ABSTRACT
Use of pneumococcal conjugate vaccines (PCVs) has greatly reduced the incidence of invasive pneumococcal disease (IPD). V114 (VAXNEUVANCE™, Merck Sharp & Dohme Corp. a subsidiary of Merck & Co. Inc. Kenilworth, NJ, USA) is a 15-valent PCV currently approved in adults in the United States, containing the 13 serotypes in licensed PCV13 and 2 additional serotypes (22F and 33F) which are important contributors to residual pneumococcal disease. This study quantified the health and economic burden of IPD attributable to V114 serotypes in hypothetical birth cohorts from Korea and Hong Kong. A Markov model was used to estimate the case numbers and costs of IPD in unvaccinated birth cohorts over 20 years. The model was applied to 3 scenarios in Korea (pre-PCV7, pre-PCV13, and post-PCV13) and to 2 scenarios in Hong Kong (pre-PCV7 and post-PCV13). For Korea, the model predicted 62, 26, and 8 IPD cases attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 scenarios, respectively. Costs of V114-type IPD fell from $1.691 million pre-PCV7 to $.212 million post-PCV13. For Hong Kong, the model estimated 62 V114-associated IPD cases in the pre-PCV7 scenario and 46 in the post-PCV13 scenario. Costs attributed to all V114 serotypes were $2.322 million and $1.726 million in the pre-PCV7 and post-PCV13 periods, respectively. Vaccine-type serotypes are predicted to cause continuing morbidity and cost in Korea (19A) and Hong Kong (3 and 19A). New pediatric pneumococcal vaccines must continue to protect against serotypes in licensed vaccines to maintain disease reduction, while extending coverage to non-vaccine serotypes.
Introduction
Streptococcus pneumoniae is a gram-positive bacterium that causes both invasive and noninvasive pneumococcal disease. Noninvasive manifestations include otitis media and non-bacteremic pneumococcal pneumonia (NBPP), while invasive pneumococcal disease (IPD) syndromes include bacteremic pneumonia, bacteremia without focus, and meningitis.Citation1,Citation2 Despite a substantial epidemiologic decline at the beginning of the 21st century, pneumococcal disease remains a significant source of morbidity and mortality in infants and children worldwide, causing an estimated 318,000 deaths in children <5 years in 2015.Citation3 In East Asia, incidence data for IPD are somewhat sparse, but reported incidence values in children <5 years range from 13/100,000 in Japan from 2003–2005Citation4 to 422/100,000 in Taiwan in 2006.Citation5 Case fatality rates in this region range from 1.6% in Japan to 8.1% in Taiwan.Citation4 In Hong Kong, the incidence of IPD in children ≤5 years was reported to be 15.6/100,000,Citation6 and the risk of death was found to be significantly increased (odds ratio 3.26) among children admitted to a pediatric intensive care unit (PICU) with pneumococcal disease compared to those without pneumococcal disease.Citation7 A 10-year analysis of data from this PICU showed a mortality rate of 20% among patients with IPD.Citation8
Global surveillance has shown that, among the more than 90 known pneumococcal serotypes, only a small number cause a majority of IPD.Citation9,Citation10 These are the serotypes contained in past pediatric pneumococcal conjugate vaccines (PCVs), which were introduced to the market in the early 2000s. The first PCV was heptavalent (PCV7) and contained serotypes 4, 6B, 9 V, 14, 18C, 19F, and 23F.Citation2 Since then, PCV13, a 13-valent vaccine, which contains all the PCV7 serotypes plus an additional 6 serotypes: 1, 3, 5, 6A, 7F, and 19A (“PCV13-specific” serotypes) has been introduced.Citation2 The introduction of PCVs via infant immunization schedules has led to substantial decreases in IPD associated with vaccine-targeted S. pneumoniae serotypes in children, and has indirectly benefited adult populations.Citation11 However, the emergence of non-PCV serotypes has been observed,Citation12–16 and select vaccine-targeted serotypes, such as 3Citation8,Citation17and 19A,Citation12,Citation13 continue to persist in East Asian populations.
In a recent global systematic review including results from 27 countries published between 2010–2015, serotypes 22F and 33F were two of the most commonly observed non-PCV13 serotypes in children with IPD,Citation16 and a new 15-valent PCV, V114 (VAXNEUVANCE™, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA), includes serotypes 22F and 33F as well as the PCV7- and PCV13-specific serotypes. V114 has been approved by the United States Food & Drug Administration for use in adults aged ≥18 years and is undergoing clinical trials in pediatric populations. To demonstrate the need for continued protection against established serotypes while also expanding serotype coverage, this study quantified the health and economic burden of IPD attributable to all 15 serotypes in V114 using two hypothetical birth cohorts from Korea and Hong Kong. IPD cases and costs attributable to all 15 V114 serotypes were estimated prior to and following PCV7 and PCV13 introduction.
