time restriction was set, and all search results until May 9th, 2021, were imported. The results were then imported into the Rayyan software, created by Qatar Computing Research Institute.
2.2. Study selection
In this systematic review, we included English articles that evaluated the efficacy and duration of COVID-19 vaccine-induced immunity and for which the full text was available. Medical news, non-medical papers, reviews, letters, commentaries, conference abstracts, and pre-clinical studies were excluded.
Two Researchers independently screened the results and selected the appropriate articles derived from selected databases based on the inclusion criteria stated above
Duplicates imported into Rayyan were then removed. Subsequently, the titles and abstracts were screened to exclude irrelevant studies and studies without associated full-text.
2.3. Data extraction
Systematic data extraction was implemented in the following manner: The first author name, year of publication, study type, country name, vaccine type, manufacturer, the interval between injections, intervention group number, placebo group number, vaccine efficacy, time to peak neutralizing antibody titers, time for optimal binding antibody responses, the duration of antibody detection in the blood, and antibody waning time. Each of the two investigators separately extracted data into the data collection sheet. Afterward, discrepancies between researchers were discussed and checked with the third reviewer to resolve the conflicts.
2.4. Quality assessment
We considered the modified JADAD scale or the Oxford quality scoring system to assess the methodological quality of the publications. We chose this scoring system as it is the preferred quality assessment tool for randomized control trials (RCT) and that most of the included studies were RCTs.Citation13
The JADAD scale mainly consists of six items which are described below in detail.
Item number 1: Was the study described as randomized? If yes, a score of 2 is given for acceptable randomization tools (e.g., computer-generated), and a score of 1 is given for inappropriate methods. If no, no score is given.
Item number 2: Was the trial stated as double-blind? If yes, a score of 2 is given for acceptable double-blinding methods (e.g., identical placebo), and a score of 1 is given for inappropriate methods. If no, no score is given.
Item number 3: Was there a description of dropouts and withdrawals? If yes, a score of 1, and if no, a score of 0 is given. Scores on the scale can range from 0 to 5, with higher numbers signifying higher quality. Studies with three or higher points are considered high quality, whereas those with less than three are considered low-quality trials.Citation14
The other four additional questions were included from the modified version of the JADAD scale: Was there a clear description of the inclusion/exclusion criteria? Was the method used to assess adverse effects described? Was the method used to assess adverse effects described? Were the methods of statistical analysis described? Each positive response is worth one point, whereas a negative response earns no points—scores on the modified JADAD scale range from 0 to 8, with higher numbers indicating higher-quality trials. Scores of 1–3 denoted poor quality, while scores of 4–8 denoted excellent quality.Citation15
Two authors (P.Sh. and Y.Kh.) independently assessed the quality of each eligible paper, which was subsequently double-checked by a third reviewer (K.M.).
3. Results
3.1. Study selection
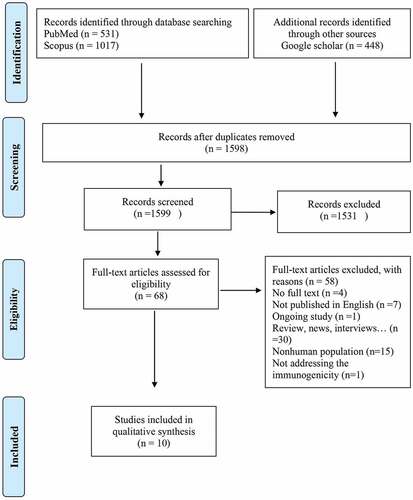
1996 studies were retrieved from the database search, of which 397 studies were duplicates, leaving us with 1599 results. We conducted title and abstract screening on the remaining articles, the number of 1531 studies then were excluded, as they addressedissues outside of our research question such as COVID-19 vaccine development, treatment, and therapeutic agents, COVID-19 prevalence, or hesitancy to vaccines. Among the remaining 68 studies, we excluded the Nonhuman population (n = 15), review articles, news and views (n = 30), Ongoing studies (n = 1), a primary language other than English (n = 7), unavailable full text (n = 4) and not addressing the immunogenicity (n = 1). Finally, 10 original articles related to the longevity of immunity following approved COVID-19 vaccines were included. ()
3.2. Study characteristics
As summarized in , we classified studies according to their first author, year of publication, country, study type, age group, vaccine type, and manufacturer. Five of the studies were randomized clinical trials (RCTs), four were cohorts, and one case series. Four studies were conducted in the USA, three studies in the UK, and one study in Belgium, Italy, Germany, Brazil, South Africa, and Eswatini as either single or multinational trials.
