ABSTRACT
The presence of maternal poliovirus antibodies may interfere with the immune response to inactivated polio vaccine (IPV), and its influence on the safety of vaccination is not yet understood. A total of 1146 eligible infants were randomly assigned (1:1) to the IPV and Sabin IPV (SIPV) groups to compare and analyze the efficacy of the two vaccines in preventing poliovirus infection. We pooled the SIPV and IPV groups and reclassified them into the maternal poliovirus antibody-positive group (MAPG; ≥1: 8) and the maternal poliovirus antibody-negative group (MANG; <1: 8). We evaluated the impact of maternal poliovirus antibodies by comparing the geometric mean titer (GMT), seroconversion rate, and geometric mean increase (GMI) of types I–III poliovirus neutralizing antibodies post-vaccination, and incidence rates of adverse reactions following vaccination between the MAPG and MANG. Respective seroconversion rates in the MAPG and MANG were 94% and 100%, 79.27% and 100%, and 93.26% and 100% (all serotypes, P < .01) for types I—III poliovirus, respectively. The GMT of all types of poliovirus antibodies in the MAPG (1319.13, 219.91, 764.11, respectively) were significantly lower than those in the MANG (1584.92, 286.73, 899.59, respectively) (P < .05). The GMI in the MAPG was significantly lower than that in the MANG (P < .05). No statistically significant difference in the incidence of local and systemic adverse reactions was observed between the MAPG and MANG. Thus, the presence of maternal poliovirus antibodies does not affect the safety of IPV but can negatively impact the immune responses in infants after IPV vaccination.
Introduction
Poliomyelitis, commonly known as polio, is an acute infectious disease that largely affects the health of children. The global goal of eliminating polio is yet to be achieved. It has been reported that there are still two countries worldwide, Pakistan and Afghanistan, with endemic polio.Citation1 If people is not eradicated from the remaining endemic areas, international trade or means of transportation are highly susceptible to causing poliovirus epidemics in the polio-eradicated countries, for example, the imported poliovirus outbreak in Xinjiang, China in 2011.Citation2 Therefore, the WHO also listed China as one of the countries with the highest risk of poliovirus eradication.Citation3
In the global polio eradication campaign, oral poliovirus vaccine (OPV) and inactivated polio vaccine (IPV) are used to prevent polio. In rare cases, the vaccine virus may recirculate and mutate. Neurovirulence is reacquired during this process, which is referred to as circulating vaccine-derived poliovirus (VDPVS). The continuous use of OPV makes it difficult to avoid the threat of VDPVs to unvaccinated populations.Citation4 To fundamentally eliminate polio, including VDPVS, the Global Polio Eradication Initiative’s (GPEI’s) polio eradication and termination strategic plan 2013–2018 recommends the introduction of at least one dose of IPV vaccine in the routine immunization program, which will eventually transform OPV immunity into full IPV immunity.Citation5,Citation6
Maternal antibodies transmitted to the infants through the placenta protect newborns from viral infections for a short period of time. However, studies have shown that after the infants are vaccinated, positive maternal antibodies can suppress the vaccine-induced immune response to a certain extent.Citation7,Citation8 Preexisting maternal antibodies may affect the level of antibodies in the infant during initial immunization such as rotavirus vaccine, OPV, and measles vaccine.Citation9–12 Currently, evidence of adverse reactions remains inconclusive, and previous studies have explored the factors influencing adverse reactions after vaccination. Some studies have shown a relationship between antibody levels after human papillomavirus vaccination and adverse reactions, although not polio vaccine.Citation1 Maternal antibody being an influencing factor for antibody levels may affect the incidence rates of adverse reactions. According to a few studies, the presence of antibodies in the body may lead to a higher incidence of adverse events after vaccination, such as the combination measles-mumps-rubella-varicella vaccine (MMRV).Citation13 The relative importance of maternal antibodies has been extensively discussed, but the impact of maternal antibodies on the safety and immunogenicity of infants vaccinated with IPV needs further study.
