ABSTRACT
CD38 is a transmembrane glycoprotein with ectoenzymatic activity and is highly and uniformly expressed on multiple myeloma (MM) cells. CD38 is expressed also at relatively low levels on normal lymphoid and myeloid cells, and in some tissues of non-hematopoietic origin. The specificity of this target has increased interest in new drugs and triggered the development of the CD38 monoclonal antibodies Daratumumab (fully human) and Isatuximab (chimeric). CD38 antibodies have pleiotropic mechanisms of action including Fc-dependent immune effector mechanisms, direct apoptotic activity, and immunomodulatory effects by the elimination of CD38+ immune-suppressor cells. Monoclonal antibody-based therapy has revolutionized MM therapy in the latest years increasing depth of response. This product review will focus on anti-CD38 monoclonal antibodies Daratumumab and Isatuximab efficacy, safety, pharmacokinetic and pharmacodynamic data from clinical trials.
Introduction
Multiple myeloma (MM) represents the second hematological cancer after non-Hodgkin’s lymphomas.Citation1 While the disease is still defined incurable, in the past 20 y, MM patients have experienced great advances in progression-free survival (PFS) and overall survival (OS).Citation2–5 In fact, OS has increased from a median of 3–4 y to a median of 8–9 y.Citation5 The introduction of novel drugs changed completely the disease perspective. These drugs are: i) the immunomodulatory (IMIDs) thalidomide, lenalidomide, and pomalidomide; ii) the proteasome inhibitors (PIs) Bortezomib, Carfilzomib, Ixazomib; iii) the monoclonal antibodies (MoA) Daratumumab, Elotuzumab, Isatuximab, Belantamab. These drugs can be used alone or combined in triplet or quadruplet, leading to overwhelming responses that can reach 90% of the treated patients.Citation4 Also, particular aggressive conditions, such as extramedullary disease, can benefit from these associations.Citation6–8 Strategies such as consolidation therapy and maintenance after autologous stem cell transplantation (ASCT) further improved PFS and OS.Citation8,Citation9 In particular, MoA directed toward CD38 have revolutionized the therapeutic landscape leading to high rates of complete remission and increasing depth of response.Citation10 The concept of minimal residual disease (MRD) has been introduced by the International Myeloma Working Group (IMWG) in the response evaluation.Citation11 Both multiparametric flow cytometry, Next-Generation Flow (NGF), and Next Generation Sequencing (NGS) can be utilized and detect MRD at 10−5 to 10−6 in the bone marrow aspirate. MRD negativity has become a surrogate for survival, and it is widely utilized in clinical trials, while it is still not mandatory in clinical practice.Citation12 The percentage of MRD negativity reached by anti CD38 MoA Daratumumab and Isatuximab has been unprecedented in multiple myeloma. This product review will focus on different aspects of these two drugs.
CD38 structure, expression, and function
The human CD38 antigen is a 46 kilodalton (kDa) type II transmembrane glycoprotein with a short N-terminal cytoplasmic tail and a long extracellular domain.Citation13 CD38 can be internalized and shed andCitation14 it also exists in a 39 kDa soluble form in biological fluids.Citation15 The gene encoding CD38 is on Chromosome 4. It is present in hematopoietic cells (its distribution seems to depend on the activation and differentiation of the cell) and can be expressed also in various tissues.Citation16 It is also expressed on regulatory T cells, regulatory B cells, and myeloid-derived suppressor cells with a high surface expression associated with compromised immune surveillance for malignancies. CD38 is present in most of the circulating T- and B-cells are CD38-, and activated B- and T-cells, it is also present on monocytes, natural killer cells, dendritic cells, and plasma cells.Citation17 Progenitor stem cells can express CD38 but not immature stem cells,Citation18 it is present also on erythrocytesCitation19,Citation20 and on platelets.Citation21 CD38 is a great target for MoA therapy in MM because MM plasma cells express higher levels of CD38 compared with normal cells. MM is not the only disease in which CD38 is expressed, it is also highly expressed in acute leukemia, chronic lymphocytic leukemiaCitation22,Citation23 and many types of lymphomas (mantle cell, follicular, diffuse large B-cell lymphoma).Citation24 Since immune suppressive cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSC) express CD38, they could be targeted by daratumumab.Citation25 Other tissues have a lower level of expression (eye, prostate, kidney, bone, pancreas, Purkinje cells, pituitary, smooth muscle cells).Citation26 CD38 functions as a receptor in signaling events, adhesion, and enzymatic activity,Citation27 in particular adhesion of CD38 is described in differentiating B-cells for interactions. Moreover, CD38 uses nicotinamide adenine dinucleotide (NAD)+ as a substrate for cyclase activity, hydrolase activity, and ectoenzymatic activity.Citation22–32 Through interactions, CD38 may play a part in regulating leukocytes’ cellular motility, adhesion, and modification of the extracellular matrix.
Daratumumab
Daratumumab (Dara) is a human immunoglobulin G1 kappa (IgG1κ) MoA from Janssen that binds to CD38 (first-in-class). Dara is used for MM treatment and is also being developed for lymphomas, leukemias therapy, and systemic AL amyloidosis. Dara can be given by intravenous (IV) infusion or subcutaneously (SC) 5 min compared with 7 h for an IV first infusion, even though short infusion times have been reported.Citation33,Citation34 SC administration can lower the rate of infusion-related reactions (IRRs) as well to reduce health care professional time spent. Dara IV received marketing authorization in November 2015 in the US and is now approved in over 90 countries worldwide for MM treatment. Dara SC is currently approved in the US and EU and is either approved or under review in several other countries worldwide. An indication for use of Dara SC for the treatment of AL amyloidosis is also approved or under review in many countries.
