ABSTRACT
This economic evaluation assesses the cost-effectiveness and budget impact of introducing a two-dose varicella vaccine in the Russian national immunization program. A static Markov model followed a simulated 2019 Russian cohort over its lifetime and compared outcomes and costs of three varicella vaccination strategies: strategy I (doses given at 12 and 15 months of age), strategy II (doses given at 1 year and 6 years of age), and a no vaccination scenario. Inputs on age-dependent clinical pathways, associated costs, and related health outcomes were collected from national sources and published literature. Results are presented as incremental cost-effectiveness ratio (ICER) from the healthcare payer and societal perspective over the lifetime of the birth cohort and the budget impact over a 10 years’ time horizon. Vaccination strategies I and II resulted in an ICER of approximately 1.7 million rubles per quality-adjusted life years gained from the healthcare payer perspective and were cost-saving from the societal perspective. From the healthcare payer perspective, the costs per varicella case averted were 5,989 and 7,140 rubles per case for strategies I and II, respectively. However, from the societal perspective, vaccination is a dominant strategy and the budget impact analysis shows significant healthcare savings over 10 years, with strategy I realizing savings of ~2 billion rubles more than strategy II. From a public health impact perspective, varicella vaccination of children at 12 and 15 months of age through the Russian NIP is expected to be cost-effective with an affordable budget impact compared to no vaccination.
Plain language summary
A graphical version of the plain language summary can be found here: 10.6084/m9.figshare.19291463
Focus on the patient
What is the context?
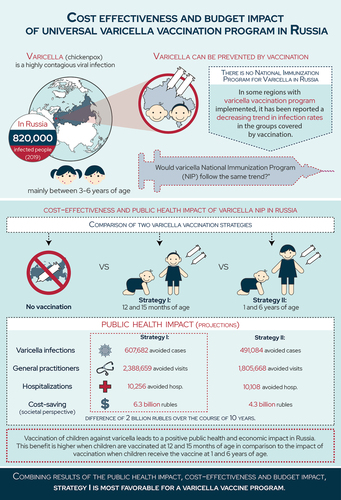
Varicella, or chickenpox, is a highly contagious infection. Though mild in children, complications can occur in older individuals, increasing the economic burden for society and public health institutions.
In 2019, approximately 0.6% of the Russian population was impacted by varicella, a vaccine-preventable disease.
In Russia, varicella vaccination is only implemented in some regions. These regions report a decreasing trend in infection rates in the groups covered by vaccination.
What is new?
This study assesses the public health and economic impact of implementing varicella vaccination in Russia through its National Immunization Program.
We compared two vaccination strategies to a no vaccination scenario:
º Strategy I: two doses at 12 and 15 months of age
º Strategy II: two doses at 1 and 6 years of age
Over a 10-year period, we found that:
º Strategy I prevented 607,682 cases, 2,388,659 general practitioner visits and 10,256 hospitalizations, and saved 6.2 million rubles
º Strategy II prevented 491,084 cases, 1,805,668 general practitioner visits and 10,108 hospitalizations, and saved 4.2 million rubles
Strategy I saves more direct (i.e., general practitioner visits, hospitalizations and treatment) and indirect (i.e., income loss, disability payments, and caregiving) costs to society than strategy II.
What is the impact?
Varicella vaccination, especially when introduced at 12 and 15 months (strategy I) in the National Immunization Program, provides public health and economic benefits.
From the healthcare payer perspective: this is a cost-effective intervention. From the societal perspective: the budget impact analysis shows significant savings.
Introduction
Varicella or chickenpox is an acute and highly contagious infection caused by varicella-zoster virus (VZV). Varicella usually presents during childhood, but adults may also contract the disease.Citation1 Varicella occurs globally and is primarily documented as a childhood disease with most children infected acquiring varicella by the age of 10 years of age.Citation1 Varicella is usually self-limited in children, but known complications such as secondary bacterial infection of skin lesions, secondary bacterial pneumonia, and central nervous system complications including aseptic meningitis, encephalitis, and cerebellar ataxia can occur.Citation1 Furthermore after the primary infection, which causes varicella, VZV becomes latent in the sensory nerve ganglia and the reactivation of VZV results in herpes zoster (shingles).Citation1 Varicella disease burden is substantial,Citation2,Citation3 and the major component of this burden is financial, which is driven by the high number of cases. The economic impact of varicella infection is two-fold: direct costs related to the treatment of initial infection and complications, and indirect costs in terms of work loss sustained by parents caring for their infected ailing children. This economic burden on society and national health systems could be alleviated through the universal implementation of varicella vaccination.Citation4
Currently, several licensed formulations of live attenuated lyophilized varicella vaccines are available, both as refrigerator-stable and frozen vaccine formulations. The vaccines are available either as monovalent (varicella only), or in combination with the measles, mumps, and rubella (MMR) vaccine.Citation5 Childhood varicella vaccination has been highly effective in decreasing the global prevalence and impact of the disease on healthcare systems and society.Citation5 The World Health Organization (WHO) recommends the introduction of varicella vaccine in the national immunization programs (NIP) of countries where VZV infection is a major public health concern.Citation5 However, resources should be sufficient to ensure reaching and sustaining vaccine coverage ≥80%.Citation5 In 1995, varicella vaccine was introduced in the NIP of the United States of America (US) following which there was a drastic reduction in the incidence of VZV infections across all age groups, suggestive of herd immunity at a vaccination coverage level of approximately 80%.Citation6 Similarly, the introduction of varicella vaccine in the NIP of Spain in 2007 decreased varicella incidence by 98.5%.Citation7 Multiple studies across regions have reported similar trends of declining incidence and disease burden in vaccinated and non-vaccinated cohorts following the successful implementation of a childhood varicella vaccination program.Citation7–10 As of 2018, 36 countries and regions had introduced universal routine varicella vaccination into their respective NIPs.Citation11
Currently, the Russian Federation does not include routine varicella vaccination in its NIP although varicella vaccinations are available in regional programs and in the private sector.Citation12 Varicella is a common childhood infection in the Russian Federation and has infected over 858,353 persons in 2017, mainly between 3 and 6 years of age.Citation13 A recent study showed that, within the Russian Federation, Moscow, a region that introduced varicella vaccination demonstrates a yearly decreasing trend of −3,1% in VZV infection, whilst Saint Petersburg, a region that didn’t introduce varicella vaccination, showed a yearly increase of 2,8%.Citation14 These results provide support for the need to include varicella vaccination in the NIP of the Russian Federation.