This study was based on the safety and primary immunization data of phase III non-inferiority clinical trial of the Sabin IPV (SIPV) vaccine to analyze whether maternal antibodies affect the safety and immunogenicity of IPV.
Methods
Study design
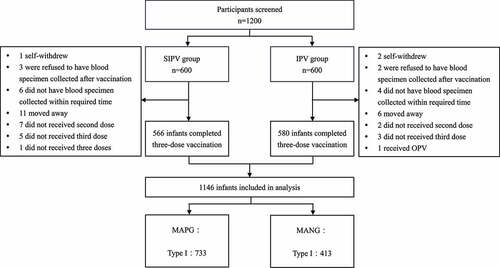
This study is a post hoc analysis based on a randomized, controlled, non-inferiority clinical trial of the Sabin strain polio vaccine (NCT03526978)Citation14 that has been carried out. The clinical trial was conducted from August 2017 to January 2018 in Pizhou and Guanyun, Jiangsu Province, China. A total of 1200 healthy 2-month-old infants were randomized (1:1) to receive the SIPV vaccine (600 infants) and IPV vaccine (600 infants) at 0, 1, and 2 months after enrollment. Blood samples were collected from all infants immediately before the first vaccination and 30 days after the third vaccination to detect neutralizing antibodies against poliovirus type I, II, and III.
All infants who had completed three doses of vaccination (at 0, 1, and 2 mouths) and had antibody test results before and after the three doses of vaccination were included in this analysis. All the infants in the group were two 2 months old, and their clinical characteristics were balanced. Poliovirus neutralizing antibodies present in the infants before vaccination were considered maternal antibodies. We divided infants into two subgroups according to the level of pre-immune antibodies, namely the maternal antibody-negative group (MANG, <1:8) and the maternal antibody-positive group (MAPG, ≥1:8).
Vaccine
The SIPV used in this study was developed by Beijing Sinovac. It was made by inoculating poliovirus type I, type II, and III (Sabin strain) into Vero cells, which were then inactivated. The antigen content of the SIPV was type I antigen 15 D-antigen unit (DU), type II antigen 45 DU, type III antigen 45 DU. The control vaccine IPV was developed by Sanofi. It was manufactured by inoculating Vero cells with type I (Mahoney strain), type II (MEF-1 strain) and type III (Saukett strain) poliovirus. The antigen content of IPV was type I antigen 40 DU, and type II antigen 8 DU, type III antigen 32 DU. Both vaccines were in liquid form, and administered at .5 mL per dose.
Determination of neutralizing titers
Venous blood samples were collected before immunization to measure the presence of antibodies against poliovirus type I, II, and III before the vaccination. One month after the immunization, venous blood samples were collected again to measure antibody levels of the three types of poliovirus. Each serum sample was heat-inactivated at 56°C for 30 min. Two-fold serial dilutions of the samples were carried out after an initial dilution of 1:4 (2 well-repeat). The viral suspension was then diluted to 100 median cell culture infective dose (CCID50) per .05 mL. Equal volumes (50 μL) of diluted serum and virus suspension were mixed. The plate was then incubated for 3 h (37℃ and 5% CO2). Subsequently, 100 µL of Hep-2 cell suspension at a cell density of .8‒1.0 × 105 cells/mL was added to each well and incubated at 35°C The cells were incubated for seven days to observe the cytopathic effect. The neutralizing antibody titer of the serum sample was determined based on the observation results. Serum with a neutralizing antibody titer ≥1:8 was considered positive.
Assessment of safety
Safety assessment mainly refers to the performance of adverse reactions after vaccination. Its main indicators include the incidence rate of systemic events, such as fever(axillary temperature ≥37.1℃), activity levels-weaken/reinforce, loss of appetite, running nose, cold and cough, rash, swallow red(redness inside throat), loose stools, indigestion, conjunctival congestion, abdominal pain, and local reactions (induration, redness, swelling, rash, and itching) within seven days after vaccination.Citation15 In this study, adverse reactions related to vaccination were analyzed, classified based on the severity according to the standard guidelines issued by the State Food and Drug Administration, and evaluated for the relationship between adverse events and immunization.
