ABSTRACT
Objective
To evaluate the safety of concomitantly administering inactivated poliomyelitis vaccine produced from Sabin strains (sIPVs) with other vaccines.
Methods
A descriptive analysis was carried out on adverse events following immunization (AEFI) based on the administration of sIPV alone or concomitant with other vaccines (from 2015 to 2020) using data from the national AEFI surveillance system of China (CNAEFIS). All adverse reactions (ADRs) of the concomitant immunization were coded using a medical dictionary for regulatory activities (MedDRA) before comparison.
Results
The CNAEFIS reported a total of 9130 sIPV-related AEFI cases, including 6842 AEFI cases collected after immunization with sIPV alone and 2288 AEFI cases collected after immunization of sIPV concomitant with other vaccines. The combination of sIPV with diphtheria, tetanus and pertussis vaccine (DTaP) was correlated with the highest frequency of AEFI, which accounted for 53.50% of all 2288 AEFI cases. After MedDRA-based coding, the most frequent ADR was fever (70.18%), followed by erythema and swelling at the injection site (6.95%), induration at the injection site (3.85%), dermatitis allergy (3.56%) and urticaria (1.55%). A statistically significant difference (P < .001) was found between sIPV immunization and sIPV immunization concomitant with other vaccines for general reactions (95.36% and 93.22%, respectively) and abnormal reactions (4.64% and 6.78%, respectively).
Conclusion
No new safety signal is found for sIPV administered concomitantly, although its administration with other vaccines may increase the occurrence of abnormal reactions. Vaccine manufacturers should focus on the safety of administering sIPV with DTaP and carry out relevant clinical studies when necessary.
1. Introduction
Vaccination is recognized as the most effective medical intervention to reduce the burden of infectious diseases in the field of public health, and it has been particularly important during the coronavirus pandemic in 2020 and beyond.Citation1,Citation2 The increase in available vaccines on the market has led to a potential safety risk when administering vaccines at the same, especially for children.Citation3 According to the WHO Expanded Program on Immunization (EPI)Citation4 and the National Immunization Program (NIP) Schedule for Children in China,Citation5 a total of 19 vaccines should be administered to children before 3 years of age. Thus, the use of a combined vaccine or a concomitant vaccination strategy for two or more vaccines is recommended to improve vaccination compliance and reduce the costs of vaccination for individuals and parents.Citation6–9 In 2015, an inactivated poliomyelitis vaccine produced from Sabin strains (sIPVs) independently developed by a Chinese manufacturer was approved for use in China.Citation10 sIPV is regarded as a powerful weapon in eliminating poliomyelitis worldwide. With increased sIPV coverage, the safety of this vaccine has been widely studied.Citation11–14 Previously, multiple domestic and international sIPV-related clinical studies have shown that sIPV has good safety and immunogenicity when administered separately.Citation15–19 However, systematic research is lacking on the immunogenicity and safety of sIPV when concomitantly vaccinated with other vaccines. According to the numerous cases of adverse events following immunization (AEFI) collected after concomitant immunization of sIPV with other vaccines from the national AEFI surveillance system in China (CNAEFIS), it is reasonable to infer that there is a considerable number of recipients who require IPV-included concomitant vaccinations; therefore, the safety of concomitant immunization strategies for sIPV and other vaccines should be investigated.
To analyze the safety of sIPV-included concomitant immunization, we performed a retrospective review and analysis on the AEFI data collected after separate sIPV immunization and sIPV-included concomitant immunization with other vaccines (from 2015 to 2020). We evaluated the safety of sIPV-included concomitant immunization and provided a reference to further guide safe sIPV immunization.
2. Materials and methods
2.1. AEFI data collection
In this study, all sIPV-related AEFI data were obtained from the CNAEFIS, which is a nationwide passive surveillance system for AEFI.Citation20,Citation21 Safety monitoring about sIPV was carried out and all monitoring data was registered in CNAEFIS since 2015 when sIPV was approved for use in China. AEFI cases that occurred at different times were reported and uploaded into the system by a responsible reporting unit according to the National AEFI guidelines.Citation22,Citation23 The data evaluated in this article covered nationwide AEFI cases after administrating sIPV alone and simultaneous with other vaccines that were reported from January 2015 to December 2020.