Methods
Model design
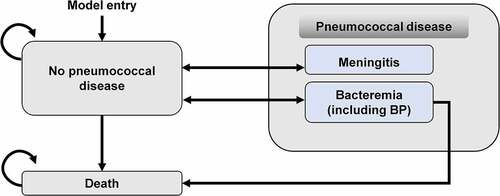
A Markov model with three health states—no pneumococcal disease, IPD (meningitis and bacteremia including bacteremic pneumonia [BP]), and death ()—was adapted from a previous analysis.Citation18 At the start of the model, an unvaccinated birth cohort enters into the “no pneumococcal disease” state and is at risk of developing IPD. The probability of an infant developing pneumococcal disease varies by age over the time horizon of the model. During each cycle, some individuals move into the IPD state based on the annual incidence. Those who are in the bacteremia (including BP) state may die due to their infection, with rates based on case fatality rates. Background mortality is applied to all states in the model. The model assumed that infants could experience only one IPD event during each year and that IPD could lead to either recovery (return to the no pneumococcal disease health state) or death. Post-meningitis sequelae, NBPP, and acute otitis media were not considered in the model. Indirect protection of adults via pediatric vaccination was not considered because the model included an unvaccinated birth cohort.
Model inputs
Cohorts of unvaccinated infants born in 2018 in Korea and Hong Kong were modeled over 20 years to estimate cases, deaths, direct medical costs, and indirect costs for IPD. Based on local census data, the total cohort consisted of 336,309 newborns in KoreaCitation19and 56,890 newborns in Hong Kong.Citation20 The model tracked each infant up to 20 years of age or death, whichever occurred first.
Epidemiologic and economic parameters were retrieved from the literature. PCVs were introduced intermittently in Korea—PCV7 in 2003, the 10-valent PCV (PCV10, which contains the PCV7 serotypes plus serotypes 1, 5, and 7F) in 2010, and PCV13 in 2010—so incidence and serotype distribution data were available for three eras: pre-PCV7, pre-PCV13, and post-PCV13.Citation21–23 Epidemiological inputs for Korea are shown in . In all three eras, the case fatality rates were 9.5% for meningitis and 5.6% for bacteremia.Citation24 In Hong Kong, PCVs were incorporated consecutively into the childhood immunization program (PCV7 in 2009, PCV10 in 2010, and PCV13 in 2011), so outcomes were only estimated for two eras, pre-PCV7 and post-PCV13, and incidence and serotype distribution data were retrieved for those two eras.Citation6,Citation25,Citation26 Epidemiological inputs for Hong Kong are shown in . In both eras, the case fatality rates were 9.0% for meningitis and 4.6% for bacteremia.Citation27,Citation28 For both analyses, the case fatality rates retrieved from the literature reflected current access to care and medical treatment for IPD.
Table 1. Epidemiological inputs for Korea and Hong Kong
Direct medical costs were obtained from the literatureCitation24,Citation28 and estimated from the healthcare perspective. Cost inputs for Korea and Hong Kong are shown in . A societal perspective considered direct medical costs and indirect costs, including productivity losses linked to premature death among children, and productivity losses among adult caregivers. However, due to lack of data on out-of-pocket costs, they were not included. For Korea, costs were updated to 2020 US dollars (USD) and discounted at 4.5% annually.Citation31 For Hong Kong, costs were updated to 2020 USD and discounted at 3% annually.Citation32,Citation33 Cost updates were performed using the medical component of the consumer price indexCitation34,Citation35and publicly available currency conversion ratios.Citation36,Citation37
Table 2. Cost inputs for Korea and Hong Kong
Model outputs
Health and economic outputs were estimated for Korea under three scenarios (pre-PCV7, pre-PCV13, and post-PCV13), and for Hong Kong under two scenarios (pre-PCV7 and post-PCV13). Disease incidence and serotype distributions corresponding to each vaccination era were applied to all 15 serotypes in V114. Outcomes included cases of IPD, mortality, and costs of IPD by serotype.
Sensitivity analysis
Deterministic sensitivity analysis was utilized to assess the impact of uncertainties around key parameters and assumptions in the pre-PCV7 scenario only. The following assumptions were explored: incidence rate of IPD ±20% (.8-1.2 the base case value), case fatality rate of IPD ±20% the base case value, direct medical costs and indirect costs associated with treating meningitis and bacteremia ±20% the base case value, and discount rates of 0% or 5%.