Table 1. Studies characteristics
There were two multinational studies conducted by J. Sadoff et al. and Merryn Voysey et al.,Citation16,Citation17 Three studies investigated the immunogenicity of the BNT162b1 vaccine, two studies studied the BNT162b2 vaccine, two studies researched the mRNA-1273 vaccine, and one study compared the BNT162b1 and the mRNA-1273 vaccine. Two studies were conducted on the AZD1222 vaccine and one on the Ad26.COV2.S vaccine.
3.3. Study quality
As represented in , articles were divided into two groups, ≥5 and ≤3, according to the JADAD quality scale; 40% of the studies scored above 4, while 60% scored below 4. ()
Table 2. Quality assessment table
3.4. Data synthesis
In this review, we demonstrate the immunogenicity of four approved vaccines, AZD1222, Ad26.COV2.S, mRNA-1273 BNT162b1, and BNT162b2. A thematic qualitative study assessing and summarizing the results of each individual vaccine was performed. We mainly represent the timeframe when antibody production following vaccines was detected and the duration the antibody response remained detectable. The key findings of the selected publications are summarized in , and a summary of the timeline of detected immune responses is indicated in .
Figure 2. This figure illustrates the timeline of the immune responses induced by the AZD122, Ad26.COV.S,mRNA-1273, and BNT16b2 vaccines. the neutralizing effect was assessed by addingthe plasma of the vaccine recipients to SARS-CoV-2 spike antigens in vitro. TheBnt16b2 vaccine has the earliest detectable immune response by producing IgG,igm, and IgA at day 7; the neutralizing effect started at day 21 and continueduntil day 105. the mRNA-1273 vaccine immune response began at day 15, while theneutralizing effect started from day 28 and persisted until day 209. TheAd26.Cov.s immune response began with T-cell responses on day 15, while the neutralizingeffect started at day 28 and persisted until day 71. the AZD122 vaccine'simmune response began at day 15, while the neutralizing effect began at day 21 andlasted until day 90.
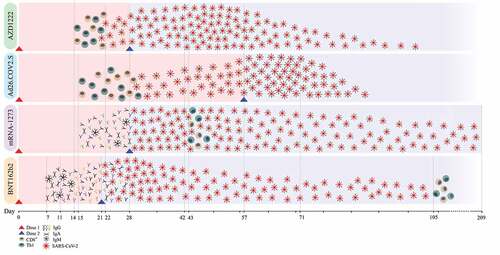
Table 3. Summary of immunologic profile following COVID-19 vaccines from selected publication
3.4.1 The immunogenicity of the AZD1222 vaccine
AZD1222 induced two types of immune response; humoral immune response, including IgG production against the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein or the S protein itself neutralizing antibodies, and a cell-mediated spike specific T-cell response after the first and the second dose.
A single dose of AZD1222 could induce anti-RBD and anti-S IgG production after 28 days, and their titers peaked 28 days after receiving the second dose. This antibody level remained stable until day 56, then decreased gradually until day 90, after which it decayed log linearly in 6 months.