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS 21.0). When the virus-neutralizing antibody titer was lower than 1:8, it was directly assigned a ratio of 1:4, which is convenient for statistical analysis, with a confidence interval of 95%. To study maternal antibodies and their effects on the safety and immunogenicity of IPV, we categorized those with a neutralizing antibody titer <1:8 before immunization as the group without maternal antibodies and those with a neutralizing antibody titer ≥1:8 after immunization as the group with maternal antibodies. Seroconversion of poliovirus neutralizing antibody was defined as positive if the neutralizing antibody titer <1:8 before immunization was converted to ≥1:8 following immunization, or if the poliovirus neutralizing antibody titer ≥1:8 before immunization was increased by four times following immunization. The chi-square test was used to compare and analyze the factors that affected the positive conversion rate of maternal antibodies, as well as the difference in the incidence rates of adverse reactions between the MANG and MAPG following IPV. Differences in post-immunization poliovirus antibody-positive conversion rate, geometric mean titer (GMT), and geometric mean increase (GMI) between the two groups were evaluated using the t-test. Differences were considered statistically significant at P < .05.
Result
Basic characteristics of study participants
shows that a total of 1146 infants were finally included in this analysis with 566 infants being vaccinated with SIPV and 580 infants were vaccinated with IPV. The seropositivity rate of maternal antibodies before vaccination in 1146 infants was 63.96% for type I poliovirus, 50.52% for type II poliovirus, and 24.61% for type IIIpoliovirus. The baseline characteristics were similar between the MAPG and MANG subjects (). The GMT of maternal antibodies before vaccination in the MAPG was 27.95 (95% CI: 26.01–30.03) for type I poliovirus, 21.92 (95% CI: 20.33–23.63) for type II poliovirus and 21.18 (95% CI: 18.85–23.81) for type III poliovirus ().
Table 1. Baseline characteristics of participants
The impact of maternal antibodies on immunogenicity
In the MAPG and MANG, the significant differences in the seroconversion rates of the three types of poliovirus-neutralizing antibodies after vaccination were observed (). In the MANG, the seroconversion rate of the three types of poliovirus in all infants were 100.0%, whereas the seroconversion rates were 94% (92.04–95.50), 79.27% (75.78–82.37), and 93.26% (89.71–95.64), respectively for types I, II, and III poliovirus in the MAPG. Significant differences were also observed in the seroconversion rates between the groups regardless of the type of poliovirus (all serotypes, P < .01).
Table 2. Comparison of GMT, GMIs and seroconversion rate of three type-specific neutralizing antibodies between two groups after three dosesa
shows the GMTs of poliovirus antibodies after vaccination in the two groups. The GMTs of different types of polioviruses weas expressed at lower levels in the MAPG than that in the MANG. The difference was considered statistically significant for all types of poliovirus antibodies between the two groups (P < .05). The GMT of the three types of polioviruses-neutralizing antibodies increased in the two groups. Among these, the GMT of type I was the highest (1584.92, 95%CI:1368.48–1835.98). In addition, the GMIs of all three types of poliovirus antibodies in infants in the MANG was significantly higher than those in the MAPG (all serotypes, P < .01). Meanwhile, the seroconversion rate in the infants of the maternal polio-virus antibody-negative was 100%, regardless of received IPV or SIPV, higher than the infants of the maternal polio-virus antibody-positive (all P < .05). These results indicate that positive maternal poliovirus antibodies before vaccination can attenuate the immune response to vaccination.
The impact of maternal antibodies on safety
During the follow-up period, among these infants, 714 infants experienced adverse reactions. Local reactions occurred in 52 of 1146 infants and systemic adverse reactions occurred in 705 of 1146 infants. Most reactions were grade 1 or 2, including 14 with induration, 42 with redness, 5 with rash, and 10 with swelling. Four infants experienced grade 3 local reactions, including one with redness and three with a rash. Among 703 infants, most of experienced systemic reactions of fever, diarrhea, and vomiting.