Results
Health and economic burden of V114-targeted pneumococcal serotypes in Korea
Cases of IPD by serotype
In the model for Korea, V114 serotypes caused 62, 26, and 8 IPD cases in the pre-PCV7, pre-PCV13 and post-PCV13 scenarios, respectively (). In the pre-PCV7 scenario, the majority of cases (46 cases, 75%) were attributable to the PCV7 serotypes. Most cases were attributable to serotype 19A in the pre-PCV13 scenario (18 cases, 67%) and the post-PCV13 scenario (4 cases, 44%). PCV13-specific serotypes increased from 15 cases in the pre-PCV7 scenario to 21 cases in the pre-PCV13 scenario. The increase was primarily due to an increase in serotype 19A from 8 cases in the pre-PCV7 scenario to 18 cases in the pre-PCV13 scenario. V114-specific serotypes 22F and 33F accounted for 0 (0%), 1 (3%), and 1 (17%) IPD cases in the pre-PCV7, pre-PCV13, and post-PCV13 scenarios, respectively.
Table 3. IPD cases attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods in Korea and Hong Konga
Mortality by serotype
The number of estimated deaths in Korea associated with V114 serotypes was 4 in the pre-PCV7 scenario, 2 in the pre-PCV13 scenario, and 1 in the post-PCV13 scenario (data not shown). Most of these deaths (75%) were attributable to PCV7-specific serotypes in the pre-PCV7 scenario. In the pre-PCV13 and post-PCV13 scenarios, deaths were attributable to the six additional serotypes in PCV13.
Costs of IPD by serotype
Total discounted costs (direct and indirect) in Korea due to V114 serotypes were estimated to be $1.691 million in the pre-PCV7 scenario, $.743 million in the pre-PCV13 scenario, and $.212 million in the post-PCV13 scenario (). PCV7-specific serotypes accounted for the majority of costs in the pre-PCV7 scenario ($1.269 million, 75%). Costs due to PCV13-specific serotypes increased from $.442 million (25%) in the pre-PCV7 scenario to $.578 million (78%) in the pre-PCV13 scenario. This was primarily due to increases in costs attributable to serotype 19A from $.211 million (12%) to $.496 million (67%). Total costs associated with V114-specific serotypes 22F and 33F were $0 in the pre-PCV7 scenario, $.020 million (3%) in the pre-PCV13 scenario, and $.035 million (17%) in the post-PCV13 scenario.
Table 4. Discounted direct and indirect costs associated with IPD in Koreaa
Sensitivity analysis
The discounted total cost was sensitive to uncertainties around all key parameters, especially the discount rate (). When the discount rate was 0% or 5%, total costs for IPD attributable to V114 serotypes increased by 290% or decreased by 11%, respectively.
Table 5. Results of sensitivity analysis for Korea and Hong Kong
Health and economic burden of V114-targeted pneumococcal serotypes in Hong Kong
Cases of IPD by serotype
In Hong Kong, V114 serotypes caused an estimated 62 and 46 IPD cases in the pre-PCV7 and post-PCV13 scenarios, respectively (). In the pre-PCV7 scenario, a majority of cases (59 cases, 96%) were attributable to PCV7 serotypes. PCV13-specific serotypes increased from 3 cases (4%) in the pre-PCV7 scenario to 43 cases (94%) in the post-PCV13 scenario. This increase was primarily due to an increase in serotype 3 from 1 case (2%) in the pre-PCV7 scenario to 38 cases (83%) in the post-PCV13 scenario. Cases due to serotype 19A increased from 0 (0%) to 5 (10%) across these time periods.
Mortality
Three deaths from IPD due to V114 serotypes were predicted in Hong Kong for the pre-PCV7 scenario, all of which were attributable to PCV7-specific serotypes (data not shown). Three deaths were predicted for the post-PCV13 scenario, 2 of which (67%) were attributable to serotype 3.
Costs of IPD by serotype
Total discounted medical costs and indirect costs in Hong Kong were estimated to be approximately $2.322 million over 20 years in the pre-PCV7 scenario and decreased to $1.726 million in the post-PCV13 scenario (). PCV7 serotypes accounted for the majority of total costs in the pre-PCV7 scenario (96%), whereas PCV13-specific serotypes accounted for the majority of the total costs in the post-PCV13 scenario (94%). The increase in PCV13-specific costs from the pre-PCV7 scenario to the post-PCV13 scenario was primarily due to increases in costs attributable to serotype 3 from $.044 million (2%) to $1.419 million (82%).