In this economic evaluation, we assess the public health impact of two different childhood varicella vaccination strategies compared to a strategy without varicella vaccination using Russian-specific demographic, epidemiologic, and cost parameters. Such a comparison could be helpful in choosing a vaccination strategy, which optimizes the public health impact while having the lowest budget impact for the introduction of varicella vaccination in the NIP of the Russian Federation.
Methods
Model overview
This economic evaluation compared the cost and outcomes of two childhood varicella vaccination strategies (strategy I—varicella vaccine doses at 12 months and 15 months of age; strategy II—varicella vaccine doses at 1 year and 6 years of age) to a strategy without childhood varicella vaccination. The impact of measures to contain the COVID-19 pandemic on varicella incidence was not considered, assuming that incidence will return to pre-pandemic values once restrictions are released.
Model structure
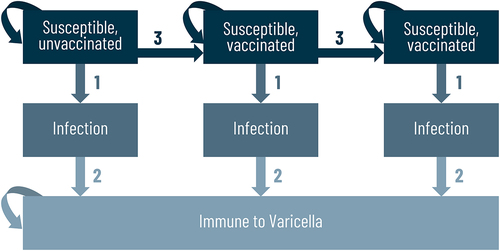
The cost-effectiveness analysis was based on a static Markov model (). The model follows the 2019 Russian birth cohort over their lifetime. The cycle length was 1 month for the first 5 years and for the remaining years it was set to 1 year. Model outcomes (costs, quality-adjusted life-years, number of events) were analyzed at the end of each cycle and aggregated over the lifetime of the birth cohort.
Figure 1. Cost-effectiveness model.
The budget impact model was built on the same Markov trace underlying the cost-effectiveness model. The budget impact model was developed to accommodate analyses up to a 10-year horizon. The annual costs per capita were obtained for each vaccination strategy from the cost-effectiveness model for each year of the budget impact analysis. The cost of each cycle was calculated and was utilized to obtain the annual cost. The total cost of each vaccination strategy in each simulation year was calculated by applying the predicted annual cost to each cohort entering the simulation (Supplementary Figure 1).
Health states and transition probabilities
The Markov model () describes the progression of a Russian birth cohort through a number of pre-defined health states, capturing all possible states of the VZV infection: persons who have not experienced varicella infection (susceptible), persons who are experiencing varicella infection (natural), persons who had a varicella infection after one or two doses of the vaccine (breakthrough infection), persons who have experienced a varicella infection (immune to varicella), and persons who have died (death due to varicella). A static Markov model was used to minimize any uncertainty around changes in the force of infection due to varicella vaccination. The risk of infection varied by cohort age of the cohort and was calculated from the current risk of infection in an unvaccinated Russian population. The model included all-cause mortality, which was applied at the beginning of each cycle, transitioning a pre-defined proportion of patients who were alive at the end of the previous cycle to an absorbing death state. Conservatively, mortality due to varicella infection complications was not included in the base case analysis.
All transition probabilities varied with time and were based on published data.Citation15 Transition from varicella susceptible to varicella infection was governed by the probability of each type of varicella, which was dependent on age, vaccine efficacy, and duration of protection against varicella. Transition between unvaccinated and vaccinated states was governed by the age and coverage rates.
Model input data and assumptions
Model input parameters and assumptions are provided in .
Table 1. Summary of base case inputs
Model population
The simulation model followed the 2019 Russian birth cohort (1,481,074 newborns,Citation22 of which 49% were female, based on the distribution of population in the Russian Federation).Citation23 All-cause mortality for the general Russian population was sourced from an official source (The Federal State Statistics Service).Citation24 For the budget impact analysis, the eligible population was calculated based on the 2019 Russian birth cohort that qualifies for vaccination and the age of vaccination depending on strategy I or II.Citation25
Vaccination strategies and related assumptions
The model compared the cost and outcomes of two different vaccination strategies: strategy I in which dose 1 of the varicella vaccine is given at 12 months of age (co-administered with the MMR vaccine) and dose 2 of the varicella vaccine is given at 15 months of age (co-administered with the pneumococcal conjugate vaccine [PCV]), and strategy II in which dose 1 is given at 1 year of age (co-administered with the MMR vaccine) and dose 2 at 6 years of age (co-administered with the MMR).