2.2. AEFI classification
According to the National AEFI guidelines, AEFI can be classified into five categories according to cause, i.e., adverse reactions (ADRs), vaccine quality events, inoculation accidents, coincidental illnesses and psychogenic reactions. Among these, ADRs include general reactions and abnormal reactions, AEFI cases that cannot be classified into the abovementioned types are defined as “to be classified”.
2.3. statistical analysis
Limited by the inherent system setting of CNAEFIS, as the enterprise user, we cannot obtain the total number of recipients who received sIPV alone and for whom sIPV was administered simultaneously with other vaccines during the data collection period. The data recorded in the CNAEFIS are the actual data reported from a real-world vaccination program, and uncertainties were observed in the sensitivity, normalization, integrity and other aspects of the reported data. The comparison of AEFI cases between sIPV immunization alone and concomitant with other vaccines was based on the ratio of AEFI cases to the total number of AEFI cases rather than the incidence of AEFI.
All AEFI cases related to sIPV vaccination were exported from the CNAEFIS, and the screened cases were divided into two large groups, i.e., sIPV alone and concomitant with other vaccines after reviewing the clinical diagnosis. Then, the filtered AEFI cases of the two groups were subdivided into various categories based on their characteristics according to the standard AEFI classification criteria.
The indicators of immunization with sIPV alone versus sIPV combined with other vaccines were retrospectively analyzed, including categories and the constituent ratio of AEFI cases, the time interval between the AEFI incidence and inoculation and outcome of the AEFI cases.
Statistical analysis was performed on the distribution of AEFI cases in the various vaccine combinations modes for sIPV and other vaccines. All ADRs (general reactions and abnormal reactions) of the sIPV-included concomitant immunization were coded by using a medical dictionary for regulatory activities (MedDRA). The coded ADRs were subjected to collection and statistical analysis according to the system organ class (SOC) and a preferred term (PT) in MedDRA. The data were then compared with the coded ADRs collected from separate sIPV immunizations.
The distributions of different categories of ADRs in the sIPV separate immunization group and various combination modes of sIPV with other vaccines were analyzed and compared to identify significant differences.
3. Results
In the results of this study, there are many different numbers with different meanings, including AEFI vs ADRs, as well as the number of cases vs the number of events. In order to avoid confusion, we distinguish them by a table, see . In addition, show the number of cases of AEFI(Corresponding to the result 3.1 to 3.3, and show the number of events of ADRs (Corresponding to the result 3.5 to 3.6).
Table 1. The number of sIPV AEFI and ADRs during 2015–2020
Table 2. Classification of sIPV AEFI during 2015–2020
Table 3. Time interval between AEFI occurrence and vaccination
Table 4. Outcome of AEFI
Table 5. Adverse reactions of sIPV when concomitantly immunized with other vaccines during 2015–2020
3.1. AEFI classification and constituent ratio
From 2015 to 2020, a total of 9130 sIPV-related AEFI cases were reported in the CNAEFIS, of which 6842 AEFI cases were related to administrating sIPV alone, including 6408 general reactions (93.66%) and 337 abnormal reactions (4.93%), and 2288 AEFI cases were related to sIPV-included concomitant vaccination, including 2086 general reactions (91.17%) and 156 abnormal reactions (6.82%) (see ).
3.2. Time interval between AEFI incidence and inoculation
The 9130 sIPV-related AEFI cases were mainly reported on Day 0 after inoculation (52.15%), followed by Day 1 after inoculation (37.21%). Similarly, the AEFI cases with sIPV alone were mainly reported on Day 0 after inoculation (55.71%), followed by Day 1 after inoculation (33.76%). The AEFI cases of sIPV-included concomitant administration were mainly reported on Day 1 after inoculation (47.51%), followed by Day 0 after inoculation (41.48%) (see ).
Table 6. Events and ratio of ADRs for sIPV concomitant immunization during 2015–2020
3.3. Outcome of AEFI cases
Among the 9130 AEFI cases reported in the CNAEFIS, 8829 cases (96.70%) recovered or improved, 221 cases (2.42%) were under treatment at the time of reporting, 3 cases (.03%) were aggravated (1 case of general reaction without recorded symptoms, 1 case of thrombocytopenic purpura and 1 case of bleeding), 10 deaths (.11%) occurred (including 9 cases of coincidental illnesses and 1 case of abnormal reaction after concomitant administration) and 67 cases (.73%) had unknown outcomes (see ).
