ABSTRACT
There are scarce data regarding influenza vaccination among people with HIV infection (PWHIV). The goal of this explorative study is to assess hesitancy toward influenza vaccination in a group of PWHIV during the COVID-19 pandemic. A questionnaire was administered to 219 patients vaccinated at our clinic during the 2020–2021 campaign. It evaluated subjects’ adherence to influenza vaccine over the last three seasonal vaccination campaigns, vaccine confidence, complacency and convenience, and the effect of the pandemic on the choice to become vaccinated. The population was divided into two groups: fully adherent to influenza vaccine (all three campaigns, 117 patients) and non-fully adherent (one or two campaigns, 102 patients). Adherence increased in the non-fully adherent group in 2020–2021, but the pandemic did not affect the choice. Misbeliefs emerged: the influenza vaccine was considered protective against SARS-CoV-2 (22.8% of the total population); almost half of all patients thought the influenza vaccine could improve their CD4 T cell level (57.3% in fully adherent, 40.2% in non-fully adherent, p < .05). In 2020–2021 campaign, three quarters of the non-fully adherent group would not have been vaccinated in a location other than our clinic (75.5% vs. 88.9% in the fully adherent group, p < .05). Conclusively, offering a secure and private space for vaccination against influenza seems to encourage vaccination; healthcare professionals should improve counseling to increase adherence and correct misbeliefs.
Introduction
Influenza virus is a common respiratory pathogen that circulates year-round and worldwide across different populations. To counteract seasonal influenza outbreaks, annual vaccination remains the primary preventive measure.Citation1 In Italy, influenza vaccination is recommended for subjects with a high risk of complications, such as children, adults with high-risk chronic conditions, people over 65 years of age (60 years since the 2020–2021 campaign), people likely to transmit influenza to the above-mentioned subjects, and workers with occupational hazards.Citation2
Symptoms of influenza infection appear to be similar in HIV and non-HIV patients. However, people living with HIV (PWHIV) seem to develop lower respiratory tract disease complications more frequently.Citation3 Thanks to antiretroviral therapy (ART), mortality after influenza infection among PWHIV decreased by three- to six-fold. Nonetheless, it remained higher than in non-HIV patients.Citation4 Vaccination is one of the cornerstones of public health since it is one of the most cost-effective methods to prevent infectious diseases. Although immune responses to most vaccines have been assessed to be somewhat less effective in PWHIV,Citation5 vaccination against several infectious diseases, including influenza, is currently recommended for HIV-infected patients.Citation6,Citation7 Despite this, adherence to influenza vaccination is suboptimal, and vaccination coverage hovers at a low level in non-mandatory settings.Citation8
In 2014, the World Health Organization’s Strategic Advisory Group of Experts (SAGE) defined both the concept of vaccine hesitancy (reluctance toward vaccination despite its availability) and the factors associated with it (confidence, complacency, and convenience).Citation9 Confidence represents the degree of trust in the effectiveness and safety of the vaccine; complacency indicates the degree to which people consider vaccination necessary to prevent a vaccine-preventable disease. Finally, convenience relates to the availability, affordability, willingness-to-pay, accessibility, ability to understand and accept vaccine-related information, and appeal of immunization services.Citation9
In 2019, the WHO classified vaccine hesitancy as a top 10 global health threat.Citation10 Since then, the determining factors of influenza vaccine hesitancy have been explored in several settings.Citation11-13 Vaccination is required every year due to waning immunity and the changing strains of the virus. Therefore, the evaluation of adherence and the assessment of consistency over time are crucial. In Italy, influenza vaccination for all PWHIV is free of charge after HIV diagnosis. Influenza vaccination is available at any general physician clinic or during public immunization campaigns. Furthermore, many HIV clinics (including ours) have a vaccination facility dedicated to administering all recommended vaccines for PWHIV. The simultaneous occurrence of both the SARS-CoV-2 pandemic and seasonal influenza epidemic caused great concern at the beginning of the 2020–2021 winter season due to clinical similarities and possible severity in case of coinfection.Citation14 Unfortunately, there are scarce data about the attitude and practice of PWHIV toward influenza vaccination, especially during the COVID-19 pandemic.