Mechanisms of action
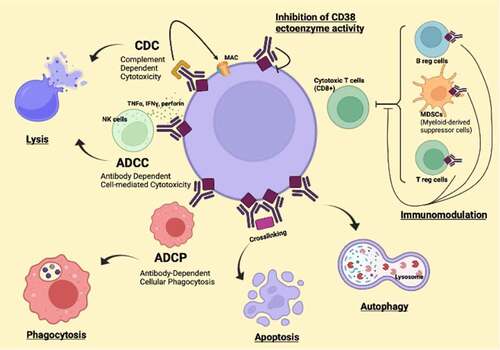
Dara can have different mechanisms of action: complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and direct cytotoxicity by induction of apoptosis by Fc gamma receptor-mediated.Citation35,Citation36 Dara can eliminate highly immunosuppressive subsets of CD38+ Tregs, CD38+ MDSCs, and CD38+ regulatory B-cells (B-regs).Citation25 The main mechanisms of action of anti-CD38 antibodies are described in .
Pharmacokinetics
The pharmacology and mechanism of action of daratumumab were assessed by extensive experiments. The pharmacology experiments focused on inhibition of CD38 enzymatic activity, ADCP, CDC, and ADCC, apoptosis, and reactivity with normal human tissues. Dara reduced tumor growth in mouse xenograft models. When Dara was combined with lenalidomide or bortezomib in MM in-vitro studies, plasma cell killing was demonstrated.Citation37 Pomalidomide or lenalidomide can also increase the activity of anti-CD38 antibodies including Dara by upregulation of CD38.Citation38
Dara’s half-life estimated following the first 16 mg/kg dose was 9 d. Based on population PK analysis, the mean half-life associated with nonspecific linear elimination was approximately 18 d. In participants with MM, Dara SC exposure showed non-inferiority in a monotherapy study using 1,800 mg (weekly for 8 weeks, biweekly for 16 weeks, monthly thereafter) as compared to 16 mg/kg Dara IV for the same dosing schedule.
Efficacy
shows the main Dara efficacy studies as monotherapy and combination therapy.Citation39–53 Dara IV clinical benefit was demonstrated in a phase 1 study of Dara monotherapy in relapsed/refractory MM patients (Study GEN501) and confirmed in a phase 2 study (Study MMY2002). Data from phase 3 studies showed Dara IV efficacy when used in combination with chemoimmunotherapy in participants with relapsed/refractory MM (RRMM) (in combination with lenalidomide and dexamethasone [Rd] [Study MMY3003], bortezomib-dexamethasone [Vd] [Study MMY3004]), pomalidomide and dexamethasone [DPd cohort of Study MMY1001], carfilzomib and dexamethasone [20160275 CANDOR study and DKd cohort of MMY1001], Dara, carfilzomib, lenalidomide, and dexamethasone [DKRd cohort of study MMY1001]), and in participants with newly diagnosed MM who were ineligible for high-dose therapy (in combination with bortezomib-melphalan-prednisone [VMP] [Study MMY3007]) and Rd [Study MMY3008], or eligible for autologous stem cell transplant (in combination with bortezomib, thalidomide, and dexamethasone [VTd] [MMY3006]). The clinical benefit of Dara SC has been demonstrated as monotherapy (MMY3012) and in participants with relapsed/refractory MM (in combination with Rd and DKd [MMY2040] and Pd [MMY3013]) as well as in participants with newly diagnosed MM (in combination with VMP and bortezomib, lenalidomide, and dexamethasone [VRd] [MMY2040]). The clinical benefit of Dara IV and SC monotherapy and combination therapy has also been demonstrated in AL amyloidosis (AMY3001) and high-risk SMM (SMM2001-CENTAURUS).
Table 1. Selected trials of daratumumab in multiple myeloma
Safety
Among the 156 MM patients treated with 16 mg/kg Dara as monotherapy in studies MMY2002, GEN501, MMY1002, the most frequently reported adverse events (TEAE) were fatigue (40%), nausea, anemia (28% each), back pain (26%), cough (24%), neutropenia (23%), pyrexia (22%), upper respiratory tract infection (22%), and thrombocytopenia (21%). Grade 3 or 4 TEAE were reported in 57% of participants treated with 16 mg/kg. Among the 156 participants, 41.7% experienced grade 3 and 15.4% grade 4 events. In the GEN503 and MMY3003, a total of 318 participants have been treated with Dara in combination with Rd (DRd) as the primary analysis. A total of 281 MM patients in Study MMY3003 received Rd alone. The most frequently reported TEAE reported in participants receiving DRd treatment were neutropenia (66%) and diarrhea (57%). Also upper respiratory tract infection (40%), anemia, fatigue (38% each), cough (36%), nasopharyngitis (33%), muscle spasms (32%), constipation (31%), thrombocytopenia (30%), nausea (29%), pyrexia, insomnia (26% each). Grade 3/4 TEAE was reported in 90% of patients who received DRd treatment. The most frequently reported were neutropenia (58%), anemia (17%), and thrombocytopenia (15%). TEAE leading to discontinuation were reported for 13% of participants in the DRd treatment group (pneumonia, 5 participants, 2%), septic shock, anemia, thrombocytopenia, and hypercalcemia (2 participants, 1% each).
A total of 226 (95.4%) MM patients in the Vd group and 240 (98.8%) participants in the DVd group had at least 1 or more TEAE (at least 10%). The most common TEAE included diarrhea, anemia, thrombocytopenia, peripheral sensory neuropathy, and upper respiratory tract infection. A total of 461 participants were treated in the CANDOR study. The safety profiles observed in the DKd (N = 308) and Kd (N = 153) arms were generally consistent with the known profiles of the study treatments administered. The most frequently reported (20% of participants in either treatment group) adverse events included thrombocytopenia (37.3%, 29.4%), anemia (32.8%, 31.4%), diarrhea (31.5%, 14.4%), hypertension (30.5%, 27.5%), upper respiratory tract infection (29.2%, 22.9%), fatigue (24.4%, 18.3%), and dyspnea (19.8%, 22.2%).