There was no significant difference in the incidence of adverse reactions between the two groups. In this study, there were 714 infants experienced vaccination-related adverse reactions. The incidence rates of adverse reactions in the MAPG were 61.12%, 64.08%, and 62.06% for the three types of polioviruses, respectively. Except for the incidence rate of adverse reactions for type III poliovirus (62.38%) in the MANG, which was higher than in the MAPG, the other two types of poliovirus in MANG showed lower incidence rate (I: 60.41%; II: 60.49%) that those in the MAPG.
There was no significant difference in the incidence rate of adverse reactions between the MAPG and MANG regardless of the poliovirus type (all serotypes, P > .05). when infants were stratified based on maternal antibody levels, a significant difference in the incidence rate of adverse reactions was not observed in the three types of poliovirus (all serotypes, P > .05).
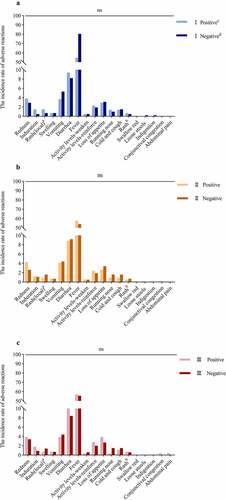
shows the incidence rate of local and systemic adverse reactions. A total of 703(61.34%) infants showed systemic adverse reactions. In the MAPG, the incidence rates of systemic adverse reactions were 60.3%, 62.9%, and 62.1% for types I, II, and III, respectively. Meanwhile, the incidence rates of systemic adverse reactions were 63.2%, 59.8%, and 61.1% for the three types of poliovirus in the MANG, respectively. For the three types of polioviruses, there was no significant difference between the MAPG and MANG with respect to the overall incidence of systemic adverse reactions (all serotypes P > .05). Moreover, there were no significant differences between the two groups in terms of adverse reaction, including vomiting, cold and cough, loss of appetite, and other reactions (all serotypes, P > .05).
In this study, 52 (4.54%) infants experienced local reactions, with redness and induration being the most common reactions, at the vaccination site. The incidence rates of local reactions in the MAPG and the MANG were 5.05% and 3.63% for type I poliovirus; 5.35% and 3.70% respectively for type II poliovirus, and 4.96% and 4.40% for type III poliovirus, respectively (all serotypes, P > .05). The incidence rates of rash and swelling at the vaccination site were too low to assess a significant difference. These results indicate that regardless of the presence or absence of maternal poliovirus antibodies in infants, the incidence rate of adverse reactions to vaccination was not significantly affected.
The incidence of local adverse reactions and systemic adverse reactions, including type I, II, and III of polio-virus, in the maternal antibody-positive group and negative group.
aRash occurring at the injection site;bRash occurring on the body;cMaternal antibody positive group; dMaternal antibody negative group
Discussion
Poliomyelitis largely endangers human health. As the target date for polio eradication is near, every infant should receive a polio vaccine that is crucial to polio eradication efforts. Factors affecting the effectiveness and safety of IPV should be considered. This study aims to assess the influence of maternal antibodies on immunogenicity and the safety of poliovirus vaccination in infants. Maternal antibodies are passed to the newborn through the placenta in the third trimester of pregnancy that protects the newborn against infections for a few months after birth.Citation16,Citation17 The most clinically relevant finding to emerge from this analysis was that the presence of maternal polio-virus antibodies could produce a lower immune effect after IPV but would not impart an adverse reaction burden.