This explorative study investigates influenza vaccination hesitancy in a group of HIV-infected patients in a single center to improve adherence during future campaigns.
Patients and methods
Setting and study participants
Between November and December 2020, a cross-sectional study was conducted among HIV-infected patients before the onset of the winter influenza season via a telephone-questionnaire administered to all PWHIV followed by our clinic (Department of Infectious and Tropical Diseases, University of Brescia, Italy) that scheduled an appointment for influenza vaccination through our outpatient offices. In our clinic counseling concerning the need for seasonal influenza vaccine administration is performed during the follow up consultation preceding the beginning of the vaccination campaign. Patients can schedule an appointment at the administrative office, the nurse front-office, or during consultation (if it occurs during the vaccination campaign). However, they can also access at the vaccination session without appointment, which is held twice a week (one in the morning and one in the afternoon). Unfortunately, unscheduled access was not feasible in the 2020–2021 campaign, due to the relative vaccine shortage compared to the request.
COVID-19 pandemic strongly affected the Lombardia region in 2020, with Brescia resulting in one of the most affected cities. This determined a delay in influenza vaccine distribution and a regional shortage of doses compared to previous years, and considering the national indication to anticipate the campaign and to extend the free-of-charge offer.Citation2,Citation15 In Lombardia, the 2020 campaign for influenza immunization started on October 19 and reached a 70.7% coverage of the recommended population target.Citation15,Citation16 Due to the above-mentioned shortage of vaccine doses and a new increase in COVID-19 cases in our center, the campaign was carried out from November 15 to 22 December 2020, with a two-week delay compared to 2019–2020. However, a 112.6% increase in available doses was registered (270 doses vs. 127 in 2019–2020, destined to HIV patients and other at-risk patients followed at our clinic).
Data collection
All the patients were called via telephone and were asked for verbal informed consent regarding participation in the survey before administering the questionnaire. A maximum of 3 call attempts was made to contact a subject who did not answer at first. During the next clinical consultation, written informed consent to the survey, anonymous data collection, and publication was obtained.
A simplified questionnaire was developed based on the WHO SAGE technical report on vaccine hesitancy. More items were included as result of semi-structured interviews, which were previously held during clinical consultations at our vaccination clinic for PWHIV from May to September 2020. These interviews revealed peculiar beliefs on the effects of vaccination on CD4 T-cell counts, as well as confusion on influenza and COVID-19. Based on that, dedicated items were included in our questionnaire.
The questionnaire (Suppl.1) was administered via telephone, and it included the following sections:
1. Demographic data: gender, age;
2. Data on influenza vaccine adherence and attitude:
- Adherence to influenza vaccinations in the previous three seasons (2017–2018, 2018–2019, 2019–2020);
- Patient’s willingness to adhere to future influenza vaccinations;
3. Data on influenza vaccine convenience of settings:
- Location where the vaccination was carried out during the previous campaigns and patients’ preferences;
4. Data on influenza vaccine confidence, convenience other than setting, and complacency:
- Main reasons for influenza vaccine adherence or, conversely, refusal;
- Knowledge about influenza vaccine and its possible relationship with HIV infection and COVID-19.
Since all the patients involved in this study were followed by our clinic, clinical and demographic data were retrieved from their electronic health records. The following HIV-related data were acquired: year of the first diagnosis, last CD4 T cell count, quantitative HIV-RNA (copies/ml), and comorbidities, if any. Comorbidities were retrieved from the clinical record. Only those which posed an indication to influenza vaccine administration by the Italian Ministry of Health were recorded:Citation2 chronic pulmonary diseases (severe asthma, COPD, pulmonary dysplasia, cystic fibrosis), cardiovascular diseases (hypertension, congenital diseases, history of stroke, heart attack or angina), diabetes and obesity (body mass index >30 kg/m2), chronic renal or adrenal insufficiency, cancer, diseases of the hematopoietic systems and hemoglobinopathies, acquired or congenital immunodeficiency (other than HIV), chronic inflammatory diseases and malabsorption syndromes, chronic liver diseases, scheduled major surgery, neuromuscular diseases, pregnancy at the beginning of the seasonal epidemic.