In the CASSIOPEIA study, the most common (at least 10%) Grade 3 or 4 TEAE during induction/ASCT/consolidation phase were neutropenia and stomatitis in both groups. The overall incidence of serious TEAE was similar for both groups (D-VTd: 46.8%; VTd: 47.4%). The most common (≥3%) TEAE reported were neutropenia (D-VTd: 3.9%; VTd: 1.5%), pneumonia (D-VTd: 3.5%; VTd: 1.7%), pyrexia (D-VTd: 2.8%; VTd: 4.3%), and pulmonary embolism (D-VTd: 1.5%; VTd: 3.7%). IRRs were presented with Dara IV. In particular, the majority of IRRs occurred at the first infusion and were Grade 1 to 2, and 4% of patients had an IRR at more than one infusion.Citation54 Severe reactions have occurred, including dyspnea, hypoxia, bronchospasm, hypertension, laryngeal edema, pulmonary edema, vomiting, nausea, nasal congestion, cough, and chills. Less common symptoms were allergic rhinitis and pyrexia. Fatal IRRs were not reported. Pre-medication of patients with antihistamines, antipyretics, and corticosteroids can reduce IRRs before treatment with Dara.
IRRs with Dara SC were less present. In clinical trials, approximately 9% (74/832) of patients experienced IRRs and occurred following the first injection (all Grade 1–2). IRRs occurring with subsequent injections were seen in <1% of patients. The median time to onset of IRRs following Dara was 3 h (range 15–83 h). The majority of IRRs occurred on the day of treatment. Delayed IRRs have occurred in <1% of patients. Signs and symptoms of IRRs may include respiratory symptoms, such as nasal congestion, chills, vomiting, nausea, cough, pruritis, allergic rhinitis, pyrexia, and chest pain. Severe reactions have occurred: bronchospasm, dyspnea, hypoxia, hypertension.
Isatuximab
Isatuximab is a chimeric humanized IgG1 monoclonal antibody from Sanofi that binds to a specific epitope on the human cell surface antigen CD38. Isatuximab is approved for the treatment of RRMM, and it can be given by intravenous infusion.
Mechanisms of action
Isatuximab mechanisms of action are Fc dependent and Fc independent. The Fc-dependent mechanisms induced by Isatuximab include ADCC, ADCP, and CDC.Citation55 ADCC is the dominant mechanism and it is mediated by activating Fcγ receptors on the surface of natural killer cells binding to the Fc regions of the antibodies.Citation56 While ADCC was seen with both CD38 high and low expression in MM cells, ADCP was seen only with CD38 high expression in MM cells, and CDC was seen in less than half of patient samples. Also, the programmed cell death 1/programmed cell death-ligand 1 (PD-1/PD-L1) pathway and MM cell-secreted transforming growth factor-beta (TGF-β) are tumor cell-related features that could suppress CD38-mediated ADCC. Isatuximab can directly activate NK cells and promote NK cell-mediated cytotoxicity via crosslinking of CD38 and CD16. Finally, Isatuximab-induced CDC was observed in cell lines with high CD38 receptor density (>250,000 molecules/cell) and limited expression of inhibitory complement regulatory proteins (CD46, CD55, and CD59; <50,000 molecules/cell).Citation57 In terms of Fc-independent activities, Isatuximab induces direct cytotoxicity against MM cells in vitro via caspase-dependent apoptosis and lysosome-mediated non-apoptotic cell killing.Citation58 Isatuximab has also demonstrated immunomodulatory effects in vitro that may contribute indirectly to the control of tumor growth in MM. Some of the demonstrated effects are MM cell lysis by NK cells direct activation or by CD8+ cells and suppression of CD38+ regulatory T cells. Isatuximab can mitigate the immunosuppressive tumor microenvironment by reducing inhibition of NK and CD8+ cells.Citation55,Citation56,Citation59
Pharmacokinetics
Isatuximab demonstrates non-linear pharmacokinetics with target-mediated drug disposition due to its binding to the CD38 receptor. After intravenous (IV) administration of Isatuximab at the approved dosage (10 mg/kg weekly for 4 weeks and then every 2 weeks), the median time to reach a steady-state is 8 weeks. Linear clearance of the drug decreases over time by 50% to a steady-state value of 9.55 mL/h (.229 L/d). The terminal half-life of Isatuximab is 28 d. At a steady state, ≥99% elimination of the drug from plasma was predicted to occur ≈2 months after the last dose. Based on a population pharmacokinetic analysis, the renal or hepatic impairment does not have a clinically meaningful effect on the pharmacokinetics of Isatuximab and does not require any dose adjustment.Citation60,Citation61
Efficacy
Main Isatuximab trials are described in . Isatuximab has been first-in human evaluated in a phase I multicenter, open-label, dose-escalation study as single-agent in RRMM.Citation62 The study enrolled 84 patients. Isatuximab was administered intravenously every 2 weeks (Q2W) or QW, in 2-week cycles. The primary objective was to determine the maximum tolerated dose (MTD), and secondary objectives were evaluation of safety/tolerability, pharmacokinetics/pharmacodynamics, and preliminary efficacy. The MTD was not reached; no cumulative adverse reactions were noted. In patients receiving Isatuximab ≥10 mg/kg, the overall response rate (ORR) was 23.8%. In high-risk patients, the ORR was 16.7%. The median (range) duration of response at doses ≥10 mg/kg was 25 (range 8–30) weeks among high-risk patients versus 36 (range 6–85) weeks for other patients. A subsequent phase I-b trial (NCT02283775) evaluated Isatuximab plus pomalidomide