3.4. Distribution of AEFI cases by vaccine type administered concomitantly with sIPV
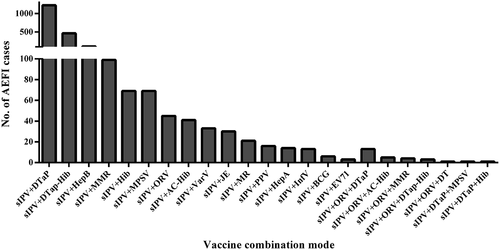
In this study, the vaccines administered simultaneously with sIPV include Adsorbed Acellular DTP Combined Vaccine (DTaP), Diphtheria, Tetanus and Acellular Pertussis-Hemophilus Influenza Type B Combined Vaccine (DTap-Hib), Measles, Mumps, And Rubella Vaccine (MMR), Hemophilus Influenza Type B Conjugate Vaccine (Hib), Hepatitis B Vaccine (Hepb), Meningococcal Groups A and C and Hemophilus Influenza Type B Conjugate Vaccine (AC-Hib), Live Oral Rotavirus Vaccine (ORV), Meningococcal Polysaccharide Vaccine (MPSV), Measles And Rubella Vaccine (MR), Varicella Attenuated Live Vaccine (Varv), Japanese Encephalitis Vaccine (JE), Pneumococcal Polysaccharide Vaccine (PPV), Inactivated Enterovirus 71 Vaccine (EV71), Hepatitis A Vaccine (Hepa), Influenza Vaccine (Infv), Bacillus Calmette Guerin Vaccine (BCG) and Diphtheria and Tetanus Vaccine (DT). shows the combination mode of sIPV-included concomitant vaccination and the number of reported AEFI cases of each combination mode. Among the concomitant vaccines, the administration of sIPV with DTaP presented the highest number of AEFI cases, i.e., 1224 AEFI cases, which accounted for 53.50% of all 2288 cases after concomitant immunization. When administrating sIPV with two other vaccines simultaneously, the concomitant administration of sIPV, DTaP and ORV had the greatest number of AEFI at 13, which accounted for .57% of all 2288 AEFI cases after concomitant immunization.
3.5. Meddra-Based coding results of ADR (general reaction and abnormal reaction)
From 2015 to 2020, a total of 7481 ADR events were reported among 6745 cases (general reactions and abnormal reactions) after immunization with sIPV alone. After concomitantly administering sIPV with other vaccines, 2388 ADR events were reported among 2242 cases. Because multiple ADR events might occur in individual cases, the term “ADR events” rather than “ADR cases” was used for the statistics on MedDRA-coded results (see ).
According to the MedDRA-based medical coding results, the ADRs with the SOC term “General Disorders and Administration Site Conditions” accounted for the highest proportion, with 5626 (75.20%) out of 7481 ADR events and 1936 (81.07%) out of 2388 ADR events occurring after administering sIPV alone and with other vaccines, respectively (see ). Some of the reactions in the CNAEFIS were generally coded as “vaccination complications” as the PT term because the vaccine name and inoculation time of the case were recorded but lacked any specific symptoms or definite diagnosis (see ).
The MedDRA-based coding results showed that relative to the separate administration of sIPV, the ADRs that were frequently reported after concomitant immunization of sIPV with other vaccines were fever, erythema and swelling at the injection site, induration at the injection site, dermatitis allergy and urticaria. The ratios of these five types of ADRs after administering sIPV alone and with other vaccines were 78.39% (5864 events) and 86.10% (2056 events), respectively (see ).
3.6. ADR (general reaction and abnormal reaction) distribution
Referring to the five most frequently reported types of ADRs after concomitant immunization of sIPV with other vaccines, i.e., fever, erythema and swelling at injection site, induration at injection site, dermatitis allergic and urticaria, the statistical analysis revealed that the number of each ADR differently when sIPV was administered alone and concomitant with other vaccines (see ).
Taking the general reactions as an example, the event frequency of general reactions after immunization with sIPV alone or together with other vaccines varied from 1 to 7134, although the proportions of total general reactions among the ADRs were almost all higher than 90%. According to the results, the proportion of general reactions observed after administering sIPV alone accounted for 95.36% while abnormal reactions account for 4.64% and the proportion of general reactions after administering sIPV with other vaccines accounted for 93.22% while abnormal reactions accounted for 6.78%. Statistically significant differences were observed in the number of general reaction and abnormal reaction events between sIPV administered alone and in combination with other vaccines (P < .001). The proportion of general reactions after administering sIPV alone was higher than that after concomitant vaccine administration (95.36% and 93.22%, respectively), although the proportion of abnormal reactions after administering sIPV alone was lower than that after concomitant vaccine administration (4.64% and 6.78%, respectively) (see ).