We classified patients as “fully adherent” (3 out of 3 campaigns) and “non-fully adherent” if they did not partake in one or more influenza vaccine seasonal campaigns.
Statistical analysis
Absolute and relative frequency was calculated for all the categorical variables, whereas continuous variables were outlined by median and interquartile range (IQR). All the information and data collected through the questionnaire were entered in an electronic form developed using EpiInfo (version 7). Statistical data analysis was performed using the software SPSS Statistics, version 27.0.1.0.
Patients were categorized as fully adherent (3 out 3 in previous campaigns) and not fully adherent (inconsistently adherent to previous campaigns) to perform univariate analysis of baseline characteristics, knowledge, and willingness responses. Chi-squared and Mann–Whitney U tests were used as appropriate (p < .05).
The study obtained ethical clearance from the local Ethics Committee (study number 4722).
Results
Patients’ characteristics ( and )
Out of the 3841 HIV-infected patients censored in December 2020 at our HIV outpatient clinic, 388 booked the administration of an influenza vaccine at our department for the 2020–2021 season, corresponding to 10.1% overall. All the patients that scheduled an appointment for vaccination were contacted for the survey: 79 (20.4%) were not reached after three phone call attempts, and 90 (23.2%) refused to participate in the survey. Two hundred nineteen patients (56.4%) participated in the survey, and all of them eventually were vaccinated at our clinic for the current influenza season (see ). The average time for survey administration was 6 minutes (range 5–8).
Figure 1. Flow-Chart of the participation in the vaccine campaign and to the survey at the HIV clinic.
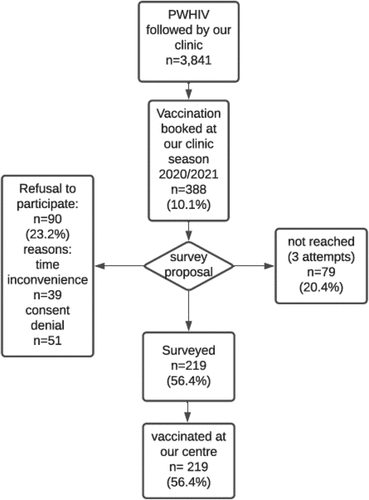
Table 1. Demographic and clinical characteristics classified per adherence to influenza vaccine in the previous 3 seasonal campaigns
The median age was 55 years (IQR 48–61), 76.3% (167/219) were male, and 26.5% (58/219) had at least one comorbidity. Age was higher in the fully adherent group (median 57 vs. 52, p < .001) (). The most frequent comorbidities were: cancer (12.3%), followed by cardiovascular disease (11%) and liver diseases (8.7%). Liver diseases were more often detected in the non-fully adherent group (13.7% vs. 4.3%, p = .01). Three patients were diagnosed with HIV in 2020, but they suffered from severe asthma (2 subjects) and from untreated, known chronic hepatitis C, all diseases for whom influenza vaccine is recommended. Years of known HIV seropositivity amounted to a median period of 18 years (IQR 8–28 years). All subjects were on stable ART with plasma HIV RNA levels <50 cp/ml, with a median CD4 T cell count of 697.5 (IQR 442–937). Only 3.7% (8/219) presented with a CD4 T cell count <200 cell/mm3.
Influenza vaccine adherence and attitude to future vaccination (data not shown)
In our study population, 14 patients (14/219, 6.4%) were never vaccinated during the 3 previous vaccination campaigns. Among others, 117/219 (53.4%) patients were “fully adherent”, while 102 (46.6%) were not adherent to at least one former campaign (“non-fully adherent”): 25 patients (25/219, 11.4%) were vaccinated in 2 campaigns out of 3 and 63 (63/219, 28.8%) in one campaign.