In the model, the direct effect of vaccination was captured by vaccine efficacy, which was applied to the probability of varicella in vaccinated individuals. Efficacy against varicella infection was set to 67.2% for the first dose and 95.4% for the second dose.Citation26,Citation27 Furthermore, we assumed similar efficacy rates for dose 1 and dose 2 regardless of vaccination strategy. Indirect effects (herd immunity) were conservatively not considered in the base case analysis. The model assumed vaccine coverage rates of 97.0% and 100.0% for the first and second doses, respectively. For both doses, it was assumed that waning starts after 10 years and half-life duration was set to 20 years for the first dose and to lifetime for the second dose.Citation26,Citation27
Risk of varicella disease
The probability of incidence was calculated based on the reported varicella incidence in 2019.Citation15 The model assumed that people could only have one varicella infection in their life-span.Citation16 A study including 2,000 samples showed that the seroprevalence of varicella virus in Russia ranges from 30% in 1-year-old children rising to more than 90.0% at 10 years of age.Citation16 Based on the official reported varicella cases in Russia, only 36.9% of the birth cohort would report a varicella case in their lifetime.Citation13 Based on expert opinion, we adjusted the age-specific probabilities of a varicella infection to reflect an additional 10.0% of the cases not captured. In order to proportionally increase the probability of a varicella infection across age groups, a constant adjustment factor of 1.362 was applied, obtained using MSExcel Goal Seek function, which resulted in 46.9% of the birth cohort having reported a varicella case over their lifetime.
Reactivation of VZV, leading to herpes zoster (HZ), was not considered in our model since the current evidence base is insufficient to demonstrate an impact of universal varicella vaccination on HZ incidence.Citation11 The number of general practitioner (GP) visits was assumed to be 4 per varicella infection and 5 in case of breakthrough infection based on expert opinion and previously reported data ().Citation18 The probability of varicella infections including breakthrough varicella infection that lead to hospitalization was obtained previously published data ().Citation17 It was assumed that the proportion of hospitalizations in infants is the same as in children aged 1–2 years.
Impact of varicella on health-related quality of life
The number of quality-adjusted life years (QALYs) lost per episode of natural and breakthrough varicella were set based on estimates published by Brisson and Edmunds (2003).Citation19 In a natural varicella case, the QALYs lost were estimated based on a study among 42 parents of children with prior history of varicella. The quality of life weighting of adults with varicella was assumed to be similar to that of a mild zoster episode. To assess QALYs lost, varicella was assumed to have an average duration of 7 days. In breakthrough varicella cases, the QALYs lost were assumed to be equal to 0.001 ().
Direct and indirect costs
The costs associated with hospitalization were acquired from official reports and treated with appropriate multipliers to account for disease severity and degree of healthcare support.Citation10,Citation20 The cost of GP visit and the varicella vaccine were sourced from official national sources.Citation20 The cost of each dose of the varicella vaccine were based on the assumption of implementing the varicella vaccination alone and did not include the costs of the co-administered vaccines. In addition, it was assumed that the varicella vaccine is administered together with other vaccines, therefore administration cost was excluded in the calculation (). All costs are presented in rubles.
For the societal perspective, indirect costs to society were accounted as income loss due to varicella case related to productivity loss and disability payments and total costs per varicella case requiring a caregiver. Indirect costs to society comprised of extended government costs (income tax lost and daily disability payments) which was estimated at 1,763 rubles per day based on the monthly average salary of 47,468 rubles in 2019.Citation28 This cost was accrued in terms of tax loss (i.e. social tax and income tax of 30% and 13%,Citation29 respectively) which was estimated at 671 rubles per day and disability pay was estimated at 1,092 rubles per day.Citation28 The proportion of cases receiving disability payment was assumed based on Shakhanina 2009,Citation30 which describes cases based on age groups and the corresponding disability payment percentage for each group. The base case value for duration of disability was set to 12 days as reported by Teplova.Citation31 A total of 21,147 rubles reflect the total societal costs per case requiring a caregiver (the proportion of varicella cases requiring a caregiver ranged from 0.00 to 1.00 [assumption] based on age).Citation31
While the societal perspective in the cost-effectiveness analysis considered the total indirect cost, the societal perspective in the budget impact analysis considered only the costs due to productivity loss of parents.