4. Discussion
In 2016, IPV was included in the Chinese NIP.Citation24 According to the provisions of NIP Immunization Procedures and Instructions for Children (2021 Edition),Citation5 NIP vaccines can be applied concomitantly according to immunization schedules or supplementary immunization principles, which provide a policy basis for simultaneous immunization with NIP vaccines. However, restrictive national policies have not been issued for the simultaneous immunization of NIP vaccines with non-NIP vaccines. Therefore, constant updates on the safety of concomitant immunization are required to provide better guidance for immunization and ensure the safety of subjects upon improved data analysis.
In this study, AEFI cases collected from 2015 to 2020 after administering sIPV alone and with other vaccines were analyzed to evaluate the safety of concomitant immunization. No significant difference in the AEFI incidence was observed between sIPV administered alone and in combination with other vaccines. General reactions accounted for the highest proportion of AEFI cases (93.66% vs. 91.17%), followed by abnormal reactions (4.93% vs. 6.82%). The AEFI cases mainly occurred within 3 days after inoculation (>96%). More than 96% of AEFI after administering sIPV concomitantly with other vaccines recovered or improved (96.87%), which is consistent with the AEFI outcomes after administering sIPV alone (96.20%).Citation24,Citation25 The AEFI cases after administering sIPV with other vaccines were consistent with those after administering sIPV alone in terms of the AEFI category, the time interval between AEFI incidence and inoculation, and disease outcomes.
We further analyzed the types of vaccines that caused AEFI after concomitant immunization. From 2015 to 2020, the vaccines administered concomitantly with sIPV included DTaP, DTap-Hib, MMR, Hib, HepB, AC-Hib, MPSV, MR, VarV, JE, PPV, EV71, HepA, InfV, BCG and DT. The combinations with the highest number of AEFI cases after immunization were sIPV+DTaP, sIPV+DTaP-Hib, sIPV+HepB and sIPV+MMR, although all of them were NIP vaccines except for DTaP-Hib. These data suggested that more attention should be focused on the safe administration of sIPV concomitantly with the above vaccines during safety monitoring, despite previous research revealing that the administration of IPV+DTaP-Hib is relatively safe.Citation26,Citation27 In a study by Clake et al.Citation28 on the safety and immunogenicity of administering IPV along with vaccines for yellow fever and measles–rubella, there were 3 (16.67%), 3 (26.67%), and 4 (16.67%) cases of fever when administering sIPV alone, MR vaccine alone, and IPV concomitant with MR vaccine, respectively, and no safety concerns were reported. There were 3418 (45.69%) cases of fever after administering sIPV alone, whereas there were 18 (81.82%) cases of fever after concomitant immunization with the sIPV and MR vaccines, which may have been associated with different study designs that included active surveillance and passive surveillance. In addition, the study also suggested that there is a high demand (73.86%) for administering sIPV combined with DTaP- or DTaP-containing vaccines. These data correlated with the high coincidence of inoculation times. According to the immunization schedules, infants should be inoculated with sIPV at the ages of 2, 3, 4 and 18 months and with DTaP and DTap-Hib at the ages of 3, 4, 5 and 18 months.