In 2020–2021 we registered a 33.8% catch-up (219/219 vs. 145/219) compared to the vaccination rate of the 2019–2020 season. Specifically, in the “non-fully adherent group”, 13.7% (14/102) of the patients received influenza vaccine for the first time in 2020–2021, and 58.8% (60/102) were non-adherent in 2019–2020 campaign.
The attitude toward future vaccination campaigns was also evaluated. Eighty-eight percent of the surveyed patients (193/219) expressed the will to receive the influenza vaccine regularly over the next three years. However, patients belonging to the fully adherent group showed a better attitude to vaccination compared to the counterpart (116/117, 99.1% vs. 77/102, 75%, p = .001).
Influenza vaccine confidence and complacency ()
Concern about developing a severe form of influenza was the main reason for vaccination among our patients (78.5%) (). Fully adherent patients were more aware of the risk of severe forms of influenza than non-fully adherent patients (103/117, 88% vs. 69/102 67.6%, p < .001). Notwithstanding, the overall presence and the number of comorbidities did not significantly influence the decision-making for vaccination (comorbidities were present in 23.1% of fully adherent vs. 30.4% of non-fully adherent patients; p = .22, see for comorbidities details).
Table 2. Beliefs in our population, classified per adherence to previous influenza vaccine seasonal campaigns
A rather peculiar finding was the one concerning a common misconception among the subjects (): almost half of the surveyed population (49.3%) stated that they were favorable to vaccination because the vaccine could somehow raise the CD4 T cell count, thus improving their HIV-related condition (57.3% in the fully adherent group vs. 40.2% in the non -fully adherent group, p = .015).
Considering the current campaign, 22.8% (50/219) patients decided to receive vaccination in the mistaken belief that the influenza vaccine could also be protective against SARS-CoV-2 (). This result was confirmed in both groups (21.6% vs. 23.9%). More than half of the subjects (112/219, 51.1%) vaccinated fearing that the clinical overlap between influenza and the SARS CoV-2 syndrome would have made difficult to pose a correct diagnosis and, consequently, treatment.
Finally, only six patients (6/102, 5.8%) did not become vaccinated in the previous campaigns due to concerns about adverse events related to the vaccine or fears related to conspiracy theories tied to the anti-vaccination environment (data not shown).
Influenza vaccine convenience ()
A significant reason that prompted our patients (79/219, 36.1%) to become vaccinated in the current campaign was the fear that healthcare facilities, in the throes of overcrowding due to COVID-19, would not have been able to offer an adequate standard of care in case of hospitalization.
Table 3. Complacency and convenience in our population, classified per adherence to previous influenza vaccine seasonal campaigns
A third of both the adherent and the non-adherent group declared that the need to guarantee continuity at work was a reason for vaccination.
For the season 2020–2021 we assessed the willingness to vaccinate in settings different than our clinic, and the availability of vaccines at our clinic was the only factor related to healthcare service that reached statistical significance. Indeed, only three quarters of the non-adherent group stated that they would have been vaccinated in a different location (75.5% vs. 88.9%, p = .009) ().
Regarding the previous campaigns, 205 patients were vaccinated at least once (data not shown). More than half of these patients (112/205, 54.6%) received vaccination at our healthcare facility exclusively, whilst 10/205 (4.8%) were vaccinated in various facilities of territorial medicine: drug stores, local prevention department, or general practitioners’ clinics. The remaining part (83/205, 40.5%) was vaccinated by both our clinic and other facilities on different campaigns.
Sixteen subjects (16/102, 15.7%) belonging to the non-fully adherent group reported not being vaccinated because the vaccination dose was not available at the time of their routine HIV-infection clinical consultation.
Discussion
To our knowledge, this paper explores for the first time the hesitancy toward the influenza vaccine in PWHIV. We evaluated adherence (during the last three seasonal campaigns), knowledge, and attitude expressed by the patients about the influenza vaccine. Additionally, we investigated whether the pandemic influenced their willingness to receive the influenza vaccine during the 2020–2021 season.