Analyses and reporting
The cost-effectiveness model measured outcomes including, the number of cases of varicella infection, the number of GP visits, and the number of hospitalizations during the lifetime of the birth cohort. The model also analyzed the lifetime costs to the healthcare payer and the society, which include lost QALYs, the impaired ability to work or engage in leisure activities due to morbidity, and other indirect costs. Cost-effectiveness was measured by calculating the incremental cost-effectiveness ratio (ICER), which is the ratio of incremental costs over incremental health benefits between the two compared strategies. To adjust the value of future costs and outcomes to present value, a discount of 3.5% was applied to all projected costs and health benefits.Citation32 The results are reported as healthcare payer and societal ICER and the costs per case averted were transformed to present value using a discount rate of 3.5%. There is no official willingness-to-pay threshold in Russia,Citation33 therefore we applied an approximate threshold of willingness to pay equal to three times GDP, i.e. 2.333 million rubles per QALY gained to facilitate the interpretations about of cost-effectiveness.Citation34
The budget impact was calculated by computing the difference in total costs accrued by vaccination strategy I or II and no vaccination. Societal costs were included in the budget impact analysis based on expert opinion. No discount rate was applied to the costs in the budget impact analysis.
One-way deterministic sensitivity analysis (DSA) was conducted to assess the sensitivity of the model to a variation in a particular model parameter or a group of parameters. The analysis was done using plausible ranges based on the available data or a percentage variation (all-cause mortality: ±25%; risk of varicella and zoster: ±10%; vaccine coverage and efficacy: ±5%; duration of protection: 0 and 100 years; costs: ±10%). Key parameters that have the highest impact on the model were identified and presented as a ‘tornado chart’. Multivariate probabilistic sensitivity analysis (PSA) was performed to explore the effects of all parameters simultaneously using a Monte Carlo simulation. An appropriate distribution was specified for each parameter of interest, transition probabilities and shares were varied using a beta distribution, number of events was varied using a normal distribution and costs were varied using a gamma distribution. The model was then run 1,000 times and at each run the values of all included parameters were drawn randomly from their respective distributions. The results of the PSA from a health payer perspective are presented in the cost-effectiveness plane and cost-effectiveness acceptability curve. All the cost and outcome simulation runs were presented in a cost-effective plane. Additionally, the results were also presented in a cost-effectiveness acceptability curve, which shows the probability of a varicella vaccination being accepted as cost-effective for various cost-effectiveness thresholds.
The model was programmed in Microsoft Excel 2010, using Visual Basic for Applications (VBA).
Results
Base case analysis
Over lifetime, vaccination of the 2019 Russian birth cohort of 1,481,074 newborns utilizing strategy I is projected to avert 607,682 cases of varicella, 2,388,659 GP visits, 10,256 hospitalizations, and realize 2,172 QALY gains when compared to the strategy without varicella vaccination (). Varicella vaccination of the same birth cohort using strategy II is expected to avert 491,084 cases of varicella, 1,805,668 GP visits, 10,108 hospitalizations, and realize 2,065 QALY gains over a lifetime horizon when compared to the strategy without varicella vaccination. Both vaccination strategies result in an ICER of approximately 1.7 million rubles per QALY gained from the healthcare payer perspective, which is below the cost-effectiveness threshold of 2.333 million rubles per QALY gained and is cost-saving considering the societal perspective, which includes indirect costs to society and the government. The total costs per varicella case averted was 5,989 and 7,140 rubles per case for strategies I and II, respectively ().
Table 2. Cost-Effectiveness results*
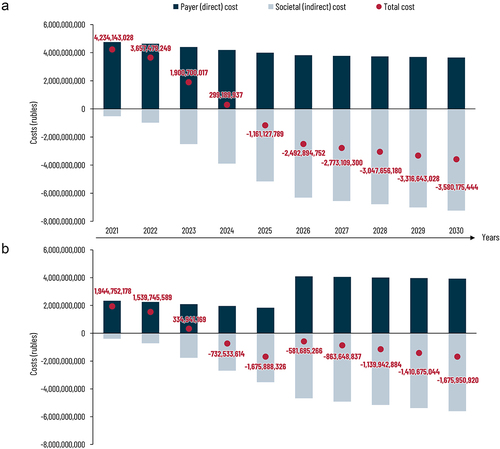
The estimated budget impact for strategy I and II from the payer (direct medical costs) and societal (direct medical costs and indirect costs) perspective is presented in a and b, respectively. A detailed description of the direct and indirect costs per year is included in Supplementary Table 1. For strategy I, the budget impact analysis from the societal perspective predicts a return on investment in 2025, after only 5 years with total savings exceeding 6.3 billion rubles during a 10-year time frame. For strategy II, the model projects a return on investment in 2024, continuing for the years after. However, since children will get their second dose at age 6, an investment has to be made in year 2026, wherein the savings are projected to be smaller but will continue to increase the years after. After this ten-year period, it is estimated that the total cost savings from strategy II could exceed 4.2 billion rubles, including the investment costs of vaccination.
Sensitivity analysis
shows the effect of varying one or more key parameters of the model on the ICER values for strategy I and II, respectively. For strategy I, the parameters with the highest impact on estimated ICER were loss of QALY due to varicella infection followed by the number of GP visits and cost of vaccination. Other parameters like the probability of varicella infection, cost of GP visit, cost of hospitalization, probability of being hospitalized due to varicella infection, and all-cause mortality factor had a minimal effect on estimated ICER. A similar trend was observed for strategy II.
Figure 3. Deterministic sensitivity analysis (a) Strategy I – Varicella vaccine doses at 12 and 15 months (b) Strategy II – Varicella vaccine doses at 1 and 6 years compared to no vaccination.