and dexamethasone in patients with RRMM. Patients enrolled had received ≥2 prior MM therapies, including lenalidomide and a proteasome inhibitor (PI). They received Isatuximab at 5, 10, or 20 mg/kg (weekly for 4 weeks, followed by every 2 weeks), pomalidomide 4 mg (days 1–21), and dexamethasone 40 mg (weekly) in 28-dy cycles until progression/intolerable toxicity. The primary objective was to determine the safety and recommended dose of Isatuximab with this combination, 45 patients were enrolled. Most of the patients were refractory to their prior regimen (91%), with 82% lenalidomide-refractory and 84% PI-refractory. ORR was 62%; median duration of response (DOR) was 18.7 months; median PFS was 17.6 months. Based on this trial, the dose of 10 mg/kg weekly/every 2 weeks Isatuximab was selected for future studies.Citation63 To confirm these data and reduce the infusion duration, we designed another phase I-b trial (NCT02283775) to evaluate the efficacy and safety of a fixed-volume infusion of Isatuximab plus pomalidomide and dexamethasone (Pd) in RRMM, who had received ≥2 prior lines of therapy. This trial was composed of two parts: part A was an Isatuximab dose-escalation study, while in the part B, Isatuximab (10 mg/kg) was administered as a fixed infusion volume of 250 mL with standard doses of Pd. The primary endpoint for Part B was the incidence of Grade (Gr) ≥3 infusion reactions (IRs) during the first six Isatuximab infusions.Citation64 Forty-seven patients were enrolled. The median duration of exposure was 36.9 weeks. The ORR in all subjects on Part B was 53.2% and 62.2% in Part A, with similar VGPR and CR rates (23.4% and 4.3% in Part B versus 22.2% and 2.2% in Part A, respectively).Citation64 In another phase Ib study, Isatuximab was combined with Carfilzomib (K) and dexamethasone in 33 RRMM.Citation65 Patients were previously treated with a median of three lines. With a median follow-up of 26.7 months, the ORR was 70%. The median PFS was 10.1 months.Citation65 Isatuximab was combined with lenalidomide and dexamethasone in another phase I b study. The trial enrolled 57 RRMM patients. Isatuximab-lenalidomide-dexamethasone was generally well tolerated, and the MTD was not reached. In the efficacy evaluable population (52/57), the ORR was 56%. Overall median PFS was 8.5 months.Citation66 Isatuximab showed efficacy and tolerability as a monotherapy and combination therapy in Phase I/II studies in RRMM. The Phase III ICARIA-MM study (NCT02990338) was a randomized, multicentre, open-label trial, which evaluated Isatuximab in combination with pomalidomide and low-dose dexamethasone (IsaPd) versus Pd alone in RRMM. Eligible participants were RRMM that had received at least two previous lines of treatment, including lenalidomide and a PI. A total of 307 patients were randomly assigned to treatment: 154 to IsaPd and 153 to Pd treatment. At a median follow-up of 11.6 months, the median PFS was 11.5 months in the IsaPd group versus 6.5 months in the Pd group, p = .001.Citation67
Table 2. Selected trials of isatuximab in multiple myeloma
The investigators conclude that the addition of Isatuximab to Pd significantly improves PFS in patients with RRMM, with acceptable toxicity in heavily pre-treated and frail patients.Citation68 In a subgroup analysis of the ICARIA study, efficacy was determined in patients with renal impairment (RI; estimated glomerular filtration rate <60 mL/min/1.73 m2). Isatuximab and pomalidomide were given with the same schedule. Median PFS for patients with RI was 9.5 months with IsaPd and 3.7 months with Pd. The ORR with and without RI was higher with IsaPd (56% and 68%) than Pd (25% and 43%). A complete renal response rate of 71.9% was observed with IsaPd and 38.1% with Pd. Isatuximab pharmacokinetics were comparable between the subgroups, suggesting no need for dose adjustment in patients with RI.Citation68
The IKEMA phase III trial (NCT03275285) evaluated the combination of Isatuximab, Carfilzomib, and Dexamethasone (IsaKd) Vs Carfilzomib dexamethasone (Kd) in RRMM. The primary endpoint was the PFS. A total of 302 patients have been randomized. Patients in the Isatuximab group received Isatuximab 10 mg/kg IV (days 1, 8, 15, and 22 in the first 28-day cycle; days 1 and 15 in subsequent cycles). In both groups, Carfilzomib was administered IV at 20 mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 on days 8, 9, 15, and 16 of cycle 1; and then 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 of subsequent cycles. The median number of previous lines of therapy was 2, and data were similar between groups and 45% of patients were refractory to immunomodulatory drugs. After a median follow-up of 2 y, the ORR was 87% in the experimental arm and 83% in the control group, without statistically significant difference. Even there was not a difference in ORR, the responses were better in IsaKd group, VGPR or better was reported in 73% of patients versus 56% in the control group (p = .0011). CR occurred in 40% versus 28% of patients. The MRD negativity rate was more than double in the IsaKd group than in the control group: 30% versus 13% p = .0004. Progression-free survival 2 and OS were not mature. Median time to first response in responders was similar in both groups: 32 d (IQR 30–40) in the IsaKd and 33 d (30–58) in the control group. Duration of response and time to next treatment was longer in the Isatuximab group than in the control group.