In our study, the seropositivity rates of infants before immunization ranged from 24.6% to 62.3% for the three poliovirus types. The population with high poliovirus antibody levels may be due to many years of polio vaccination programs in China that led to the presence of maternal poliovirus antibodies in infants. The presence of maternal antibodies is a factor that affects the immunity of infants after vaccination. Treating maternally transmitted antibodies as preexisting antibodies may affect the immune response after vaccination. This phenomenon of maternal antibodies interference has weakened the performance of many vaccines such as adenovirus-based, flu, and pertussis vaccines.Citation18–20 In our study, except for the GMT of type III poliovirus that showed a non-significant difference between the two groups, other data, including GMI and seroconversion rate, were significantly different for the three types of poliovirus between the two groups. These results corroborate the finding that maternal poliovirus antibodies can interfere with the immune response to polio vaccination in infants. In addition, our study included two types of IPV, and the phenomenon that maternal antibody could reduce antibody levels after immunization was observed in both types. Stratified analysis of vaccine-type did not affect the final results, indicating that adequate weakening effect of maternal antibodies on the immunogenicity was observed for both IPV.
Maternal antibodies in infants can inhibit the immune responses to several vaccines, particularly IPV. 21 There have been a few studies on the inhibitory effect of maternal antibodies on antibody levels in infants after polio vaccination.Citation22–25 The influence of maternal antibodies was studied extensively by Merryn et al.Citation21 There were 20 antigens that could inhibit the immune response of prime vaccination in their findings. The phenomenon of inducing a weakened immune response to IPV was observed, and the GMTs decreased with an increase in maternal antibody levels. Our results indicated that maternal antibodies can affect the level of antibody level after immunization. Delayed IPV vaccination has a higher seroconversion rate, as maternal antibodies decline with time in infants.Citation22 This result should be considered for the routine immunization programs involving polio vaccines.
The most prominent finding to emerge from the analysis was that the presence of maternal antibodies is not an influencing factor for adverse reactions in infants vaccinated with IPV. Our study did not find a significant difference between the two groups, regardless of the reaction type.Citation26,Citation27 However, most previous studies have endpoints for the adverse events after the second immunization, and there are few studies focused on the relationship between maternal antibodies and the safety after vaccination. Our research is the first, to the best of our knowledge, to explored whether the presence of maternal antibodies is an influencing factor for adverse reactions after IPV. Our study showed that maternal antibodies had no effect on the incidence of adverse reactions after polio vaccination based on either IPV or SIPV. Moreover, the type of IPV did not affect the outcome. Fever is a common adverse event associated with polio vaccination in infants.Citation28 In the present study, among all adverse events, the incidence rate of fever was higher in the SIPV group than in the IPV group, which might be due to the higher concentration of D-antigen in SIPV.
Since China included OPV vaccination in its immunization program in 1978, most mothers had received OPV when they were newborns; therefore, most infants in China have detectable maternal poliovirus antibodies at the time of birth.
Moreover, by comparing the seroconversion rate of maternal poliovirus antibodies, we found that the positive rates of maternal antibodies in infants in the two regions were different in type I and type II. This might be due to the immunization gap between the regions when the mother was at the age of vaccination. Considering that maternal antibodies will affect the immune response of vaccinated infants, the difference in the positive rate of maternal antibodies between the regions may cause a differences in the positive rates of poliomyelitis antibodies in infants from different regions.
The present study had a number of limitations need to be noted regarding the present study. Our data were derived from a phase III clinical trial that used the Sabin strains to measure the neutralizing antibodies induced by the two vaccines. The Salk strains used for producing the control IPV was not used in the assay, which might have affected antibody titers. Nevertheless, data of SIPV clinical trials have shown satisfactory cross-neutralization ability among different wild poliovirus strains.Citation14 Since mothers have been vaccinated a long time back, it is difficult for us to collect information about their poliovirus antibody levels. In addition, we did not collect the birth information of the infants included in the study, such as the infant’s BMI, pregnancy method, and pregnancy time. Considering that these may be factors may affect an infant’s maternal antibodies levels, future studies should collect relevant information to provide a more comprehensive analysis for similar research in the future. Notwithstanding the relatively limited sample size, this study offers valuable results on the impact of maternal antibodies.