In our study population we observed an increase in the adherence to the influenza vaccine during the 2020–2021 seasonal campaign compared to previous campaigns.
Several studies, which focused on the general population, on groups at risk, and general practitioners, identified hesitancy as a critical obstacle to vaccination.Citation17-21 The lack of confidence, usually due to the fear of potential side effects and doubts about the safety and efficacy of the influenza vaccine, puts the success of vaccination programs at risk.Citation22,Citation23
Vaccination coverage is a growing concern in Italy, and coverage against influenza in people over 65 years of age and chronic patients hovers around 55% and 25–30%, respectively, in recent seasons. However, an increase has been registered in the 2020–2021 seasonal campaign: up to 65.3% among the elderly and 23.6% for the general population.Citation24,Citation25
In this survey, the setting of vaccination for PWHIV emerged as a crucial factor for vaccination adherence, possibly serving the purpose of maintaining their own privacy. In Italy, influenza vaccination is free of charge for all PWHIV independently of age or other comorbidities. However, it is necessary to state the HIV status at the moment of vaccination, thus exposing patients to social stigma. Offering vaccination in HIV clinics, hence assuring more privacy, might prove helpful to increase adherence to all vaccinations, not just influenza.
Contrary to previous studies, the presence and number of comorbidities did not positively influence decision-making among our population.Citation26 Comorbidities for whom vaccination is usually recommended and the risks they entail are not correctly perceived by the patients.Citation27,Citation28 This is confirmed both by recent literature about the Italian context, which highlights a clear gap in knowledge and information,Citation29 and by our study, in which patients with coexisting liver diseases were inconsistently adherent. Conversely, HIV patients with comorbidities may choose to receive influenza vaccine administration in other settings, as they do not need to declare HIV. This could be an additional explanation for the lack of association between comorbidities and influenza vaccine uptake. Even in this scenario, the fear of social stigma remains crucial in the choice to receive influenza vaccine.
Although the COVID-19 pandemic did not seem to be a determining factor in adherence, the belief that the influenza vaccine was protective for SARS CoV-2 emphasizes the importance of improving both communication and counseling for patients.
Indeed, only half of the population that participated in the survey was brought to vaccination by the counseling offered by healthcare professionals. This endorses the fact that a proactive attitude toward vaccination by healthcare professionals and informational campaigns should be considered instrumental in boosting adherence to vaccination.
The decision-making process followed by the patients to become vaccinated is deeply affected by beliefs and perceptions. Approximately half of the subjects stated that they were favorable to vaccination because it could improve their immune system, particularly the CD4 T cells count. In the literature, a transitory CD4 T cells count increase, linked to the physiological response to vaccination, is described in PWHIV after the administration of several different vaccines (including influenza vaccine).Citation30,Citation31 Adequate counseling about the meaning of this transitory response must be offered.
The attitude toward vaccination is generally positive, although the presence of a social desirability bias cannot be excluded. Patients receiving influenza immunization in 2019 were more likely to accept the vaccine in 2020. This seems to confirm the theory of “tendency of persistence”, in which vaccination in the prior year is considered one of the most important predictors of adherence to future vaccinations.Citation32
The hesitancy toward future vaccinations observed in our study represents the target for more incisive and precise counseling. Vulnerable groups are generally less hesitant, and their frequent access to the hospital environment may favor contact with medical counseling and awareness campaigns.Citation33 Therefore, informative strategies are going to be crucial in the forthcoming seasonal campaigns since an increase in complacency and reduced adherence are expected due to the low report of influenza cases globally in 2020–2021.Citation34
The most important limitation of this study is the missing data about PWHIV who did not book vaccination at our clinic, which precluded the possibility to deeply understand the relationship between comorbidities and vaccination, and the exploratory nature of the study, with a limited number of patients. Additionally, the survey depended on self-report data, which may be unreliable and may be affected by the social desirability bias (patients may have over-reported previous vaccinations or the intention to be vaccinated in the future). Furthermore, in 2020, the number of vaccine doses available at our HIV clinic was low compared to the request, because of the shortage and the distribution delay of vaccines in peripheral vaccination sites, which induced non-HIV patients to book vaccine administration at our site. This possibly hindered and delayed vaccine administration to HIV patients. National data have not shown a reduction in the vaccination coverage,Citation24 but inequity in dose distribution may potentially impact the benefits of the influenza vaccination campaign.Citation34 For all these reasons, results should be cautiously generalized, especially in non-Italian contexts, where HIV services and influenza vaccine administration in PWHIV may differ (i.e., not free of charge). Notwithstanding these limitations, to our knowledge, this is the first study carried out in Italy that evaluates practices and attitudes toward vaccination among PWHIV.