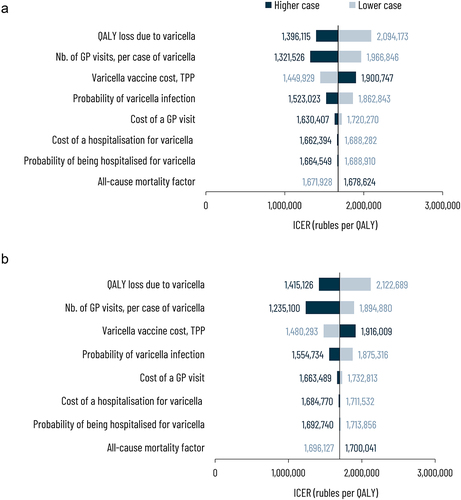
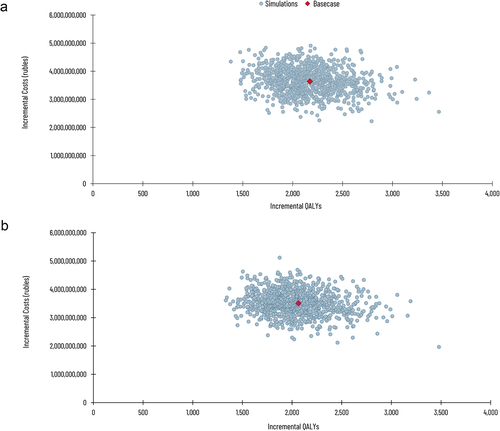
The cost-effectiveness plane displays the incremental costs and incremental outcomes obtained with each simulation (). From the healthcare payer perspective, both vaccination strategies were always more effective and more costly than the no vaccination strategy (). The probability of being cost-effective under specific threshold values is presented in Supplementary Figure 2. For varicella vaccination using strategy I, considering a threshold of 2,000,000 rubles, about 78.4% of all simulations in the PSA show cost-effectiveness. The probability of being cost-effective decreases as the ICER threshold decreases; a threshold of 1,500,000 rubles per QALY has an approximately 26.7% chance of being cost-effective in this analysis. For strategy II, about 74.4% and 23.5% of the simulations are deemed cost-effective under a threshold of 2,000,000 and 1,500,000 rubles per QALY, respectively.
Discussion
The introduction of new vaccines into NIPs and sustaining delivery of routine vaccination stays vitally important, especially in the coronavirus disease era, to alleviate any further burden on national healthcare resources. Prevention of varicella through vaccination has been sidelined as the disease is assumed to have lower mortality rates than other diseases.Citation5 However, an increasing number of countries have begun to recognize the substantial burden of disease incurred due to VZV. This includes the impact on quality of life of the affected children and their caregivers, as a consequence of which varicella vaccination has been implemented through the national immunization calendars in several countries.Citation11 Considering the regional epidemiological trends from Russia,Citation15 further attention is warranted toward the prevention of varicella in the country.
Since 2013, varicella vaccination has been implemented in Russia according to epidemiological indications and as part of regional immunization programs in some regions. Historically, one of the first regions that introduced varicella vaccination into its regional immunization calendar was Sverdlovsk region in 2008.Citation35,Citation36 The first experience of varicella immunization was analyzed in the capital of the region—Ekaterinburg: it was demonstrated that varicella vaccination was effective and safe for planned vaccination (children 3–6 years of age) and for post-exposure immunization. Specifically, >3,500 children 3–6 years of age attending kindergartens were immunized against varicella with 98% protective efficacy. Coverage levels reached in the vaccine target group were 54.0%.Citation35 Later, the Sverdlovsk region diverted its financial resources for varicella vaccination in one district (Kachkanar), which has the highest incidence of varicella infection.Citation37 The varicella incidence was analyzed from 1990 to 2016 in Kachkanar and compared with the average incidence in Sverdlovsk region. After the implementation of varicella immunization in Kachkanar, the incidence dropped from 1204.7 per 100,000 population (2007, before vaccination start) to 20.6 per 100,000 general population (2012, after 3 years of successful implementation of vaccination) which was 35 times lower than average incidence in Sverdlovsk region.
The program was extremely successful but in 2013–2016, due to limited financial resources, the region saw an increase in varicella as high as the average incidence documented in the Sverdlovsk region.Citation37 Other regional varicella vaccination programs have also met with success and show a marked decline in varicella incidence and burden compared to those regions without a varicella vaccination program. Importantly, achieving a high vaccination coverage was crucial in order to have an impact on varicella incidence.Citation14,Citation38 Collectively, these regional experiences with a varicella vaccination program emphasize the need to consider the inclusion of varicella vaccination in the Russian Federations’ national schedule of vaccinations.