Conclusion
Poliovirus antibodies enter the newborn’s body through the placenta during pregnancy, and provide short-term protection to the newborn against infection. The presence of maternal antibodies in infants does not affect the safety of IPV but weakens the immune response after vaccination to a certain extent. The positive rates of maternal antibodies in different regions may differ. In the early stages of the global goal of eradicating polio and the preexistence of poliomyelitis virus antibodies in the population, the vaccination rate of newborns should be universalized, comprehensive, and jointly promote the steps of global eradication of polio.
Abbreviations
| GMT | = | geometric mean titers |
| GPEI | = | Global Polio Eradication Initiative |
| IPV | = | inactivated polio vaccine |
| MMRV | = | measles-mumps-rubella-varicella vaccine |
| MANG | = | negative maternal antibody group |
| OPV | = | oral polio vaccine |
| MAPG | = | positive maternal antibody group |
| SIPV | = | inactivated polio vaccine based on Sabin strain |
| VDPVs | = | vaccine‐derived polio-virus |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhuang CL, Lin ZJ, Bi ZF, Qiu LX, Hu FF, Liu XH, Lin BZ, Su YY, Pan HR, Zhang TY, et al. Inflammation-Related adverse reactions following vaccination potentially indicate a stronger immune response. Emerg Microbes Infect. 2021;10(1):365–7. doi:10.1080/22221751.2021.1891002.
- Luo HM, Zhang Y, Wang XQ, Yu WZ, Wen N, Yan DM, Wang HQ, Wushouer F, Wang HB, Xu AQ, et al. Identification and control of a poliomyelitis outbreak in Xinjiang, China. N Engl J Med. 2013;369(21):1981–90. doi:10.1056/NEJMoa1303368.
- De Jesus NH. Epidemics to eradication: the modern history of poliomyelitis. Virol J. 2007;4(1):70. doi:10.1186/1743-422x-4-70.
- Minor P. Vaccine-Derived poliovirus (VDPV): Impact on poliomyelitis eradication. Vaccine. 2009;27(20):2649–52. doi:10.1016/j.vaccine.2009.02.071.
- Aylward B, Yamada T. The polio endgame. N Engl J Med. 2011;364(24):2273–75. doi:10.1056/NEJMp1104329.
- Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine — Worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(35):934–38. doi:10.15585/mmwr.mm6535a3.
- du Chatelet IP, Merchant AT, Fisher-Hoch S, Luby SP, Plotkin SA, Moatter T, Agboatwalla M, Mc Cormick JB. Serological response and poliovirus excretion following different combined oral and inactivated poliovirus vaccines immunization schedules. Vaccine. 2003;21(15):1710–18. doi:10.1016/s0264-410x(02)00523-6.
- Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25(34):6296–304. doi:10.1016/j.vaccine.2007.06.020.
- Caceres VM, Strebel PM, Sutter RW. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin Infect Dis. 2000;31(1):110–19. doi:10.1086/313926.
- Szenborn L, Tischer A, Pejcz J, Rudkowski Z, Wojcik M. Passive acquired immunity against measles in infants born to naturally infected and vaccinated mothers. Med Sci Monit. 2003;9:CR541–546.
- Moon S-S, Groome MJ, Velasquez DE, Parashar UD, Jones S, Koen A, van Niekerk N, Jiang B, Madhi SA. Prevaccination rotavirus serum IgG and IgA are Associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis. 2016;62(2):157–65. doi:10.1093/cid/civ828.
- World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine. Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and the Gambia. J Infect Dis. 1995;171(5):1097–106. doi:10.1093/infdis/171.5.1097.
- Vesikari T, Baer M, Willems P. Immunogenicity and safety of a second dose of measles-mumps-rubella-varicella vaccine in healthy children aged 5 to 6 years. Pediatr Infect Dis J. 2007;26(2):153–58. doi:10.1097/01.inf.0000250689.09396.21.