Conclusion
Vaccine hesitancy represents a problem for global healthcare systems, and the scarce awareness of diseases’ severity represents an important barrier to vaccine uptake, especially in people facing chronic conditions.
Chronic diseases offer clinicians a close relationship to patients, which should be exploited to foster them to become vaccinated. PWHIV should be more actively encouraged to receive vaccination, especially when affected by comorbidities. The organization of dedicated and flexible vaccine sessions in HIV clinics with a private and secure environment seems to positively affect influenza vaccine administration. Fears connected with the COVID-19 pandemic did not significantly influence the administration of the influenza vaccine. Our findings highlight the need for culturally appropriate and effective messages and approaches tailored to the concerns of PWHIV. In conclusion, influenza vaccine hesitancy in this vulnerable group is problematic, and a deeper effort by healthcare professionals toward proper counseling is imperative. Further research is needed to develop proper strategies to motivate vaccination recipients.
Authors' contribution
Conceptualization, VM and EQR; methodology, VM and EQR; validation, VM and EQR; formal analysis, VM; investigation, VM and EQR; data curation, CM and SS; writing—original draft preparation, VM and EQR; writing—review and editing, SS, CM, DC, EF, and FC; visualization, SS; supervision, EQR; project administration, EQR. All authors have read and agreed to the published version of the manuscript.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Institutional review board satement
The study obtained ethical clearance from the Ethics Committee of Spedali Civili of Brescia, Italy (study number 4722).
Supplemental Material
Download MS Word (29 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings are available from the corresponding author upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2046434
Additional information
Funding
References
- Budd A, Blanton L, Grohskopf L, Campbell A, Dugan VD, Wentworth E, Brammer L Surveillance Manual - Chapter 6: Influenza [Internet]. [accessed 2022 Sep 14]. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt06-influenza.html .
- Italian Ministry of Health. Ministero della Salute DIREZIONE GENERALE DELLA PREVENZIONE SANITARIA Prevenzione e controllo dell’influenza: raccomandazioni per la stagione 2020-2021 [Internet]. [accessed 2021 Sep 14]. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=74451&parte=1%20&serie=null .
- Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, Dawood H, Chhagan M, Haffejee S, Variava E, et al. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009-2013. PloS One. 2015;10:e0118884. doi:10.1371/journal.pone.0118884.
- González Álvarez DA, López Cortés LF, Cordero E. Impact of HIV on the severity of influenza. Expert Rev Respir Med. 2016;10:463–7. doi:10.1586/17476348.2016.1157474.
- Crum-Cianflone NF, Iverson E, Defang G, Blair PJ, Eberly LE, Maguire J, Ganesan A, Faix D, Duplessis C, Lalani T, et al. Durability of antibody responses after receipt of the monovalent 2009 pandemic influenza A (H1N1) vaccine among HIV-infected and HIV-uninfected adults. Vaccine. 2011;29:3183–91. doi:10.1016/j.vaccine.2011.02.040.
- Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi:10.1016/S1473-3099(09)70175-6.
- Ryom L, Cotter A, de Miguel R, Béguelin C, Podlekareva D, Arribas JR, Marzolini C, Mallon P, Rauch A, Kirk O, et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21:617–24. doi:10.1111/hiv.12878.