Neutralizing antibodies and T-cell spike-specific responses were induced around 14–22 days after the first dose. The T-cell spike-specific responses were not significantly enhanced by receiving the second dose and persisted until days 90.Citation17,Citation18
3.4.2 The immunogenicity of the Ad26.COV2.S vaccine
Neutralizing antibodies and anti-spike binding IgG titers were detected in more than 90% of participants 29 days after the first vaccination dose. Then these percentages were increased to more than 96% of the participants with detecting the highest titers in the 18–55 age group. These levels of antibodies remained stable until day 71.Citation16
The T-cell responses were measured indirectly via the detection of interferons and interleukins. While T helper 1 cells (Th1) response, detected via the measurement of interferon-γ; IL-2; or both, was induced at day 15 from the first dose of vaccination with higher response rates in the high dose recipients and the 18–55 age group. No T helper 2 cells (Th2) were observed. The CD8+ T cells response was also present as the younger participants who received higher doses had higher response rates with an exception to the ≥65 years age group having higher response rates with low doses.Citation16
3.4.3 The immunogenicity of the mRNA-1273 vaccine
The mRNA-1273 vaccine (Moderna) induced neutralizing antibodies, anti-spike, anti-RBD IgG, and IgM approximately 15 days after receiving the 2nd dose and persisted between 3 to 6 months after this dose.Citation19–21
3.4.4 The immunogenicity of the BNT162b1 and BNT162b2 vaccines
Both the BNT162b1 and the BNT162b2 vaccines were produced by Pfizer–BioNTech companies.
The former induced low levels of neutralizing antibodies and anti-RBD-binding IgGs 21 days after the first dose. Then, seven days after receiving the second dose, both the neutralizing antibodies and anti-RBD-binding IgGs substantially increased in a dose-dependent manner. Fourteen days after the second dose, neutralizing antibody titers continued to rise. In comparison, the RBD-binding IgGs started to drop. 21 days after the second dose, both neutralizing antibodies and anti-RBD-binding IgGs dropped in all age groups and doses except for the 18–55 age group who received the 1 μg dose of the vaccine, which elicited a stable titer.Citation22,Citation23
BNT162b2 is an upgraded version of BNT162b1 with IgA production and better immunogenicity. While the production of neutralizing antibodies is induced three weeks after receiving the first dose, anti-RBD-binding, anti S1, and S2 IgGs are induced 11–21 days after the first dose. The production of anti S1 IgA is induced 7–11 days from the first dose.Citation24,Citation25
After receiving the second dose, anti-RBD-binding, anti S1, and S2 IgG, and IgM are induced at day 7 to reach a peak at day 14. The anti-S1 IgA hit a peak 7 days after the second dose. From day-14 to day 29 after the second vaccine dose, antibody titers gradually decreased; however, from day 29 to day 44, they reduced significantly and approached baseline levels. Thus, the BNT162b2 vaccine approximately provided two months of protection.Citation24,Citation25
4. Discussion
Vaccines direct the immune system toward providing immunity against infections. Various vaccines mainly aim for disease prevention and not necessarily full protection against specific infections.Citation26 While the ‘sterilizing immunityʻ provided by vaccines might wane in the long run, the protection against either the disease or the disease progression (severity) can remain for a longer period because of the immune memory.Citation27 This might make vaccines good candidates against the death toll and the burden of the COVID-19 infection. However, the condition is a bit challenging when it comes to the COVID-19 vaccination. Initially, the effectiveness of these vaccines, meaning the efficacy of a vaccine for preventing the disease in real-world situations not under certain controlled conditions -as it represents the efficacy of vaccines- is still questionable.Citation28 Although eight vaccines have the emergency use listing (EUL) of the WHO, which means they have reliable trials guaranteeing their 50% or more efficacy, their effectiveness is disputable mostly due to the emergence of new COVID-19 variants and the differences in the characteristics of vaccine recipients.Citation28,Citation29 Hence, knowing the relationship between the time and immunogenicity of various COVID-19 vaccines can take the world a step toward curbing this pandemic down.