The international sharing of regulatory information of medical products for humans (MedDRA) is a rich and highly specific set of standardized medical terminology developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).Citation29 MedDRA is widely adopted in databases and individual case safety reports for drug regulatory authorities and pharmaceutical enterprises. In this study, a total of 6824 cases AEFI were reported after sIPV alone and 2288 cases AEFI after sIPV with other vaccines. By comparison, a total of 6745 cases ADRs were reported after sIPV alone, and 2242 cases ADRs after sIPV with other vaccines. In addition to ADRs, AEFI also includes other adverse events not related to vaccination, such as coincidental illnesses and psychogenic reactions. In this study, we focused on vaccine-related adverse events, known as ADRs (General and abnormal reactions in AEFI). To compare the differences in the number of events of specific ADRs occurring after administrating sIPV alone and concomitant with other vaccines, we coded all reported ADRs by MedDRA terms and further nominated and analyzed these ADRs at the PT level. Our results indicated among the ADRs after administering sIPV with other vaccines, fever accounted for the highest proportion (70.18%), followed by erythema and swelling at the injection site (6.95%), induration at the injection site (3.85%), allergic dermatitis (3.56%) and urticaria (1.55%). In a phase III clinical trial of sIPV sponsored by the Institute of Medical Biology Chinese Academy of Medical Sciences,Citation15 the local ADRs after inoculation with sIPV mainly included redness (1.3%) and pain (1.3%). The systemic ADRs mainly included fever (43.8%), diarrhea (7.5%), agitation (10.7%) and vomiting (5.3%). Compared to the results in this study, the proportions of the three most reported ADRs were higher than those in previous reports, i.e., fever (70.18%), erythema and swelling at the injection site (6.95%) and induration at the injection site (3.85%). These data were consistent with the characteristics of a relatively high incidence of local redness and induration after immunization with the DTaP vaccine alone.Citation30 In addition, the data from Zhiqun Li et al.Citation27 suggested that from 2011 to 2017 in the Guangdong area, the various symptoms after DTaP-IPV/Hib administration included fever (48.1%), swelling at injection site (22.2%), induration at injection site (7.4%), and point rash (16.0%). In this study, however, the various symptoms for stand-alone immunization and concomitant immunization were fever (45.69% and 70.18%, respectively), swelling at injection site (22.74% and 6.95%, respectively), induration at injection site (6.74% and 3.85%, respectively), and allergic dermatitis (2.23% and 3.56%, respectively). It follows that the proportions of fever, swelling at injection site, induration at injection site after DTaP-IPV/Hib immunization were much closer to the proportions after administering sIPV alone while the proportion of allergic dermatitis (which is regarded as point rash by Zhiqun Li et al.) was far higher than that for administering sIPV alone or in combination. However, the lower number of cases in this study could be a cause.
To verify the differences in the number of ADRs after immunization with sIPV alone and in combination with other vaccines, the number of events of the five types of the most frequently reported ADRs was compared, and a difference was found in the frequency of ADRs. Taking fever as an example, the proportion of fever in ADRs after administering sIPV alone and in combination with other vaccines was 45.69% and 70.18%, respectively. Except for the vaccine combination modes with less than 10 ADRs reported, the combination of sIPV+ORV showed the lowest proportion (47.73%) while the combination of sIPV+MMR showed the highest proportion (86.87%) of fever. However, according to the ADR types, the administration of sIPV alone and in combination with other vaccines demonstrated a high proportion of general reactions, with ratios of 95.36% and 93.22%, respectively, and there were statistically significant differences between them (P < .001). Fever accounted for the most frequently reported general reaction, with a proportion of >47%, which was consistent with the monitoring results obtained at the provincial level.Citation24 Statistically significant differences were found in the ratio of abnormal reactions in all ADRs after the administration of sIPV alone (4.64%) and with other vaccines (6.78%), suggesting that the concomitant administration of sIPV with other vaccines may increase the frequency of abnormal reactions.
This study has some limitations. As a passive surveillance method, CNAEFIS has inherent drawbacks, including potentially biased reporting and a lack of control groups.Citation31, Citation32 Compared to the national AEFI monitoring results,Citation25 no new safety signal was found for sIPV-included concomitant immunization. It should be noted that the death of a 2-month-old boy was recorded on the day of immunization with sIPV+ORV, and it was recorded as an “abnormal reaction” in the system. Limited records in the CNAEFIS and measures to protect the privacy of recipients meant that no additional relevant information was available; therefore, the case was not further analyzed. Additionally, 1 case of ataxia, 1 case of neurological symptoms and 1 case of bronchitis were recorded in this study. These rare ADRs were not observed following sIPV immunization only. Due to the limited information available, it was impossible for us to further confirm and assess the safety signal. However, these data suggest that concomitant immunization with sIPV and other vaccines may aggravate ADRs and result in ADRs that do not occur following the administration of sIPV alone.
In conclusion, the characteristics of AEFI reported after sIPV-included concomitant immunization are consistent with those reported after separate sIPV immunization. Our preliminary data suggest that sIPV-included concomitant immunization is feasible. However, it is undeniable that certain AEFI cases that were not observed in clinical studies and for separate sIPV immunization were reported when concomitantly administering sIPV with other vaccines; thus, concomitant vaccination potentially increases the occurrence of abnormal reactions. Because the data used in this research are from passive surveillance in a real-world setting, there are certain limitations in the results. To further clarify sIPV-included concomitant immunizations, it is particularly important for vaccine manufacturers to perform studies for sIPV-included concomitant immunization, especially for sIPV in combination with DTaP, DTaP-Hib and MMR, because these combinations showed higher AEFI proportions due to the overlap of inoculation time points.