- Sticchi L, Bruzzone B, Caligiuri P, Rappazzo E, Lo Casto M, de Hoffer L, Gustinetti G, Viscoli C, di Biagio A. Seroprevalence and vaccination coverage of vaccine-preventable diseases in perinatally HIV-1-infected patients. Hum Vaccines Immunother. 2015;11:263–69. doi:10.4161/hv.36162.
- World Health Organization (WHO). Report of the sage working group on vaccine hesitancy [Internet]. 2014 [accessed 2022 Jan 24]. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf .
- World Health Organization (WHO). Ten Threats to global health in 2019 [Internet]. 2019 [accessed 2022 Jan 24] . https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- Dini G, Toletone A, Sticchi L, Orsi A, Bragazzi NL, Durando P. Influenza vaccination in healthcare workers: A comprehensive critical appraisal of the literature. Hum Vaccines Immunother. 2018;14:772–89. doi:10.1080/21645515.2017.1348442.
- Domnich A, Cambiaggi M, Vasco A, Maraniello L, Ansaldi F, Baldo V, Bonanni P, Calabrò GE, Costantino C, de Waure C, et al. Attitudes and beliefs on influenza vaccination during the COVID-19 pandemic: results from a representative Italian survey. Vaccines. 2020;8(4):711. doi:10.3390/vaccines8040711.
- Lytle KL, Collins SP, Feldstein LR, Baughman AH, Brown SM, Casey JD, Erickson HL, Exline MC, Files DC, Gibbs KW, et al. Influenza vaccine acceptance and hesitancy among adults hospitalized with severe acute respiratory illnesses, United States 2019-2020. Vaccine. 2021;39:5271–76. doi:10.1016/j.vaccine.2021.07.057.
- Dadashi M, Khaleghnejad S, Abedi Elkhichi P, Goudarzi M, Goudarzi H, Taghavi A, Vaezjalali M, Hajikhani B. COVID-19 and influenza co-infection: a systematic review and meta-analysis. Front. Med. 2021;8:681469. doi:10.3389/fmed.2021.681469.
- GIMBE Foundation. Report osservatorio GIMBE n. 3/2021 La vaccinazione antinfluenzale in Italia [Internet]. Bologna; 2021 [accessed 2022 Jan 29]. https://www.gimbe.org/osservatorio/Report_Osservatorio_GIMBE_2021.03_Vaccinazione_antinfluenzale_in_Italia.pdf .
- Regione Lombardia. Informazioni sulla campagna vaccinale [Internet]. 2020 [accessed 2021 Sep 14]. https://www.ats-brescia.it/campagna-vaccinale-antinfluenzale .
- Nowak SA, Gidengil CA, Parker AM, Matthews LJ. Association among trust in health care providers, friends, and family, and vaccine hesitancy. Vaccine. 2021;39:5737–40. doi:10.1016/j.vaccine.2021.08.035.
- González-Block MÁ, Pelcastre-Villafuerte BE, Riva Knauth D, Fachel-Leal A, Comes Y, Crocco P, Noboa L, Rodríguez Zea B, Ruoti M, Díaz Portillo SP, et al. Influenza vaccination hesitancy in large urban centers in South America. Qualitative analysis of confidence, complacency and convenience across risk groups. PloS One. 2021;16:e0256040. doi:10.1371/journal.pone.0256040.
- Hudson A, Montelpare WJ. Predictors of vaccine hesitancy: implications for COVID-19 public health messaging. Int J Environ Res Public Health. 2021;18(15):8054. doi:10.3390/ijerph18158054.
- Ziello A, Scavone C, di Battista ME, Salvatore S, di Giulio Cesare D, Moreggia O, Allegorico L, Sagnelli A, Barbato S, Manzo V, et al. Influenza vaccine hesitancy in patients with multiple sclerosis: a monocentric observational study. Brain Sci. 2021;11(7):890. doi:10.3390/brainsci11070890.