In Russia, varicella vaccination is licensed since 2019 with a two dose schedule and a minimum interval of 6 weeks between doses.Citation26 However, expert consensus from two meetings on varicella vaccination recommends a varicella vaccination program for children using a two dose schedule at 12 months and 6 years of age.Citation39,Citation40 Therefore, we assessed the public health impact of two different childhood varicella vaccination strategies compared to a strategy without varicella vaccination, which can be helpful in choosing the optimal vaccination strategy with the highest public health impact and the lowest budget impact in the Russian Federation. The results from this economic evaluation show that vaccinating children at 12 and 15 months of age results in larger public health benefits, compared to vaccination at 1 and 6 years of age. Under current model inputs, the base case shows a difference of approximately 117,000 additional cases avoided for strategy I, over strategy II. This subsequently leads to more savings in direct medical costs, due to less GP visits and need for hospitalization. However, we acknowledge that any decision regarding dosing strategy is multifactorial including also other considerations such as ease of incorporation of new vaccines into the current NIP. Both strategies show cost-saving results when looking at the societal perspective of the vaccination strategies (see Plain Language Summary for a simple overview of main findings of this research). Differences between ICERs from a payer perspective are also relatively similar. The ICER is one of the key decision parameters to evaluate the clinical and cost-effectiveness of a medical program. In Russia, there is no pre-determined ICER threshold level for decision-makers; thus, we applied an approximate threshold of willingness to pay equal to three times GDP i.e. 2.333 million rubles per QALY gained to facilitate the interpretations about cost-effectiveness. In countries without an acceptable threshold, cost-effectiveness curves can also be useful in determining the probability of the vaccination program being cost-effective under different threshold values. Analysis of the incremental cost-effectiveness curve of both vaccination strategies shows that strategy I has a higher probability of being cost-effective compared to strategy II. From a budget impact perspective, both strategies provide a return on investment. For strategy I, the return on investment is achieved at year 5 and for strategy II at year 4. This can be explained by the fact that for strategy I, the government is assumed to invest vaccination costs for one cohort all in the same year (at month 12 and 15), while for strategy II costs are spread over different years (year 1 and 6). However, it is important to note that the total cost-savings for strategy I is 6.3 billion rubles and for strategy II is 4.3 billion rubles; this translates to a difference of 2 billion rubles over the course of 10 years. Combining results of the public health impact, cost-effectiveness and budget impact, strategy I is most favorable for a varicella vaccine program. From an implementation perspective, varicella vaccination introduction can be easily done by co-administrating with existing antigen vaccines in the NIP (MMR and PCV).
The study has several limitations. This economic evaluation utilized a static model instead of a dynamic model, which means that herd immunity was unaccounted for in the model. Reported varicella cases are considered fairly low compared with published values from other regions. In most countries with temperate climates, over 90% of children and adolescents will have been infected with varicella by the age of 15 years.Citation16,Citation41,Citation42 Therefore, based on expert opinion we increased the overall incidence from the reported 36.9% to 46.9%, which is still lower than values observed in other countries. Mortality due to varicella infections was not included in this model, as we could not find reliable sources of varicella associated mortality rates in Russia. Both decisions may have led to an underestimation of the public health impact of varicella vaccination at the population level. The current results can be considered conservative with more favorable outcomes expected if herd immunity and mortality due to varicella were considered in the model and if higher varicella incidence values were assumed. The number of GP visits had a significant impact on the estimated ICER values for both vaccination strategies. The values were based on a previously published study,Citation18 not an epidemiological study, and can be considered a limitation.
In conclusion, our analysis showed that the inclusion of varicella vaccination of children at 12 and 15 months of age into the Russian NIP is expected to be cost-effective with an affordable budget impact.
Contributorship
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Clinical trial registration
NCT Number (www.clinicaltrials.gov).
Previous congress activities
Preliminary results present in this manuscript have been presented at ARCIDC (All-Russian Congress on Infectious Diseases in Children) 2019.
Supplemental Material
Download TIFF Image (1.9 MB)Supplemental Material
Download TIFF Image (1.6 MB)Supplemental Material
Download MS Word (17 KB)Acknowledgements
The authors thank Jorge Gomez for reviewing the manuscript. The authors thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination and design support for the digital illustrations/animations, on behalf of GSK. Fanélie Bauer coordinated publication development and editorial support. Amrita Ostawal (independent medical writer for Business & Decision Life Sciences) provided medical writing support.
Disclosure statement
Evgeniy Shpeer, Mikhail Scherbakov, Désirée van Oorschot, Alen Marijam, and Ekaterina Safonova are employed by and hold shares in the GSK group of companies. Vladimir Tatochenko received an honorarium from the GSK group of companies for participation to advisory boards. Alla Rudakova received an honorarium from the GSK group of companies for participation to advisory boards and travel fees. Evgeniy Shpeer, Mikhail Scherbakov, Désirée van Oorschot, Alen Marijam, Ekaterina Safonova, and Vladimir Tatochenko declare no other financial and non-financial relationship and activities. Nikolay Briko declares no financial and non-financial relationship and activities.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2045152
Additional information
Funding
References
- Centers for Disease Control and Prevention. Varicella. in: the Pink Book: Epidemiology and prevention of vaccine-preventable diseases. Washington D.C: National Center for Immunization and Respiratory Diseases; 2020 [accessed 2021 May 2]. https://www.cdc.gov/vaccines/pubs/pinkbook/varicella.html .
- Riera-Montes M, Bollaerts K, Heininger U, Hens N, Gabutti G, Gil A, Nozad B, Mirinaviciute G, Flem E, Souverain A, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017;17:353. doi:10.1186/s12879-017-2445-2 .