At a median follow-up of 20.7 months, the addition of Isatuximab to Kd showed a significant improvement in PFS with an HR of .53 (99% CI .32–.89, p = .0007). At 2 y, the estimated PFS was 68·9% (95% CI 60.7–75.8) in the IsaKd group versus 45.7% (35.2–55.6) in the control group.Citation69
Safety
Isatuximab in monotherapy and combination with other drugs was generally well tolerated. The most frequent adverse events were IRRs that occurred in 51% of enrolled patients. IRRs were mostly grade 1/2, occurred predominantly during Cycle 1, and led to treatment discontinuation in two patients.Citation62 After the introduction of mandatory prophylactic treatment, 36/73 patients (49.3%) experienced IRRs. The IRRs mostly resolved in 1 d, either spontaneously or with few medications. The most common symptoms (≥5%) reported during IRRs were chills, dyspnea, nausea, headache, chest discomfort, and pyrexia, all of which were grade 1/2 in intensity. Except for IRRs, the most common (>10%) TEAE of any grade, were fatigue, nausea, upper respiratory tract infection, and cough. Among hematological toxicity, the most frequent grade 3/4 TEAEs were lymphopenia, anemia, thrombocytopenia, and neutropenia, the incidence, and severity of these events did not appear to be dose-dependent.Citation62 These data were confirmed with the safety profile shown in phase III trials, ICARIA and IKEMA. The incidence of TEAEs was similar in the ICARIA study, both for patients enrolled in the experimental arm and the control group (respectively 99% vs 98%).Citation67 Incidence of TEAEs was consistent with the data from IKEMA, where the incidence of any grade TEAEs in the IsaKd arm was 97% while in Kd was 96%.Citation69 The two Isatuximab-based triplets showed similar side effects, the most frequent non-hematological TEAEs of any grade in the Isatuximab combination therapy recipients included IRRs, respiratory infections (upper respiratory tract infection, pneumonia, and bronchitis), and dyspnea.Citation67,Citation69 Most of the Isatuximab recipients enrolled in ICARIA and IKEMA study experienced IRRs, they mainly occurred in the first infusion of the first cycle, with a manageable profile, most of them were grade 1–2 and resolved in the same infusion day. Anaphylactic reactions occurred in <1% of the patients. IRRs led to the discontinuation of Isatuximab in <1% of patients in IKEMA,Citation69 similar to the ICARIA study. Other significant non-hematological TEAEs were upper respiratory tract infections, 36% in the IKEMA trail and 28% in the ICARIA, most of them were grade 1–2, only 3–4% were TEAEs of grade 3–4.Citation67,Citation69 In both phase III trials, a minimal increased risk of pneumonia was observed in the Isatuximab group compared to the control group. About 20% of patients enrolled in Isatuximab group in the ICARIA study developed pneumonia vs 17% in the control group, similar data were seen in the IKEMA study (29% versus 23%).Citation67,Citation69 In the IKEMA study, an increased rate of hypertension was observed in both groups, 37% in the experimental arm and 31% in the control group. Hematological AEs in the IKEMA were in the Isatuximab group: anemia in 99% of patients (grade 3 or more 22%), neutropenia in 55% (19% grade 3–4), thrombocytopenia 94% (30% grade 3–4), data were almost similar in the control group.Citation69 In the ICARIA study, the main difference in hematological toxicities was the incidence in both groups of neutropenia, reported in 96% of patients in Isatuximab group and 93% in the control group, 85% and 70% were grade 3–4, respectively.Citation67
New anti CD38 monoclonal antibodies
MOR202 is a human IgG1λ CD38 antibody derived from a phage library (MorphoSys AG, Planegg, Germany) and has demonstrated high efficacy in-vitro and in-vivo in preclinical studies.Citation70,Citation71 MOR202 has anti-myeloma activity via ADCC and ADCP but does not have CDC. In a recent multicenter phase 1–2 study,Citation71 In this study, 91 refractory/relapsed MM patients with a median of 3 previous lines of therapy were treated (35 with MOR202 monotherapy, 56 with combinations with dexamethasone (N = 18), with pomalidomide and dexamethasone (N = 21), with lenalidomide and dexamethasone (N = 17)). Different treatment schedules were used for MOR202 given IV at doses between .001 mg/kg and 16 mg/kg in a 3 + 3 design. The recommended dose was 16 mg/kg in 30-min infusions. IRRs were more present in MOR202 monotherapy without steroids (14/35, 40%) than in combination therapy (4/56, 7%).
The more common TEAE were lymphopenia grade 3 or higher in 35/91 (38%) patients and neutropenia in 30 (33%). Serious adverse events (SAE, defined as events requiring hospitalization or life-threatening, including events resulting in death) occurred in 51/91 (56%) patients (7 in the group as monotherapy, 4 in the group combined with dexamethasone, 13 in the group combined with pomalidomide dexamethasone, 17 in the lenalidomide dexamethasone). SAE were mostly due to IRRs (hypersensitivity, tachycardia, pyrexia, 18/91, 20%) and pneumonia (4/91,4%). Regarding efficacy, in the monotherapy or dexamethasone group, 9/35 (26%) had stable disease, while an ORR was seen in 26/56 (46%) in the combination group (4 CR, 5 VGPR, 12 PR, 5 SD). The median duration of response was 16.7 months.
TAK-079 is a fully human, non-agonistic, IgG1 from Takeda, a cell-depleting monoclonal antibody that binds human CD38 with high affinity. It has been reported to act with ADCC, ADCP, and CDC. Recent data on TAK-079 showed an ORR of 33% at the dose of 600 mg sc in relapsed/refractory MM patients with a median of 4 prior lines of therapy. TAK −079 seems to have the advantage of the sc route of administration and a promising safety profile (no IRRs, no significant hematologic toxicity).Citation72
Conclusion
Anti-CD38 monoclonal antibodies represent the first immunotherapeutic approach in multiple myeloma. These new drugs have increased the therapeutic choice for clinicians. The safety profile is good, and efficacy is unprecedented when these drugs are combined with other agents such as proteasome inhibitors and immunomodulatory drugs. Future perspectives should focus on patient selection and timing of therapy, together with the understanding of mechanisms of resistance with the intent to prolong efficacy and survival.