- Figueroa-Parra G, Esquivel-Valerio JA, Santoyo-Fexas L, Moreno-Salinas A, Gamboa-Alonso CM, de Leon-Ibarra AL, Galarza-Delgado DA. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum Vaccines Immunother. 2021;17:1420–25. doi:10.1080/21645515.2020.1816108.
- Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191–203. doi:10.1016/j.vaccine.2015.04.041.
- Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF joint reporting form data-2015–2017. Vaccine. 2018;36:3861–67. doi:10.1016/j.vaccine.2018.03.063.
- Italian Ministry of Health. Vaccinazione antinfluenzale-Confronti coperture nella Popolazione GENERALE al 2020-2021 [Internet]. 1999 [accessed 2022 Jan 24]. https://www.salute.gov.it/imgs/C_17_tavole_19_3_0_file.pdf .
- Bonanni P, Angelillo IF, Villani A, Biasci P, Scotti S, Russo R, Maio T, Vitali Rosati G, Barretta M, Bozzola E, et al. Maintain and increase vaccination coverage in children, adolescents, adults and elderly people: let’s avoid adding epidemics to the pandemic: appeal from the board of the vaccination calendar for life in Italy: maintain and increase coverage also by re-organizing vaccination services and reassuring the population. Vaccine. 2021;39:1187–89.
- Gerussi V, Peghin M, Palese A, Bressan V, Visintini E, Bontempo G, Graziano E, de Martino M, Isola M, Tascini C. Vaccine hesitancy among Italian patients recovered from COVID-19 infection towards influenza and Sars-Cov-2 vaccination. Vaccines. 2021;9:172. doi:10.3390/vaccines9020172.
- Alcusky MJ, Pawasauskas J. Adherence to guidelines for Hepatitis B, Pneumococcal, and influenza vaccination in patients with diabetes. Clin Diabetes. 2015;33:116–22. doi:10.2337/diaclin.33.3.116.
- Martinez-Huedo MA, Lopez-De-Andrés A, Mora-Zamorano E, Hernández-Barrera V, Jiménez-Trujillo I, Zamorano-Leon JJ, Jiménez-GarcíGarcíA R. Decreasing influenza vaccine coverage among adults with high-risk chronic diseases in Spain from 2014 to 2017. Hum Vaccines Immunother. 2020;16:95–99. doi:10.1080/21645515.2019.1646577.
- Napolitano F, della Polla G, Capano MS, Augimeri M, Angelillo IF. Vaccinations and chronic diseases: knowledge, attitudes, and self-reported adherence among patients in Italy. Vaccines. 2020;8:560. doi:10.3390/vaccines8040560.
- Thitilertdecha P, Khowawisetsut L, Ammaranond P, Poungpairoj P, Tantithavorn V, Onlamoon N. Impact of vaccination on distribution of T cell subsets in antiretroviral-treated HIV-infected children. Dis Markers. 2017;2017:1–8. doi:10.1155/2017/5729639.
- Pasricha N, Datta U, Chawla Y, Singh S, Arora SK, Sud A, Minz RW, Saikia B, Singh H, James I, et al. Immune responses in patients with HIV infection after vaccination with recombinant Hepatitis B virus vaccine. BMC Infect Dis. 2006;6:65. doi:10.1186/1471-2334-6-65.
- Nowalk MP, Zimmerman RK, Shen S, Jewell IK, Raymund M. Barriers to pneumococcal and influenza vaccination in older community-dwelling adults (2000–2001). J Am Geriatr Soc. 2004;52:25–30. doi:10.1111/j.1532-5415.2004.52006.x.
- Abramson ZH, Cohen-Naor V. Factors associated with performance of influenza immunization among the elderly. Isr Med Assoc J. 2000;2:902–07.
- Palache A, Rockman S, Taylor B, Akcay M, Billington JK, Barbosa P. Vaccine complacency and dose distribution inequities limit the benefits of seasonal influenza vaccination, despite a positive trend in use. Vaccine. 2021;39:6081–87. doi:10.1016/j.vaccine.2021.08.097.