Sensitivity analysis
The discounted total cost was sensitive to uncertainties around all key parameters, especially the discount rate (). When the discount rate was 0% and 5%, total costs for IPD attributable to various serotypes increased by 185% and decreased by 36%, respectively.
Discussion
This study showed that PCV-targeted serotypes of S. pneumoniae are predicted to cause ongoing morbidity and cost in both Korea and Hong Kong. The models predicted that PCV13-specific serotypes of S. pneumoniae persisted to various degrees after implementation of routine PCV13 vaccination. Specifically, serotype 19A in Korea and serotype 3 in Hong Kong still caused a majority of IPD cases in the most recent period. Serotypes specific to the V114 vaccine, 22F and 33F, were predicted to impart additional morbidity and costs in Korea.
Our findings are consistent with the literature. Prior to the introduction of PCVs, the majority of IPD was caused by serotypes in PCV7.Citation38 In Korea, approximately 60% of IPD cases were due to PCV7 serotypes prior to PCV7 introduction,Citation39 and in Hong Kong, 89.6% of IPD cases were caused by PCV7 serotypes before PCV7 introduction.Citation17 Our results showed that IPD cases associated with PCV7 serotypes still persist. Thus, it is important to retain protection against PCV7 serotypes in current and future vaccine formulations.
Our findings indicated that S. pneumoniae serotype 19A is dominant in Korea, and serotype 3 is currently dominant in Hong Kong, despite these serotypes being included in PCV13. These results are in agreement with recent epidemiological studies from both and Hong KongCitation8,Citation17,Citation18 and Korea.Citation13 A survey of incidence rates in Hong Kong from 1995 to 2017 found that IPD cases due to serotype 3 were steadily increasing across all vaccine eras.17 Consistent with this, several studies have demonstrated a lack of PCV13 effective ness against IPD caused by serotype 3,40 and studies from the United States and the United Kingdom have highlighted the persistence of serotype 3 after PCV13 introduction.41,42 Regarding serotype 19A, Data from 2010–2015 in Korea showed that, although serotype 19A prevalence decreased steadily, it remained as prevalent as many non-PCV serotypes in 2015 and was the most prevalent serotype overall during this time period.Citation13 Elsewhere, the prevalence of IPD caused by serotype 19A has plateaued in recent years,Citation41,Citation42,Citation43 and this serotype remains a persistent cause of IPD in Europe.Citation16
Regarding serotype 3, a survey of incidence rates in Hong Kong from 1995 to 2017 found that IPD cases due to serotype 3 were steadily increasing across all vaccine eras.Citation17 Consistent with this, several studies have demonstrated a lack of PCV13 effectiveness against IPD caused by serotype 3,Citation18,Citation40 and studies from the United States and the United Kingdom have highlighted the persistence of serotype 3 after PCV13 introduction.Citation40,Citation44,Citation45,Citation46 Together, these findings highlight the importance of retaining the protection against the vaccine-type serotypes as new PCVs are developed. Moreover, higher-valency PCVs have been found to elicit lower immune responses relative to first-generation PCVs for the shared serotypes,Citation47 and this phenomenon, known as ‘geometric mean concentration creep’, becomes more pronounced as more serotypes are added.Citation48 New PCVs will need to incorporate strategies for better targeting of persistent serotypes.
Interestingly, although serotypes 22F and 33F are two of the most commonly observed non-PCV13 serotypes in children with IPD,Citation16 we observed only a minimal burden from these serotypes in the current analysis. This is likely because these serotypes were rarely or never observed in most serotyping studies from Korea and Hong Kong,Citation8,Citation12,Citation13,Citation17,Citation49,Citation50 suggesting a geographically tailored approach to surveillance, vaccination policy, and vaccine development will be needed. However, even in locations where serotypes 22F and 33F are rare, their invasiveness, which is comparable to that of serotype 19A,Citation51 may prioritize them for targeting by new PCVs. In some locations, the antibiotic resistance of both persistent and emergent serotypes may be the greater immediate concern. For example, in a prospective study at Seoul National University Children’s Hospital from 2010 to 2015, 91% of nasopharyngeal S. pneumoniae isolates from children exhibited multi-drug resistance.Citation13 Isolates from Korean children attending daycare centers in 2014 showed an 82% multi-drug resistance rate.Citation15 In a multi-center study of S. pneumoniae isolates collected from Korean hospitals between 2014 and 2016, serotype 19A had one of the highest rates of multi-drug resistance.Citation52
Our results show that PCV-targeted serotypes of S. pneumoniae will cause ongoing costs in both Korea and Hong Kong. Similar to the prevalence trends, total costs were expected to decrease from the pre-PCV7 scenario to the post-PCV13 scenario with costs decreasing by 87% in Korea and 26% in Hong Kong. Of interest, in Korea total costs associated with V114-specific serotypes 22F and 33F increased by 17% from the pre-PCV7 scenario to the post-PCV13 scenario. The costs associated with emerging serotypes 22F and 33F will likely vary by location, with these serotypes persisting in Korea.