- Hu Y, Wang J, Zeng G, Chu K, Jiang D, Zhu F, Ying Z, Chen L, Li C, Zhu F, et al. Immunogenicity and safety of a sabin strain-based inactivated polio vaccine: a Phase 3 clinical trial. J Infect Dis. 2019;220(10):1551–57. doi:10.1093/infdis/jiy736.
- Herve C, Laupeze B, Del Giudice G, Didierlaurent AM, Da Silva FT. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4(1). doi:10.1038/s41541-019-0132-6.
- Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine. 2003;21(24):3389–92. doi:10.1016/s0264-410x(03)00339-6.
- Lindsey B, Jones C, Kampmann B. Bridging the gap: maternal immunisation as a means to reduce neonatal deaths from infectious diseases. Pathog Glob Health. 2012;106(3):137–38. doi:10.1179/204777312x13462106637684.
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–93. doi:10.1016/s0140-6736(08)61591-3.
- Sasaki S, He X-S, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. Plos One. 2008;3(8):e2975. doi:10.1371/journal.pone.0002975.
- Barug D, Pronk I, van Houten MA, Versteegh FGA, Knol MJ, van de Kassteele J, Berbers GAM, Sanders EAM, Rots NY. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19(4):392–401. doi:10.1016/s1473-3099(18)30717-5.
- Voysey M, Kelly DF, Fanshawe TR, Sadarangani M, O’-Brien KL, Perera R, Pollard AJ. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses an individual participant meta-analysis. JAMA Pediatr. 2017;171(7):637–46. doi:10.1001/jamapediatrics.2017.0638.
- Dayan GH, Thorley M, Yamamura Y, Rodriguez N, McLaughlin S, Torres LM, Seda A, Carbia M, Alexander LN, Caceres V, et al. Serologic response to inactivated poliovirus vaccine: a randomized clinical trial comparing 2 vaccination schedules in Puerto Rico. J Infect Dis. 2007;195(1):12–20. doi:10.1086/508427.
- Asturias EJ, Dueger EL, Omer SB, Melville A, Nates SV, Laassri M, Chumakov K, Halsey NA. Randomized trial of inactivated and live polio vaccine schedules in Guatemalan infants. J Infect Dis. 2007;196(5):692–98. doi:10.1086/520546.
- Cramer JP, Jimeno J, Han HH, Lin S, Hartmann K, Borkowski A, Saez-Llorens X. Safety and immunogenicity of experimental stand-alone trivalent, inactivated Sabin-strain polio vaccine formulations in healthy infants: a randomized, observer-blind, controlled phase 1/2 trial. Vaccine. 2020;38(33):5313–23. doi:10.1016/j.vaccine.2020.05.081.
- Tang R, Chu K, Hu Y, Chen L, Zhang M, Liu S, Ma H, Wang J, Zhu F, Hu Y, et al. Effect of maternal antibody on the infant immune response to inactivated poliovirus vaccines made from Sabin strains. Hum Vaccines Immunother. 2019;15(5):1160–66. doi:10.1080/21645515.2019.1572410.
- Andrews NJ, Walker WT, Finn A, Heath PT, Collinson AC, Pollard AJ, Snape MD, Faust SN, Waight PA, Hoschler K, et al. Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and non-adjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. Vaccine. 2011;29(45):7913–19. doi:10.1016/j.vaccine.2011.08.076.
- Grabenstein JD, Manoff SB. Pneumococcal polysaccharide 23-valent vaccine: Long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine. 2012;30(30):4435–44. doi:10.1016/j.vaccine.2012.04.052.
- Kang G, Tang F, Wang Z, Hu R, Yu J, Gao J. Surveillance of adverse events following the introduction of inactivated poliovirus vaccine made from Sabin strains (sIPV) to the Chinese EPI and a comparison with adverse events following inactivated poliovirus vaccine made from wild strains (wIPV) in Jiangsu, China. Hum Vaccines Immunother. 2021;17(8):2568–74. doi:10.1080/21645515.2021.1898306.