Vaccines induce immunity via the utilization of two components, a pathogen-specific immunogen, and an adjuvant. The former component carries the viral protein, while the latter is in charge of activating innate immunity and providing a second signal for T cell activation. An ideal adjuvant precisely activates innate immunity and does not lead to systemic inflammation resulting in severe adverse effects.Citation30 In mRNA vaccines, mRNA serves as both the pathogen-specific immunogen and the adjuvant. These vaccines contain purified, in vitro-transcribed single-stranded mRNA with modified nucleotides. They bind less effectively to toll-like receptors (TLR) and immune sensors; therefore, restricting the excessive production of type I interferon and its inhibitory impact on cellular translation.Citation31
This scenario differs in adenovirus (AdV) vaccines, as both vaccine components—the immunogen and the adjuvant- exist and are not the same like mRNA vacccines. Both components are embedded in the viral component encoding the immunogenʻs DNA. AdV particles stimulate innate immune cells like dendritic cells and macrophages via binding to multiple pattern-recognition receptors and in particular to TLR9 resulting in the production of type I interferon.Citation32
Vaccine-induced type I interferon facilitates the differentiation of CD4+ and CD8+ effector T cells, leading to cytotoxic and inflammatory mediators and the CD4+ T follicular helper (TFH) cells that promote the differentiation of B cells into antibody-secreting plasma cells.Citation30
While mRNA and AdV vaccines induce optimal immunogenicity theoretically, the situation might differ in the real world. Thus, in this review, we discuss immunogenicity and particularly the relation between the timing of receiving vaccine shots and the presence of detectable amounts of anti-SARS-CoV-2 antibodies and T cell responses.
Starting with mRNA vaccines, the BNT162b2 showed the quickest vaccine-induced immune response; 7 to 11 days, for anti-spike IgA, and 11 to 21 days, for anti-spike IgG, after receiving the first dose of vaccine.Citation20,Citation24,Citation25
It is also worth mentioning, the mRNA-1273 and the BNT162b2 vaccines induced the production of the anti-spike IgAs, which might be effective in preventing the asymptomatic spread.Citation33
However, the BNT162b2 and the Ad26.COV2.S have a short duration of antibody persistence of about 2 months after the second dose.Citation24,Citation25 Findings of several cohorts and clinical trials have demonstrated that both symptomatic and asymptomatic COVID-19 infection after vaccination are not unexpected because vaccines could not be 100% effective. Host immune responses and susceptibility of new variants of SARS-CoV-2 for infection could be the cause of this failure. Given that immune response is multifractional and complex, it depends on both humoral and cellular immunity and it varies from person to person.Citation34 The antibody persistence time of the mRNA-1273 vaccine is about 180 days (six months), following the AZD122 vaccine with 90 days.Citation17–19
Indeed the antibody response raised by vaccines is roughly affected by not only the time but also the emergence of new SARS-CoV-2 variants; therefore, to mitigate the spread of this infection in the long run, a more effective immune response is needed.Citation35,Citation36
The T-cell response can serve as an optimal response in the long run; as was shown by Bange et al, 2021 higher levels of CD8+ T cell-mediated immunity was linked to the improvement of survival and the reduction of fatality, disease severity, or viral load among the patients with hematological malignancy receiving anti-CD20 therapy with low titers of anti-SARS-CoV-2-specific IgG considering the protective effects of CD8+ T cell-mediated immunity.Citation37
Moreover, T-cell response can fight better with SARS-CoV-2 new variants due to the variation of the HLA-specific T-cell epitopes among individuals and their wide distribution across proteins; thus, escaping from T-cell response is much harder.Citation36
Fortunately, the vaccines studied in this review elicit T-cell immunity providing a backup mechanism coping with new SARS-CoV-2 variants and the waning of humoral immunity—each one of the Ad26.COV2.S, AZD1222, mRNA-1273 vaccines elicit T-cell responses respectively, 15 days, 14–22 days, and 43 days from receiving the first vaccination dose.