There are several limitations of this study. First, the use of an unvaccinated cohort in combination with post-PCV13 incidence data provides an estimate of what will happen in the current era in the absence of external influences. The actual outcomes will, of course, be affected by local vaccination rates and other factors. In Korea, reported PCV coverage has ranged from 74% as of 2010Citation53 to 98% as of 2015.Citation54 Although a survey of Hong Kong parents of primary school children found that only 42% reported having had their children vaccinated with PCVs,Citation55 most studies from Hong Kong claim nearly universal infant vaccination with PCVs.Citation17,Citation27,Citation28,Citation56,Citation57 The high vaccination rates in both locations would be expected to affect the serotype replacement patterns over time, especially if new PCVs are introduced.
Secondly, although we assumed constant IPD incidence over 20-year time horizon, the incidence of IPD may shift over time in response to the invasiveness of emergent pneumococcal serotypes and to widespread health events such as the COVID-19 pandemic. In Korea, IPD cases decreased by 22% in the first half of 2020 compared to the previous 5 years.Citation58 In Hong Kong, cases of IPD decreased by 75% in 2020 compared to the previous five years, likely due to mask wearing adopted during the pandemic (similar results were observed in Singapore and Taiwan).Citation59
Other limitations of our analysis include the fact that noninvasive syndromes such as NBPP and acute otitis media were not considered in the model. Similarly, sequelae of meningitis, such as deafness or other long-term disability, were not included. The cost calculations also did not include direct non-medical costs accrued by families and caregivers, such as transportation and lodging. Indirect costs associated with productivity loss were estimated using conservative values for earnings, and for caregivers, only absenteeism was including in the productivity loss calculation. Furthermore, the average income for individuals under 20 years of age was assumed to be zero. As a result, the cost calculations presented here are likely an underestimation. Our Markov model did not account for transmission dynamics, outcomes such as nasopharyngeal carriage, and the indirect protective effect of PCVs on unvaccinated groups.. Finally, the many IPD cases attributed to non-vaccine-type serotypes in all three time periods likely caused our model to underestimate the health and economic burden of IPD attributable V114 serotypes.Citation60
In conclusion, our health and economic model showed that PCV-targeted serotypes, particularly serotypes 3 and 19A, continue to be associated with substantial IPD-related morbidity and costs after the introduction of PCVs. The persistence of PCV-targeted serotypes, and the morbidity and costs associated with emerging serotypes 22F and 33F, will likely vary by location. Thus, future pediatric PCVs must include serotypes contained in currently licensed PCVs to maintain disease reduction as well as expand serotype coverage to key non-vaccine serotypes that have emerged. Furthermore, surveillance of serotype prevalence should guide vaccine design and vaccination policy.
Acknowledgments
The authors thank Melissa Stauffer, PhD, in collaboration with ScribCo, for medical writing assistance.
Disclosure statement
Salini Mohanty, Tianyan Hu, GyongSeon Yang, Tsz K Khan, Kwame Owusu-Edusei, and Isaya Sukarom are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Additional information
Funding
References
- Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012 Jul;25(3):409–7. doi:10.1128/CMR.00018-12.
- Scelfo C, Menzella F, Fontana M, Ghidoni G, Galeone C, Facciolongo NC. Pneumonia and invasive pneumococcal diseases: the role of pneumococcal conjugate vaccine in the era of multi-drug resistance. Vaccines (Basel). 2021 Apr 22;9(5). doi:10.3390/vaccines9050420.
- Wahl B, O’-Brien KL, Greenbaum A, et al. Burden of streptococcus pneumoniae and haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018 Jul;6(7):e744–e757. doi:10.1016/S2214-109X(18)30247-X.
- Lin TY, Shah NK, Brooks D, Garcia CS. Summary of invasive pneumococcal disease burden among children in the Asia-Pacific region. Vaccine. 2009 Dec 9 ;28(48):7589–605. doi:10.1016/j.vaccine.2010.07.053.
- Bravo LC. Overview of the disease burden of invasive pneumococcal disease in Asia. Vaccine. 2010 Nov 10 ;27(52):7282–91. doi:10.1016/j.vaccine.2009.04.046.
- Ho PL, Chiu SS, Cheung CH, Lee R, Tsai TF, Lau YL. Invasive pneumococcal disease burden in Hong Kong children. Pediatr Infect Dis J. 2006 May;25(5):454–55. doi:10.1097/01.inf.0000215004.85582.30.