Acknowledgements
We thank all patients and nurses at Hematology Siena.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–9. doi:10.3322/caac.21654. PMID 33433946
- Gozzetti A, Le Beau MM. Fluorescence in situ hybridization: uses and limitations. Semin Hematol. 2000;37(4):320–33. doi:10.1016/s0037-1963(00)90013-1. PMID:11071355
- Gozzetti A, Candi V, Papini G, Bocchia M. Therapeutic advancements in multiple myeloma. Front Oncol. 2014;4:241. doi:10.3389/fonc.2014.00241. PMID: 25237651
- Ocio EM, Richardson PG, Rajkumar SV, Palumbo A, Mateos MV, Orlowski R, Kumar S, Usmani S, Roodman D, Niesvizky R, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia. 2014;28(3):525–42. doi:10.1038/leu.2013.350. PMID: 24253022
- Mohty M, Terpos E, Mateos MV, Cavo M, Lejniece S, Beksac M, Bekadja MA, Legiec W, Dimopoulos M, Stankovic S, et al. EMMOS investigators. Multiple myeloma treatment in real-world clinical practice: results of a prospective, multinational, noninterventional study. Clin Lymphoma Myeloma Leuk. 2018;18(10):e401–e419. doi:10.1016/j.clml.2018.06.018. PMID: 30030033
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20. doi:10.1182/blood-2007-10-116129. PMID: 17975015
- Castillo JJ, Jurczyszyn A, Brozova L, Crusoe E, Czepiel J, Davila J, Dispenzieri A, Eveillard M, Fiala MA, Ghobrial IM, et al. IgM myeloma: a multicenter retrospective study of 134 patients. Am J Hematol. 2017;92(8):746–51. doi:10.1002/ajh.24753. PMID: 28383205
- Gozzetti A, Cerase A. Novel agents in CNS myeloma treatment. Cent Nerv Syst Agents Med Chem. 2014;14(1):23–27. doi:10.2174/1871524914999140818111514. PMID: 25134940
- Gozzetti A, Cerase A, Lotti F, Rossi D, Palumbo A, Petrucci MT, Patriarca F, Nozzoli C, Cavo M, Offidani M, et al. GIMEMA (Gruppo Italiano Malattie Ematologiche dell’adulto) myeloma working party. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012 Mar 15; 118(6):1574–84. 10.1002/cncr.26447. Epub 2011 Aug 25. PMID: 21932386
- Kocoglu MH, Badros AZ. Newly diagnosed multiple myeloma: current treatment strategies, emerging therapeutic approaches and beyond. Expert Rev Hematol. 2020 ;13(6):669–86. doi:10.1080/17474086.2020.1756258. PMID: 32290719.
- Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016 ;17(8):e328–e346. doi:10.1016/S1470-2045(16)30206-6. PMID: 27511158
- Gozzetti A, Raspadori D, Bacchiarri F, Sicuranza A, Pacelli P, Ferrigno I, Tocci D, Bocchia M. Minimal residual disease in multiple myeloma: state of the art and applications in clinical practice. J Pers Med. 2020 Sep 10;10(3):120. 10.3390/jpm10030120. PMID: 32927719; PMCID: PMC7565263
- Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human CD38: a glycoprotein in search of a function. Immunol Today. 1994 ;15(3):95–97. doi:10.1016/0167-5699(94)90148-1. PMID: 8172650.
- Funaro A, Reinis M, Trubiani O, Santi S, Di Primio R, Malavasi F. CD38 functions are regulated through an internalization step. J Immunol. 1998 Mar 1;160(5):2238–47. PMID: 9498763
- Funaro A, Horenstein AL, Calosso L, Morra M, Tarocco RP, Franco L, De Flora A, Malavasi F. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int Immunol. 1996 ;8(11):1643–50. doi:10.1093/intimm/8.11.1643. PMID: 8943558.
- Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk Res. 2001;25(1):1–12. doi:10.1016/S0145-2126(00)00093-X.
- Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194(1):48–60. doi:10.1034/j.1600-065X.2003.00041.x.
- Prosper F, Stroncek D, Verfaillie CM. Phenotypic and functional characterization of long-term culture-initiating cells present in peripheral blood progenitor collections of normal donors treated with granulocyte colony-stimulatingfactor. Blood. 1996;88(6):2033–42. doi:10.1182/blood.V88.6.2033.bloodjournal8862033.
- Lee HC, Zocchi E, Guida L, Franco L, Benatti U, De Flora A. Production and hydrolysis of cyclic ADP-ribose at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993 Mar 15;191(2):639–45. 10.1006/bbrc.1993.1265. PMID: 8461019
- Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee HC, De Flora A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1459–65. 10.1006/bbrc.1993.2416. PMID: 8250903
- Ramaschi G, Torti M, Festetics ET, Sinigaglia F, Malavasi F, Balduini C. Expression of cyclic ADP-ribose-synthetizing CD38 molecule on human platelet membrane. Blood. 1996 Mar 15;87(6):2308–13. PMID: 8630392. 10.1182/blood.V87.6.2308.bloodjournal8762308
- Xu Y, McKenna RW, Asplund SL, Kroft SH. Comparison of immunophenotypes of small B-cell neoplasms in primary lymph node and concurrent blood or marrow samples. Am J Clin Pathol. 2002 ;118(5):758–64. doi:10.1309/11J6-0U42-VF4E-WA02. PMID: 12428797.
- Keyhani A, Huh YO, Jendiroba D, Pagliaro L, Cortez J, Pierce S, Pearlman M, Estey E, Kantarjian H, Freireich EJ. Increased CD38 expression is associated with favorable prognosis in adult acute leukemia. Leuk Res. 2000 ;24(2):153–59. doi:10.1016/s0145-2126(99)00147-2. PMID: 10654451.
- Rodig SJ, Vergilio JA, Shahsafaei A, Dorfman DM. Characteristic expression patterns of TCL1, CD38, and CD44 identify aggressive lymphomas harboring a MYC translocation. Am J Surg Pathol. 2008 ;32(1):113–22. doi:10.1097/PAS.0b013e3180959e09. PMID: 18162778.
- Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016 Jul 21; 128(3):384–94. 10.1182/blood-2015-12-687749. Epub 2016 May 24. PMID: 27222480
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/cd38 gene family in physiology and pathology. Physiol Rev. 2008 ;88(3):841–86. doi:10.1152/physrev.00035.2007.
- Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004 ;121(4):482–88. doi:10.1309/74R4-TB90-BUWH-27JX.
- Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12(11–12):317–23. doi:10.2119/2006-00086.Lee.
- Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999 Sep 15;94(6):1840–47. PMID: 10477712. 10.1182/blood.V94.6.1840
- Chini EN, Chini CC, Kato I, Takasawa S, Okamoto H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem J. 2002 Feb 15;362(Pt 1):125–30. 10.1042/0264-6021:3620125. PMID: 11829748
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993 Nov 12;262(5136):1056–59. 10.1126/science.8235624. PMID: 8235624
- Takasawa S, Tohgo A, Noguchi N, Koguma T, Nata K, Sugimoto T, Yonekura H, Okamoto H. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J Biol Chem. 1993 Dec 15;268(35):26052–54.