Lastly, the BNT162b1 and the BNT162b2 vaccines promote T-cell immunity respectively after 7 days and 12 weeks from receiving the second vaccination dose. Look at and .
Although the function of these T-cell responses is still debatable and more studies are needed to approve their effectiveness and function, their presence might provide hopeful promises toward mitigating the spread of this pandemic and controlling its burden.
5. Limitations
Various limitations surround this review. To begin with, the focus of this review is mainly on the mRNA and viral vector vaccines; other EUL vaccines are missed due to the lack of data. Moreover, the reported results of this study are not comparable between various vaccines due to study protocol variations; for instance, we cannot say that only BNT162b2 and mRNA-1273 produce IgA. Other vaccines might have this capability, but it was not reported because it was not a part of the study protocol. Furthermore, some of the included studies have small sample sizes resulting in enormous differences between the reported outcomes and the real-world outcomes, making them unreal and biased for the general population ().
6. Conclusion
AZD1222, Ad26.COV2.S, mRNA-1273, BNT162b1, and BNT162b2 vaccines have an acceptable immunogenicity and vaccine persistence of up to two months after the second dose (except for Ad26.COV2.S, which is administered at only one dose).
In summary, in this systematic review, we summarize key immunological data following four of the currently approved COVID-19 vaccines, while the immunogenicity of other vaccines is being explored at ongoing trials. More studies are needed on wider populations with various genetic and environmental backgrounds to emphasize and generalize these results.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO coronavirus (COVID-19) dashboard [Internet]. [accessed 2021 Sep 10]. https://covid19.who.int/
- Copper FA, de Vázquez CC, Bell A, Mayigane LN, Vedrasco L, Chungong S, de Vázquez CC. Preparing for COVID-19 vaccine roll-out through simulation exercises. Lancet Glob Health. 2021; 9(6):e742–10. doi:10.1016/S2214-109X(21)00051-6.
- Burki TK. Challenges in the rollout of COVID-19 vaccines worldwide. Lancet Respir Med. 2021;9(4):e42–3. doi:10.1016/S2213-2600(21)00129-6.
- Criteria for COVID-19 vaccine prioritization [Internet]. [accessed 2021 Sep 10]. https://www.who.int/publications/m/item/criteria-for-covid-19-vaccine-prioritization
- Lai CKC, Lam W. Laboratory testing for the diagnosis of COVID-19. Biochem Biophys Res Commun. 2021;538:226–30. doi:10.1016/j.bbrc.2020.10.069.
- Paces J, Strizova Z, Smrz D, Cerny J. COVID-19 and the immune system. Physiol Res. 2020;69:379–88. doi:10.33549/physiolres.934492.
- Salehi-Vaziri M, Jalali T, Farahmand B, Fotouhi F, Banifazl M, Pouriayevali MH, Sadat Larijani M, Afzali N, Ramezani A. Clinical characteristics of SARS-CoV-2 by re-infection vs. reactivation: a case series from Iran. Eur J Clin Microbiol Infect Dis. 2021;40(8):1713–19. doi:10.1007/s10096-021-04221-6.
- Murchu EO, Byrne P, Carty PG, De Gascun C, Keogan M, O’-Neill M, Harrington P, Ryan M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev Med Virol. 2021:e2260. doi:10.1002/rmv.2260.
- Giurgea LT, Memoli MJ. Navigating the Quagmire: Comparison and interpretation of COVID-19 vaccine phase 1/2 clinical trials. Vaccines (Basel) 2020;8(4):746 doi:10.3390/vaccines8040746.
- Xing K, Tu X-Y, Liu M, Liang Z-W, Chen J-N, Li J-J, Jiang L-G, Xing F-Q, Jiang Y. Efficacy and safety of COVID-19 vaccines: a systematic review. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:221–28.
- Yan Z-P, Yang M, Lai C-L. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals. 2021;14(5):406. doi:10.3390/ph14050406.