Disclosure statement
We confirm that all of the listed authors have actively participated in the present study, and have read and approved the submitted manuscript. The authors do not have any potential conflicts of interest to declare.
References
- Burrell CJ, Howard CR, and Murphy FA. Vaccines and vaccination. Fenner and Whiteʻs medical virology. Fifth ed. London: Academic Press; 2017. pp. 155–8.
- Wang J, Jing R, Lai X, Zhang H, Yun L, Knoll MD, Fang H. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8(3):482. doi:10.3390/vaccines8030482.
- Koslap-Petraco MB, Judelsohn RG. Societal impact of combination vaccines: experiences of physicians, nurses, and parents. J Pediatr Health Care. 2008;22:300–09. doi:10.1016/j.pedhc.2007.09.004.
- Pan A, Organization WH. Expanded program on immunization. Int J Epidemiol. 1979;19:219–20.
- Commission NH . Procedures and instructions for childrenʻs immunization with vaccines in the National Immunization Program (2021 edition). Chin J Viral Dis. 2021;11(4):241–245 .
- Vaudaux B. Combined vaccines and vaccine combinations. Rev Med Suisse Romande. 1998;118:377–93.
- Peter G. Lessons learned from a review of the development of selected vaccines. Pediatrics. 1999;104(4):942–50. doi:10.1542/peds.104.4.942.
- Hutchins SS, Escolan J, Markowitz LE, Hawkins C, Orenstein WA, Morgan RA, Preblud SR, Orenstein WA. Measles outbreak among unvaccinated preschool-aged children: opportunities missed by health care providers to administer measles vaccine. Pediatrics. 1989;83(3):369–74. doi:10.1542/peds.83.3.369.
- King GE, Hadler SC. Simultaneous administration of childhood vaccines: an important public health policy that is safe and efficacious. Pediatr Infect Dis J. 1994;13(5):394–407. doi:10.1097/00006454-199405000-00012.
- WPRO. WPRO | New poliovirus vaccine licensed in China. WPRO | WHO Western Pacific Region.
- Verdijk P, Rots NY, van Oijen MG, Weldon WC, Oberste MS, Okayasu H, Sutter RW, Bakker WAM. Safety and immunogenicity of a primary series of Sabin-IPV with and without aluminum hydroxide in infants. Vaccine. 2014;32(39):4938–44. doi:10.1016/j.vaccine.2014.07.029.
- He H, Wang Y, Deng X, Yue C, Tang X, Li Y, Liu Y, Yin Z, Zhang G, Chen Z, et al. Immunogenicity of three sequential schedules with Sabin inactivated poliovirus vaccine and bivalent oral poliovirus vaccine in Zhejiang, China: an open-label, randomised, controlled trial. Lancet Infect Dis. 2020;20:1071–79. doi:10.1016/S1473-3099(19)30738-8.
- Sutter RW, Bahl S, Deshpande JM, Verma H, Ahmad M, Venugopal P, Rao JV, Agarkhedkar S, Lalwani SK, Kunwar A, et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet (London, England). 2015;386(10011):2413–21. doi:10.1016/S0140-6736(15)00237-8.
- Li Z, Ding W, Guo Q, Ze L, Zhu Z, Song S, Li W, and Liao G. Analysis of the dose-sparing effect of adjuvanted Sabin-inactivated poliovirus vaccine (sIPV). Hum Vaccin Immunother . 2018;14(8):1.
- Liao G, Li R, Li C, Sun M, Li Y, Chu J, Jiang S, Li Q. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, positive-controlled trial. J Infect Dis. 2012;205(2):237–43. doi:10.1093/infdis/jir723.
- Liao G, Li R, Li C, Sun M, Jiang S, Li Y, Mo Z, Xia J, Xie Z, Che Y, et al. Phase 3 trial of a Sabin strain–based inactivated poliovirus vaccine. J Infect Dis. 2016;214:1728–34. doi:10.1093/infdis/jiw433.