- Hon KL, Luk MP, Fung WM, et al. Mortality, length of stay, bloodstream and respiratory viral infections in a pediatric intensive care unit. J Crit Care. 2017 Apr;38:57–61. doi:10.1016/j.jcrc.2016.09.019.
- Hon KL, Chan KH, Ko PL, Cheung MHY, Tsang KYC, Chan LCN, Chan RWY, Leung TF, Ip M, et al. Change in pneumococcus serotypes but not mortality or morbidity in pre- and post-13-valent polysaccharide conjugate vaccine era: epidemiology in a pediatric intensive care unit over 10 years. J Trop Pediatr. 2018 Oct 1 ;64(5):403–08. doi:10.1093/tropej/fmx084.
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005 Feb;5(2):83–93. doi:10.1016/S1473-3099(05)70083-9.
- Song JY, Nahm MH, Moseley MA. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci. 2013 Jan;28(1):4–15. doi:10.3346/jkms.2013.28.1.4.
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74. doi:10.1080/21645515.2015.1118593.
- Chan KC, Subramanian R, Chong P, Nelson EAS, Lam HS, Li AM, Ip M, et al. Pneumococcal carriage in young children after introduction of PCV13 in Hong Kong. Vaccine. 2016 Jul 19;34(33):3867–74. doi:10.1016/j.vaccine.2016.05.047.
- Lee JK, Yun KW, Choi EH, Kim SJ, Lee SY, Lee HJ. Changes in the serotype distribution among antibiotic resistant carriage streptococcus pneumoniae isolates in children after the introduction of the extended-valency pneumococcal conjugate vaccine. J Korean Med Sci. 2017 Sep;32(9):1431–39. doi:10.3346/jkms.2017.32.9.1431.
- Ahn JG, Choi SY, Kim DS, Kim KH. Changes in pneumococcal nasopharyngeal colonization among children with respiratory tract infections before and after use of the two new extended-valency pneumococcal conjugated vaccines. Infect Dis (Lond). 2015 Jun;47(6):385–92. doi:10.3109/00365548.2014.1001997.
- Choe YJ, Lee HJ, Lee H, et al. Emergence of antibiotic-resistant non-vaccine serotype pneumococci in nasopharyngeal carriage in children after the use of extended-valency pneumococcal conjugate vaccines in Korea. Vaccine. 2016 Sep 14 ;34(40):4771–76. doi:10.1016/j.vaccine.2016.08.030.
- Balsells E, Guillot L, Nair H, Kyaw MH, Borrow R. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi:10.1371/journal.pone.0177113.
- Ho PL, Law PY, Chiu SS. Increase in incidence of invasive pneumococcal disease caused by serotype 3 in children eight years after the introduction of the pneumococcal conjugate vaccine in Hong Kong. Hum Vaccin Immunother. 2019;15(2):455–58. doi:10.1080/21645515.2018.1526555.
- Lo SW, Gladstone RA, van Tonder AJ,et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: An international whole-genome sequencing study. Lancet Infect Dis. 2019 Jul;19(7):759–769.
- Rubin JL, McGarry LJ, Strutton DR, Klugman KP, Pelton SI, Gilmore KE, Weinstein MC, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010 Nov 10 ;28(48):7634–43. doi:10.1016/j.vaccine.2010.09.049.
- Korean Statistical Information Service. Population. 2018 [ accessed 2021 Jul 12]. https://kosis.kr/index/index.do
- Hong Kong Census and Statistics Department. Population Estimates; [ accessed 2021 Jul 12]. https://www.censtatd.gov.hk/en/web_table.html?id=1A
- Lee S, Bae S, Lee KJ, Yu JY, Kang Y. Changes in serotype prevalence and antimicrobial resistance among invasive Streptococcus pneumoniae isolates in Korea, 1996–2008. J Med Microbiol. 2013 Aug;62(8):1204–10. doi:10.1099/jmm.0.058164-0.
- Cho EY, Choi EH, Kang JH, et al. Early Changes in the Serotype Distribution of Invasive Pneumococcal Isolates from Children after the Introduction of Extended-valent Pneumococcal Conjugate Vaccines in Korea, 2011-2013. J Korean Med Sci. 2016 Jul;31(7):1082–88. doi:10.3346/jkms.2016.31.7.1082.