- Sagili Anthony DP, Sivakumar K, Venugopal P, Sriram DK, George M. Can mRNA vaccines turn the tables during the COVID-19 pandemic? Current status and challenges. Clin Drug Investig. 2021;41(6):499–509. doi:10.1007/s40261-021-01022-9.
- Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–75. doi:10.2522/ptj.20070147.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4.
- Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimerʻs disease drug trials. Dement Geriatr Cogn Disord. 2001;12(3):232–36. doi:10.1159/000051263.
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384(19):1824–35. doi:10.1056/NEJMoa2034201.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE et al. Single-Dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdox1 nCov-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91. doi:10.1016/S0140-6736(21)00432-3.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G et al. Safety and immunogenicity of ChAdox1 nCov-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Doria-Rose N, Suthar MS, Makowski M, Oʻ-Connell S, McDermott AB, Flach B, Ledgerwood JE, Mascola JR, Graham BS, Lin BC et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–61. doi:10.1056/NEJMc2103916.
- Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi:10.1056/NEJMc2032195.
- Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–22. doi:10.1038/s41586-021-03324-6.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–93. doi:10.1038/s41586-020-2639-4.
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D et al.COVID-19 vaccine BNT162b1 elicits human antibody and T H 1 T cell responsesNature 2020 [ accessed 2021 Jul 15]. 586(7830):594–99. doi:10.1038/s41586-020-2814-7.
- Danese E, Montagnana M, Salvagno GL, Peserico D, Pighi L, De Nitto S, Henry BM, Porru S, Lippi G. Comprehensive assessment of humoral response after Pfizer BNT162b2 mRNA Covid-19 vaccination: a three-case series. Clin Chem Lab Med. 2021;59(9):1585–91. doi:10.1515/cclm-2021-0339.
- Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, Haljasmägi L, Rumm P, Maruste R, Kärner J, et al. Declined antibody responses to COVID-19 mRNA vaccine within first three months. medRxiv [Internet] 2021 [cited 2021 Jul 15]. 2021.04.19.21255714. https://www.medrxiv.org/content/full10.1101/2021.04.19.21255714v2.full
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86(2):140–46. doi:10.2471/blt.07.040089.
- Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine. 2000;19:877–85. doi:10.1016/s0264-410x(00)00224-3.
- Vaccine efficacy, effectiveness and protection [Internet]. [accessed 2021 Nov 22]. https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection
- WHO issues emergency use listing for eighth COVID-19 vaccine [Internet]. [accessed 2021 Nov 22]. https://www.who.int/news/item/03-11-2021-who-issues-emergency-use-listing-for-eighth-covid-19-vaccine
- Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–97. doi:10.1038/s41577-021-00526-x.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. doi:10.1038/nrd.2017.243.
- Sayedahmed EE, Elkashif A, Alhashimi M, Sambhara S, Mittal SK. Adenoviral vector-based vaccine platforms for developing the next generation of Influenza vaccines. Vaccines (Basel). 2020;8. doi:10.3390/vaccines8040574.
- Bleier BS, Ramanathan M Jr, Lane AP. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol–head Neck Surg. 2021;164(2):305–07. doi:10.1177/0194599820982633.
- Alidjinou EK, Gaillot O, Guigon A, Tinez C, Lazrek M, Bocket L, Engelmann I, Hober D. SARS-CoV-2 infection long time after full vaccination is related to a lack of neutralizing antibodies. Diagn Microbiol Infect Dis. 2022;102(1):115565. doi:10.1016/j.diagmicrobio.2021.115565.
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–80. doi:10.1038/s41586-021-03777-9.
- Noh JY, Jeong HW, Kim JH, Shin E-C. T cell-oriented strategies for controlling the COVID-19 pandemic. Nat Rev Immunol. 2021;21(11):687–88. doi:10.1038/s41577-021-00625-9.
- Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, Greenplate AR, Hwee MA, Porterfield F, Owoyemi O et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27(7):1280–89. doi:10.1038/s41591-021-01386-7.