- Sengupta N, Breuer J. A global perspective of the epidemiology and burden of Varicella-Zoster virus. Curr Pediatr Rev. 2009;5:207–11. doi:10.2174/157339609791317315 .
- Dbaibo G, Tatochenko V, Wutzler P. Issues in pediatric vaccine-preventable diseases in low- to middle-income countries. Hum Vaccin Immunother. 2016;12:2365–77. doi:10.1080/21645515.2016.1181243 .
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper June 2014. Recommendations Vaccine. 2016;34:198–99. doi:10.1016/j.vaccine.2014.07.068 .
- Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, Maupin TJ, Goldman GS, Tabony LJ, Brodovicz KG, et al. Varicella disease after introduction of varicella vaccine in the United States 1995-2000. Jama. 2002;287:606–11 .
- García Cenoz M, Castilla J, Chamorro J, Martínez-Baz I, Martínez-Artola V, Irisarri F, Arriazu M, Ezpeleta C, and Barricarte A. Impact of universal two-dose vaccination on varicella epidemiology in Navarre, Spain 2006 to 2012. Eurosurveillance. 2013;18:20552. doi:10.2807/1560-7917.ES2013.18.32.20552 .
- Heywood AE, Wang H, Macartney KK, McIntyre P. Varicella and herpes zoster hospitalizations before and after implementation of one-dose varicella vaccination in Australia: an ecological study. Bull World Health Organ. 2014;92:593–604. doi:10.2471/BLT.13.132142 .
- Marin M, Watson TL, Chaves SS, Civen R, Watson BM, Zhang JX, Perella D, Mascola L, Seward JF. Varicella among adults: data from an active surveillance project 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94–s100. doi:10.1086/522155 .
- Quian J, Rüttimann R, Romero C, Dall’-Orso P, Cerisola A, Breuer T, Greenberg M, Verstraeten T. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997-2005. Arch Dis Child. 2008;93:845–50. doi:10.1136/adc.2007.126243 .
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Human Vaccine Immunotherapeutics. 2019;15:645–57. doi:10.1080/21645515.2018.1546525 .
- World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2020 global summary. Geneva: World Health Organization; 2020 [accessed 2021 May 5]. http://apps.who.int/immunization_monitoring/globalsummary/schedules .
- Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor). On the state of sanitary and epidemiological welfare of the population in the Russian Federation in 2017. 2018 [accessed 2021 June 3]. https://www.rospotrebnadzor.ru/documents/details.php?ELEMENT_ID=10145 .
- Baryshev MA, Chernyavskaya OP, Saltykova TS. Experience of the varicella vaccine introduction into regional vaccination schedule of the Russian Federation. Epidemiol Vaccinal Prev. 2019;18:67–74. doi:10.31631/2073-3046-2019-18-6-67-74 .
- Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor). Государственный доклад О состоянии санитарно-эпидемиологического благополучия населения в российской федерации в 2019 году; 2020 [accessed 2021 July 15]. https://www.rospotrebnadzor.ru/documents/details.php?ELEMENT_ID=14933 .
- Vankova O, Ulanova T, Loparev V. P1438 seroprevalence and genotyping of varicella zoster virus strains in the Russian Federation. Int J Antimicrob Agents. 2007;29. doi:10.1016/S0924-8579(07)71277-3 .
- Peredelskaya EA, Safyanova TV, Druchanov MM. Clinical and epidemiological characteristics of chickenpox in children aged 0–17 in Barnaul. J Infectol. 2021;13:66–70. doi:10.22625/2072-6732-2021-13-1-66-70.
- Druzhinina T. Chickenpox in the Yaroslavl region. Economic efficiency of varicellation of one age-group of children. Pediatr Pharmacol (New York). 2012;9:14–21. doi:10.15690/pf.v9i5.450 .
- Brisson M, Edmunds WJ. Varicella vaccination in England and Wales: cost-utility analysis. Arch Dis Child. 2003;88:862–69. doi:10.1136/adc.88.10.862 .
- Government of the Russian Federation. Decree of the Government of the Russian Federation of December 8, 2017 No. 1492 “on the program of state guarantees of free provision of medical care to citizens for 2018 and for the planning period of 2019 and 2020”. Moscow: Ministry of Health of the Russian Federation; 2017 [accessed 2021 May 9]. http://www.garant.ru/products/ipo/prime/doc/71729300/ .
- State Register of Medicine. State register of maximum selling prices. Russian Federation; 2009 [accessed 2021 Apr 13]. http://grls.rosminzdrav.ru/PriceLims.aspx?Torg=%d0%92%d0%b0%d1%80%d0%b8%d0%bb%d1%80%d0%b8%d0%ba%d1%81&Mnn=&RegNum=&Mnf=&Barcode=&Order=&PageSize=8&orderby=pklimprice&orderType=desc&pagenum=1 .
- Emiss. Number of births (without stillbirths) per year. Russia: State Statistics [accessed 2021 May 6]. http://apps.who.int/immunization_monitoring/globalsummary/schedules .
- Katz AL, Webb SA. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485 .
- RosInfoStat. Mortality statistics according to Rosstat: RosInfoStat; 2021 [accessed 2021 Augt 13]. https://rosinfostat.ru/smertnost/#i-4.