- Korea Disease Control and Prevention Agency. Serotypes of Pneumococci Isolated from Invasive Infections of Korean Children (2 Year Study); [ accessed 2021 Aug 13]. https://www.prism.go.kr/homepage/
- Zhang XH, Leeuwenkamp O, Oh KB, Lee YE, Kim CM. Cost-Effectiveness analysis of infant pneumococcal vaccination with PHiD-CV in Korea. Hum Vaccin Immunother. 2018 Jan 2 ;14(1):85–94. doi:10.1080/21645515.2017.1362513.
- Ho PL, Chiu SS, Ang I, Lau YL. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae before and after introduction of 7-valent pneumococcal conjugate vaccine, Hong Kong, 1995-2009. Vaccine. 2011 Apr 12;29(17):3270–75. doi:10.1016/j.vaccine.2011.02.025.
- Hong Kong Centre for Health Protection. Scientific Committee on Vaccine Preventable Diseases. Updated Recommendations on the Use of 13-valent Pneumococcal Conjugate Vaccine in Childhood Immunisation Programme; [ accessed 2021 Jul 14]. https://www.chp.gov.hk/files/pdf/updated_recommendation_on_the_use_of_pcv3_in_hkcip_march2019_accessibility.pdf
- Lee KK, Rinaldi F, Chan MK, Chan STH, So TMT, Hon EKL, Lee VWY, et al. Economic evaluation of universal infant vaccination with 7vpcv in Hong Kong. Value in Health. 2009 Nov-Dec;12(Suppl 3):S42–48. doi:10.1111/j.1524-4733.2009.00626.x.
- Wu DB, Roberts C, Lee VW, Hong L-W, Tan KK, Mak V, Lee KKC, et al. Cost-Effectiveness analysis of infant universal routine pneumococcal vaccination in Malaysia and Hong Kong. Hum Vaccin Immunother. 2016;12(2):403–16. doi:10.1080/21645515.2015.1067351.
- Myers AL, Hall M, Williams DJ, Auger K, Tieder JS, Statile A, Jerardi K, McClain L, Shah SS, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013 Jul;32(7):736–40. doi:10.1097/INF.0b013e318290bf63.
- Olarte L, Barson WJ, Barson RM, et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin Infect Dis. 2015 Sep1;61(5):767–75. doi:10.1093/cid/civ368.
- Heath Insurance Review and Assessment Service. Guidelines for Economic Assessment of Drugs; [ accessed 2021 Aug 17]. https://repository.hira.or.kr/handle/2019.oak/2541
- Chan TS, Cheng SS, Chen WT, Hsu DC, Chau RWY, Kang SH, Alsumali A, Kwong Y-L, et al. Cost-Effectiveness of letermovir as cytomegalovirus prophylaxis in adult recipients of allogeneic hematopoietic stem cell transplantation in Hong Kong. J Med Econ. 2020 Dec;23(12):1485–92. doi:10.1080/13696998.2020.1843321.
- Yuen MF, Liu SH, Seto WK, Mak L-Y, Corman SL, Hsu DC, Lee MYK, Khan TK, Puenpatom A, et al. Cost–utility of All-Oral Direct-Acting Antiviral Regimens for the Treatment of Genotype 1 Chronic Hepatitis C Virus-Infected Patients in Hong Kong. Dig Dis Sci. 2021 Apr;66(4):1315–26. doi:10.1007/s10620-020-06281-8.
- Census and Statistics Department, Government of the Hong Kong Special Administrative Region. Consumer Prices; [ accessed 2021 Aug 13]. https://www.censtatd.gov.hk/en/scode270.html#section4
- Federal Reserve Economic Data. Consumer Price Index for All Urban Consumers: Medical Care in U.S. City Average; [ accessed 2021 Aug 13]. https://fred.stlouisfed.org/series/CPIMEDSL
- World Currency Exchange Rates and Currency Exchange Rate History; [ accessed 2021 Aug 13]. https://www.exchange-rates.org/Rate/KRW/USD/12-31-2018
- Internal Revenue Service. Yearly Average Currency Exchange Rates; [ accessed 2021 Aug 13]. https://www.irs.gov/individuals/international-taxpayers/yearly-average-currency-exchange-rates
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O'Brien KL, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010 Oct 5;7(10):e1000348. doi:10.1371/journal.pmed.1000348.
- Choe YJ, Choi EH, Lee HJ. The changing epidemiology of childhood pneumococcal disease in Korea. Infect Chemother. 2013 Jul;45(2):145–58. doi:10.3947/ic.2013.45.2.145.