- Barr H, Dempsey J, Waller A, Huang Y, Williams N, Sharma N, Benson DM, Rosko AE, Efebera YA, Hofmeister CC Ninety-Minute daratumumab infusion is safe in multiple myeloma. Leukemia. 2018;32(11):2495–518. doi:10.1038/s41375-018-0120-2. Epub 2018 Mar 31. PMID: 29679038.
- Gozzetti A, Bacchiarri F, Sammartano V, Defina M, Sicuranza A, Mecacci B, Zappone E, Cencini E, Fabbri A, Raspadori D, et al. Long-term safety of rapid Daratumumab infusions in multiple myeloma patients. Front Oncol. 2020;10:570187. doi:10.3389/fonc.2020.570187. PMID: 33415072.
- Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, et al. Antibody-Mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. Mabs. 2015;7(2):311–21. doi:10.1080/19420862.2015.1007813. PMID: 25760767.
- Overdijk MB, Jansen JH, Nederend M, van Bueren JJ L, Groen RW, Parren PW, Leusen JH, Boross P. The therapeutic CD38 monoclonal antibody Daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016 Aug 1;197(3):807–13. 10.4049/jimmunol.1501351. Epub 2016 Jun 17. PMID: 27316683
- van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, Lokhorst HM, Mutis T The therapeutic human CD38 antibody daratumumab improves the anti-myeloma effect of newly emerging multi-drug therapies. Blood Cancer J. 2011 ;1(10):e41. doi:10.1038/bcj.2011.42. Epub 2011 Oct 28. PMID: 22829073.
- Busch L, Mougiakakos D, Büttner-Herold M, Müller MJ, Volmer DA, Bach C, Fabri M, Bittenbring JT, Neumann F, Boxhammer R, et al. Lenalidomide enhances MOR202-dependent macrophage-mediated effector functions via the vitamin D pathway. Leukemia. 2018 ;32(11):2445–58. 10.1038/s41375-018-0114-0. Epub 2018 Mar 28. PMID: 29654274
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, et al. Targeting CD38 with Daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015 Sep 24;373(13):1207–19. 10.1056/NEJMoa1506348. Epub 2015 Aug 26. PMID: 26308596
- Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, Lokhorst HM, Voorhees PM, Richardson PG, Chari A, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016 Jul 7;128(1):37–44. 10.1182/blood-2016-03-705210. Epub 2016 May 23. PMID: 27216216; PMCID: PMC4937359
- Usmani SZ, Nahi H, Plesner T, Weiss BM, Bahlis NJ, Belch A, Voorhees PM, Laubach JP, van de Donk NWCJ, Ahmadi T, et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020 ;7(6):e447–e455. 10.1016/S2352-3026(20)30081-8. PMID: 32470437
- Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, et al. POLLUX investigators. Daratumumab, Lenalidomide, and Dexamethasone for multiple myeloma. N Engl J Med. 2016 Oct 6;375(14):1319–31. 10.1056/NEJMoa1607751. PMID: 27705267
- Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, et al. CASTOR investigators. Daratumumab, Bortezomib, and Dexamethasone for multiple myeloma. N Engl J Med. 2016 Aug 25;375(8):754–66. 10.1056/NEJMoa1606038. PMID: 27557302
- Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, Weiss BM, Krishnan A, Lentzsch S, Comenzo R, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017 Aug 24;130(8):974–81. 10.1182/blood-2017-05-785246. Epub 2017 Jun 21. PMID: 28637662; PMCID: PMC5570682
- Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Yang H, Klippel Z, Zahlten-Kumeli A, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020 ;396(10245):186–97. doi:10.1016/S0140-6736(20)30734-0. Erratum in: Lancet. 2020 Aug 15;396(10249):466. PMID: 32682484
- Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, Béné MC, Broijl A, Caillon H, Caillot D, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10192):29–38. doi:10.1016/S0140-6736(19)31240-1. Epub 2019 Jun 3. Erratum in: Lancet. 2019 Jun 14; PMID: 31171419
- Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020 Jan 11;395(10218):132–41. 10.1016/S0140-6736(19)32956-3. Epub 2019 Dec 10. PMID: 31836199
- Mateos MV, Nahi H, Legiec W, Grosicki S, Vorobyev V, Spicka I, Hungria V, Korenkova S, Bahlis N, Flogegard M, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020 ;7(5):e370–e380. 10.1016/S2352-3026(20)30070-3. Epub 2020 Mar 23
- Dimopoulos MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, Moreau P, Baldini L, Symeonidis A, Bila J, et al. APOLLO trial investigators. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021 ;22(6):801–12. 10.1016/S1470-2045(21)00128-5. PMID: 34087126
- Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, et al. MAIA trial investigators. Daratumumab plus Lenalidomide and Dexamethasone for untreated myeloma. N Engl J Med. 2019 May 30;380(22):2104–15. 10.1056/NEJMoa1817249. PMID: 31141632
- Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, Chari A, Silbermann R, Costa LJ, Ld A Jr, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020 Aug 20;136(8):936–45. 10.1182/blood.2020005288. PMID: 32325490
- Mateos MV, Kumar S, Dimopoulos MA, González-Calle V, Kastritis E, Hajek R, De Larrea CF, Morgan GJ, Merlini G, Goldschmidt H, et al. International myeloma working group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020 Oct 16;10(10):102. 10.1038/s41408-020-00366-3. PMID: 33067414; PMCID: PMC7567803
- Landgren CO, Chari A, Cohen YC, Spencer A, Voorhees P, Estell JA, Sandhu I, Jenner MW, Williams C, Cavo M, et al. Daratumumab monotherapy for patients with intermediate-risk or high-risk smoldering multiple myeloma: a randomized, open-label, multicenter, phase 2 study (CENTAURUS). Leukemia. 2020 ;34(7):1840–52. 10.1038/s41375-020-0718-z. Epub 2020 Feb 5. PMID: 32024950; PMCID: PMC7326703
- Janssen. DARZALEX® (daratumumab) injection, for intravenous use [package insert]; [ accessed 2021 Nov] https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX-pi.pdf
- Zhu C, Song Z, Wang A, Srinivasan S, Yang G, Greco R, Theilhaber J, Shehu E, Wu L, Yang ZY, et al. Isatuximab acts through Fc-dependent, independent, and direct pathways to kill multiple myeloma cells. Front Immunol. 2020 Aug 14;11:1771. 10.3389/fimmu.2020.01771. PMID: 32922390; PMCID: PMC7457083
- Feng X, Zhang L, Acharya C, An G, Wen K, Qiu L, Munshi NC, Tai YT, Anderson KC. Targeting CD38 suppresses induction and function of T Regulatory cells to mitigate immunosuppression in multiple myeloma. Clin Cancer Res. 2017 Aug 1;23(15):4290–300. 10.1158/1078-0432.CCR-16-3192. Epub 2017 Mar 1. PMID: 28249894; PMCID: PMC5540790
- Song Z, Yang G, Wang A, Greco R, Theilhaber J, Shehu E, Ajona D, Paiva B, Zhu C, Wiederschain D et al. Isatuximab-Induced multiple myeloma cell killing through effector functions is dependent on CD38 expression and complement inhibitors. Cancer Res. 2019;79:13 Supplement.
- Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, Dasilva N, Song Z, Yang G, Adrian F, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016 ;30(2):399–408. 10.1038/leu.2015.240. Epub 2015 Sep 4. PMID: 26338273
- Tai YT, Anderson KC. Targeting CD38 alleviates tumor-induced immunosuppression. Oncotarget. 2017 Dec 26;8(68):112166–67. 10.18632/oncotarget.22992. PMID: 29348814; PMCID: PMC5762499
- SanofI-Aventis. SARCLISA (isatuximab): EU summary of product characteristics; 2021 [accessed 2021 Apr 22]. https://www.ema.europa.eu
- SanofI-Aventis. SARCLISA® (isatuximab-irfc): US prescribing information; 2021 [accessed 2021 Apr 22]. https://www.fda.gov/drugs
- Martin T, Strickland S, Glenn M, Charpentier E, Guillemin H, Hsu K, Mikhael J. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 2019 Mar 29;9(4):41. 10.1038/s41408-019-0198-4. PMID: 30926770; PMCID: PMC6440961
- Mikhael J, Richardson P, Usmani SZ, Raje N, Bensinger W, Karanes C, Campana F, Kanagavel D, Dubin F, Liu Q, et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood. 2019 Jul 11;134(2):123–33. 10.1182/blood-2019-02-895193. Epub 2019 Mar 12. PMID: 30862646; PMCID: PMC6659612
- Usmani SZ, Karanes C, Bensinger WI, D’-Souza A, Raje N, Tuchman SA, Sborov D, Laubach JP, Bianchi G, Kanagavel D, et al. Final results of a phase 1b study of isatuximab short-duration fixed-volume infusion combination therapy for relapsed/refractory multiple myeloma. Leukemia. 2021 May 28. 10.1038/s41375-021-01262-w. Epub ahead of print. PMID: 34050260
- Martin TG, Shah N, Richter J, Vesole DH, Wong SW, Huang CY, Madduri D, Jagannath S, Siegel DS, Biran N, et al. Phase 1b trial of isatuximab, an anti-CD38 monoclonal antibody, in combination with carfilzomib as treatment of relapsed/refractory multiple myeloma. Cancer. 2021 Jun 1;127(11):1816–26. 10.1002/cncr.33448. Epub 2021 Mar 18. PMID: 33735504; PMCID: PMC8252002
- Martin T, Baz R, Benson DM, Lendvai N, Wolf J, Munster P, Lesokhin AM, Wack C, Charpentier E, Campana F, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017 Jun 22;129(25):3294–303. 10.1182/blood-2016-09-740787. Epub 2017 May 8. PMID: 28483761; PMCID: PMC5482100
- Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, Leleu X, Schjesvold F, Moreau P, Dimopoulos MA, et al; ICARIA-MM study group. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019 Dec 7;394(10214):2096–107. doi: 10.1016/S0140-6736(19)32556-5. Epub 2019 Nov 14. Erratum in: Lancet. 2019 Dec 7;394(10214):2072. PMID: 31735560.
- Dimopoulos MA, Leleu X, Moreau P, Richardson PG, Liberati AM, Harrison SJ, Miles Prince H, Ocio EM, Assadourian S, Campana F, et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis. Leukemia. 2021 ;35(2):562–72. 10.1038/s41375-020-0868-z. PMID: 32444867; PMCID: PMC7862055
- Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Špička I, Baker R, Kim K, et al. IKEMA study group. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021 Jun 19;397(10292):2361–71. 10.1016/S0140-6736(21)00592-4. PMID: 34097854
- Boxhammer R, Weirather J, Steidl S, Endell J. MOR202, a human anti-CD38 monoclonal antibody, mediates potent tumoricidal activity in vivo and shows synergistic efficacy in combination with different antineoplastic compounds. Blood. 2015;126:3015.
- Raab MS, Engelhardt M, Blank A, Goldschmidt H, Agis H, Blau IW, Einsele H, Ferstl B, Schub N, Röllig C, et al. MOR202, a novel anti-CD38 monoclonal antibody, in patients with relapsed or refractory multiple myeloma: a first-in-human, multicentre, phase 1-2a trial. Lancet Haematol. 2020 ;7(5):e381–e394. 10.1016/S2352-3026(19)30249-2. PMID: 32171061
- Krishnan AY, Patel KK, Hari P, Jagannath S, Niesvizky R, Silbermann RW, Berg DT, Li Q, Allikmets K, Stockerl-Goldstein K. A phase Ib study of TAK-079, an investigational anti-CD38 monoclonal antibody (mAb) in patients with relapsed/refractory multiple myeloma (RRMM): preliminary results. J Clin Oncol 2020;38. doi:10.1200/JCO.2020.38.15_suppl.8539. Abstract #8539