- Federal State Statistics Service (ROSSTAT). Fertility according to rosstat data: RosInfoStat; 2021 [accessed 2021 July 14]. https://rosinfostat.ru/rozhdaemost/ .
- GlaxoSmithKline Biologicals SA. Инструкция по медицинскому применению лекарственного препарата варилрикс®/varilrix®: GlaxoSmithKline Biologicals SA; 2020 [accessed 2021 July 16]. https://gskpro.com/content/dam/global/hcpportal/ru_RU/varilrix/pdf/varilrix_pil_gds_v012_2017_05_11_aw_2017_06_19.pdf .
- Povey M, Henry O, Riise Bergsaker MA, Chlibek R, Esposito S, Flodmark CE, Gothefors L, Man S, Silfverdal SA, Štefkovičová M, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine or one dose of monovalent varicella vaccine: 10-year follow-up of a phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect Dis. 2019;19:287–97. doi:10.1016/S1473-3099(18)30716-3 .
- Federal Statistics Service. Key figure- Russia. Russian Federation; 2019 [accessed 2021 Mar 6]. https://www.gks.ru/free_doc/new_site/m-sotrudn/eng_site/Key_Figures.pdf .
- Federal Tax Service of Russia. Налог на доходы физических лиц (НДФЛ); 2021 [accessed 2021 July 15]. https://www.nalog.gov.ru/rn77/taxation/taxes/ndfl/ .
- Shakhanina IL, Gorelov AV, Lytkina IN, Tolkushin AG. Economic assessment of vaccine prevention of children pox on the example of Moscow. Epidemiology Infect Dis. 2009;3:49–56 .
- Teplova TE, Teplova EG Организацияи совершенствование медицинскогообеспеченияигосударственногосанитарноэпидемиологического надзора. предупреждение и ликвидация последствий чрезвычайных ситуаций. 2012: medicine of extreme situations. [accessed 2021 July 27]. https://cyberleninka.ru/article/n/ekonomicheskaya-effektivnost-vaktsinoprofilaktiki-vetryanoy-ospy-v-usloviyah-priblizhennyh-k-obscherossiyskim/viewer .
- International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world - Russian Federation 2018. 2018: ISPOR Russia HTA regional chapter. [accessed 2021 May 4]. https://tools.ispor.org/PEguidelines/countrydet.asp?c=18&t=4 .
- Teptsova TS, Musina NZ, Omelyanovsky VV. Evaluation of the reference value of the incremental parameter “cost-effectiveness” for Russian healthcare system (in Russ.). FARMAKOEKONOMIKA Modern Pharmacoeco Pharmacoepidemiol. 2020;13:367–76. doi:10.17749/2070-4909/farmakoekonomika.2020.071 .
- Yagudina RI, Sorokovikov IV. Methodology for conducting cost-benefit analysis in pharmacoeconomic research. 2012: Методология фармакоэкономического анализа. [accessed 2021 July 27]. https://cyberleninka.ru/article/n/metodologiya-provedeniya-analiza-zatraty-poleznost-pri-provedenii-farmakoekonomicheskih-issledovaniy .
- Ksenofontova O, Rozhkova L, Savvinova T, Kharitonov A. Experience of vaccine prevention for varicella in Yekaterinburg. Pediatr Pharmacol (New York). 2010;7:34–36 .
- Tatochenko V, Ksenofontova O, Rozhkova L, Savvinova T, Kharitonov A. Post-Exposure immunological prevention against varicella. Pediatr Pharmacol (New York). 2010;7:30–33 .
- Smirnova SS, Yuzhanina TS, Stepanova EA, Rupysheva TA. ВЕТРЯНАЯ ОСПА: РИСК-ОРИЕНТИРОВАННАЯ МОДЕЛЬ УПРАВЛЕНИЯ ЭПИДЕМИЧЕСКИМ ПРОЦЕССОМ '‘Flowpox: A risk-based epidemic control model’’. Far East J Infect Pathol. 2020;39:63–68 .
- Filippov O, Bolshakova N, Elagina T, Novikova Y, Shapovalova R, Aristova A. Regional schedule of vaccination in Moscow: history, development, prospects. Epidemiol Vaccinal Prev. 2020;19:63–75. doi:10.31631/2073-3046-2020-19-4-63-75 .
- Experts Nuo I Resolutions of the expert council on vaccine prevention. Moscow: Ministry of Health of the Russian Federation. [accessed 2021 July 24]. https://www.pediatr-russia.ru/information/vaktsinatsiya/natsionalnyy-nezavisimyy-ekspertnyy-sovet-po-immunoprofilaktike/natsionalnyy-soyuz-ekspertov-v-sfere-immunoprofilaktiki-.php .
- Vishneva EA, Kostinov MP, Mazankova LN, Malinnikova EY, Namazova-Baranova LS, Plakida AV, Privalova TE, Rtishchev AY, Tatochenko VK, Fedoseenko MV, et al. Resolution of the expert forum of Russian Federation '‘chickenpox: serious infection in Russian Federation, which can be prevented due to vaccination. Curr Pediatr. 2019;18:491–94 .
- Seward JF, Jumaan AO. VSV: persistence in the population. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007 .
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. doi:10.1038/nrdp.2015.16 .