- Chu K, Ying Z, Wang L, Hu Y, Xia J, Chen L, Wang J, Li C, Zhang Q, Gao Q, et al. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: A phase II, randomized, dose-finding trial. Vaccine. 2018;36:6782–89. doi:10.1016/j.vaccine.2018.09.023.
- Capeding MR, Gomez-Go GD, Oberdorfer P, Borja-Tabora C, Bravo L, Carlos J, Tangsathapornpong A, Uppala R, Laoprasopwattana K, Yang Y, et al. Safety and immunogenicity of a new inactivated polio vaccine made from Sabin strains: a randomized, double-blind, active-controlled, phase 2/3 seamless study. J Infect Dis. 2020. doi:10.1093/infdis/jiaa770.
- Hu Y, Wang J, Zeng G, Chu K, Jiang D, Zhu F, Ying Z, Chen L, Li C, Zhu F, et al. Immunogenicity and safety of a Sabin strain–based inactivated Polio vaccine: a phase 3 clinical trial. J Infect Dis. 2019;220(10):1551–57. doi:10.1093/infdis/jiy736.
- Guo B, Page A, Wang H, Taylor R, Mcintyre P. Systematic review of reporting rates of adverse events following immunization: an international comparison of post-marketing surveillance programs with reference to China. Vaccine. 2013;31:603–17. doi:10.1016/j.vaccine.2012.11.051.
- Liu D, Wu W, Li K, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33(32):4041–46. doi:10.1016/j.vaccine.2015.04.060.
- Health CMo. National guideline for the Surveillance of adverse events following immunization. Chin J Vaccin Immun 2011;017:72–81.
- Wu W, Liu D, Nuorti JP, Li K, Xu D, Ye J, Zheng J, Cao L, Wang H. Deaths reported to national surveillance for adverse events following immunization in China, 2010–2015. Vaccine. 2019;37(9):1182–87. doi:10.1016/j.vaccine.2019.01.009.
- Kang G, Tang F, Wang Z, Hu R, Yu J, and Gao J. Surveillance of adverse events following the introduction of inactivated poliovirus vaccine made from Sabin strains (sIPV) to the Chinese EPI and a comparison with adverse events following inactivated poliovirus vaccine made from wild strains (wIPV) in Jiangsu, China. Hum Vaccin Immunother . 2021;17(8):2568–2574.
- Jiang R, Liu X, Sun X, Wang J, and Sun M. Immunogenicity and safety of the inactivated poliomyelitis vaccine made from Sabin strains in a phase IV clinical trial for the vaccination of a large population. Vaccine . 2021;39(9):1463–1471.
- White C, Halperin SA, Scheifele DW. Pediatric combined formulation DTaP–ipv/hib vaccine. Expert Rev Vaccines. 2009;8:831–40. doi:10.1586/erv.09.59.
- Li Z, Xu J, Tan H, Zhang C, Chen J, Ni L, Yun X, Huang Y, Wang W. Safety of pentavalent DTaP-IPV/Hib combination vaccine in post-marketing surveillance in Guangzhou, China, from 2011 to 2017. Int J Infect Dis. 2020;99:149–55. doi:10.1016/j.ijid.2020.07.019.
- Clarke E, Saidu Y, Adetifa JU, Adigweme I, Hydara MB, Bashorun AO, Moneke-Anyanwoke N, Umesi A, Roberts E, Cham PM. Safety and immunogenicity of inactivated poliovirus vaccine when given with measles–rubella combined vaccine and yellow fever vaccine and when given via different administration routes: a phase 4, randomised, non-inferiority trial in the Gambia. Lancet Glob Health. 2016;4(8):e534–47. doi:10.1016/S2214-109X(16)30075-4.
- Fescharek DR, Kübler J, Elsasser U, Frank M, G??thlein, P. Medical Dictionary for Regulatory Activities (MedDRA). Int J Pharm Med. 2004;18(5):259–69. doi:10.2165/00124363-200418050-00001.
- Li L, Yang R, Cai J, Zheng J. [Evaluation of the safety of diphtheria, tetanus and acellular pertussis containing combination vaccines in Chengdu, 2015-2019]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(9):958–62. doi:10.3760/cma.j.cn112150-20200417-00593.
- Singleton JA. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. Vaccine. 1999;17(22):2908–17. doi:10.1016/S0264-410X(99)00132-2.
- Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398–405. doi:10.1016/j.vaccine.2015.07.035.