- Pilishvili TPCV13 effects on disease caused by serotype 3. Presentation to the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention, Atlanta, Georgia;. 2019 Feb 28;Atlanta, Georgia: Advisory Committee on Immunization Practices; [accessed 2021 Jun 9]. https://www.cdc. gov/vaccines/acip/meetings/downloads/min-archive/min-2019- 02-508.pdf
- European Centre for Disease Prevention and Control. Surveillance atlas of infectious diseases; [ accessed 2021 Jun 9]. https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018 Apr;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020 Apr;26(4): 512 e510-512 e511. doi:10.1016/j.cmi.2019.09.008.
- Pilishvili T. PCV13 effects on disease caused by serotype 3. Presentation to the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention, Atlanta, Georgia; 2019 Feb 28; Atlanta, Georgia: Advisory Committee on Immunization Practices; [accessed 2021 Jun 9]. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2019-02-508.pdf
- Beall B, Chochua S, Gertz RE Jr., et al. A Population-Based Descriptive Atlas of Invasive Pneumococcal Strains Recovered Within the U.S. During 2015-2016. Front Microbiol. 2018;9:2670. doi:10.3389/fmicb.2018.02670.
- Kandasamy R, Voysey M, Collins S, et al. Persistent Circulation of Vaccine Serotypes and Serotype Replacement After 5 Years of Infant Immunization with 13-Valent Pneumococcal Conjugate Vaccine in the United Kingdom. J Infect Dis. 2020 Mar 28;221(8):1361–70. doi:10.1093/infdis/jiz178
- Yeh SH, Gurtman A, Hurley DC, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010 Sep;126(3):e493–505. doi:10.1542/peds.2009-3027.
- Kwambana-Adams BA, Mulholland EK, Satzke C, group I. State-of-the-art in the pneumococcal field: Proceedings of the 11(th) International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-11). Pneumonia (Nathan); Melbourne, Australia. 2020;12:2.
- Park M, Kim HS, Shin KS, Kim HS, Park JY, Song W, Cho HC, Lee KM, Kim J-S, et al. Changes in the incidence of Streptococcus pneumoniae bacteremia and its serotypes over 10 years in one hospital in South Korea. Vaccine. 2014 Nov 12;32(48):6403–07. doi:10.1016/j.vaccine.2014.09.062.
- Kim SH, Bae IK, Park D, et al. Serotype Distribution and Antimicrobial Resistance of Streptococcus pneumoniae Isolates Causing Invasive and Noninvasive Pneumococcal Diseases in Korea from 2008 to 2014. Biomed Res Int. 2016;2016:6950482.
- Balsells E, Dagan R, Yildirim I, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: A systematic review and meta-analysis. J Infect. 2018 Nov;77(5):368–78. doi:10.1016/j.jinf.2018.06.004.
- Park DC, Kim SH, Yong D, et al. Serotype Distribution and Antimicrobial Resistance of Invasive and Noninvasive Streptococcus pneumoniae Isolates in Korea between 2014 and 2016. Ann Lab Med. 2019 Nov;39(6):537–44. doi:10.3343/alm.2019.39.6.537.
- Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013 Sep;28(9):1283–88.
- Sohn S, Hong K, Chun BC. Evaluation of the effectiveness of pneumococcal conjugate vaccine for children in Korea with high vaccine coverage using a propensity score matched national population cohort. Int J Infect Dis. 2020 Apr;93:146–50. doi:10.1016/j.ijid.2020.01.034.
- Hon KL, Tsang YC, Chan LC, Ng DKK, Miu TY, Chan JY, Lee A, Leung TF, et al. A community-based cross-sectional immunisation survey in parents of primary school students. NPJ Prim Care Respir Med. 2016 Apr 7;26(1):16011. doi:10.1038/npjpcrm.2016.11.
- Wong CKH, Liao Q, Guo VYW, Xin Y, Lam CLK. Cost-Effectiveness analysis of vaccinations and decision makings on vaccination programmes in Hong Kong: A systematic review. Vaccine. 2017 May 31;35(24):3153–61. doi:10.1016/j.vaccine.2017.04.050.
- Chan D. Immunisation Coverage for Children Aged Two to Five: Findings of the 2015 Immunisation Survey. Communicable Diseases Watch; [ accessed 2021 Jun 4]. https://www.chp.gov.hk/files/pdf/cdw_v14_6.pdf
- Yun HE, Ryu BY, Choe YJ. Impact of social distancing on incidence of vaccine-preventable diseases, South Korea. J Med Virol. 2021 Mar;93(3):1814–16. doi:10.1002/jmv.26614.
- Teng JL, Fok KM, Lin KP, Chan E, Ma Y, Lau SK, Woo PC. Substantial Decline in Invasive Pneumococcal Disease During Coronavirus Disease 2019 Pandemic in Hong Kong. Clinical Infectious Diseases. 2022 Jan 15;74(2):335–8.