ABSTRACT
The quadrivalent human papillomavirus (4vHPV) vaccine has shown confirmative effectiveness in preventing HPV-related diseases among women and men around the globe. The phase III, randomized, double-blind efficacy study (Base study, NCT00834106) conducted in China showed 100% efficacy against HPV 16/18-related cervical intraepithelial neoplasia and efficacy against HPV persistent infection for 78 months. Participants aged 20–45 years who received three doses of 4vHPV vaccine or placebo during the base study were selected and invited for this long-term follow-up (LTFU) study to assess the long-term effectiveness of the 4vHPV vaccine in preventing HPV-related diseases. A total of 368 participants were included in this LTFU study with a median follow-up of 94 months. Among 27 participants (Vaccine vs. Placebo: 8 vs. 19) who underwent colposcopy and biopsy due to cervical cytological abnormalities or HPV infection, no HPV-16/18-related cases of cervical intraepithelial neoplasia (CIN), vulvar intraepithelial neoplasia (VIN), or vaginal intraepithelial neoplasia (VaIN) was observed in the vaccine group while two HPV-16-related cases (CIN1/VaIN) were observed in the placebo group. There were another two HPV-related cases (non-vaccine HPV types) found in the placebo group. Consistent with the findings from global studies that suggested long-term efficacy of 4vHPV vaccine, our study showed continued protective effect of 4vHPV vaccine against HPV-related precancerous diseases through a median follow-up time of 94 months with the longest follow-up time of 125 months after completing three doses of vaccination among Chinese women 20–45 years of age.
Introduction
Human papillomavirus (HPV) is responsible for 690,000 new cancer cases (age-standardized incidence rates 8.0 cases per 100,000 person-years), including 570,000 cervical cancer cases per year worldwide based on the 2018 estimate.Citation1 8.6% and 0.8% of all cancers in women and men are attributable to HPV, respectively.Citation2 In China, HPV causes 110,000 new cancer cases in 2018, approximately 14.1% of all cancer cases attributable to infectious pathogens indicating a high disease burden.Citation1 Vaccination with HPV vaccines is a key measure to prevent the morbidity and mortality associated with HPV. By now, there are three prophylactic HPV vaccines including human papillomavirus bivalent (Types 16, 18) vaccine, human papillomavirus quadrivalent (Types 6, 11, 16, 18) vaccine (4vHPV), and human papillomavirus 9-valent (9vHPV) vaccine approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA),Citation3,Citation4 and have been widely used globally. In China, the 4vHPV vaccine and 9vHPV vaccine were licensed in May 2017 and May 2018, respectively. Another domestically produced bivalent HPV vaccine (Types 16, 18) was approved by China’s National Medical Products Administration (NMPA) on 31Dec 2019.Citation5
Although the 9vHPV vaccine has been used in females and males through 45 years in the US and Europe, it is only approved for use in young women aged 16–26 years currently in China, thus the 4vHPV vaccine is still playing a critical role in protecting Chinese women above that age from getting HPV infection and HPV 6/11/16/18-related lesions. HPV 16 and 18 together account for 71% of cervical cancer cases globally,Citation2 and HPV 6 and 11 account for >90% of the genital wart cases globally.Citation6 The efficacy and safety of 4vHPV vaccine in Chinese women aged 20 to 45 years have been demonstrated in the phase III clinical trial (NCT00834106), a randomized, placebo-controlled, double-blinded multicenter study.Citation7,Citation8 At the end of the 78-month follow-up, efficacy in the per-protocol efficacy (PPE) population was 100% (95% CI: 32.3, 100; 0 vs 7 cases) against HPV16/18-related cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) and 100% (95% CI: 65.2, 100; 0 vs 12 cases) against HPV16/18-related CIN grade 1 or worse (CIN 1+).Citation7
The long-term effectiveness and immunogenicity of 4vHPV vaccine in young women have been reported recently in Nordic countries. The long-term follow-up (LTFU) study based on the FUTURE II efficacy study (NCT00092534) suggested durable protection offered by 4vHPV vaccine against HPV 16/18-related high-grade cervical dysplasia in trial participants vaccinated as adolescents or young adults. The effect was shown to last longer than 12 years, with a trend toward continued protection through 14 years post-vaccination.Citation9 After the approval of the 4vHPV vaccine in 2017 in China, long-term effectiveness in Chinese women has not been examined yet. Here we report a LTFU study conducted among the trial participants of the aforementioned phase III base study to examine long-term effect of the 4vHPV vaccine in Chinese women vaccinated at the age of 20–45 years.
Methods
Study design and participants
In the base study, a total of 3006 Chinese female participants aged 20 through 45 years were enrolled and randomized in a ratio of 1:1 to receive three doses of 4vHPV vaccine or placebo, administered at day 1, month 2, and month 6. Detailed study procedures and results were published previously.Citation7,Citation8 The first dose was administered from 3 January 2009 through 31 July 2009, and the third dose was administered from 4 July 2009 through 31 May 2010. The participants were followed until 78 months after the first vaccination for the assessment of efficacy and until 90 months for the assessment of safety.
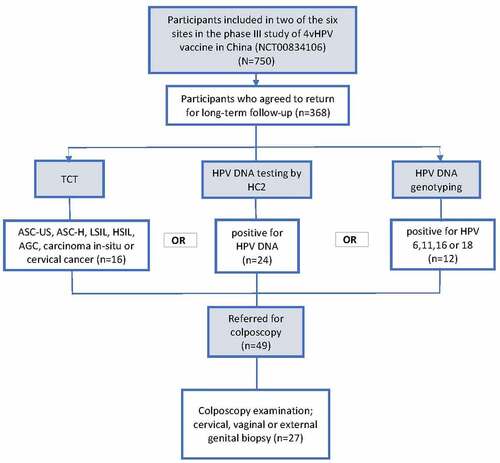
The base study was conducted at six sites and completed in September 2016. The LTFU study was performed among participants from two of the six sites. All participants from these two sites (N = 750) were contacted and invited to return for the long-term follow-up study and test for HPV infection and cervical cytology. Participants with cervical cytological abnormality or HPV infection would be referred for colposcopy and cervical biopsy. Participants in the placebo group who have received catch-up 4vHPV vaccine at the time of the LTFU were excluded. This study has been approved by the ethical committee of Peking University People’s Hospital and all participants enrolled in this LTFU study had provided written informed consent.
Study procedures
Cervical exfoliated cell samples were collected using broom-type collection devices (Hologic, Inc.) and subject to Thinprep cytological test (TCT), hybrid capture 2 (HC2) high-risk HPV DNA test (QIAGEN, USA), and HPV genotyping. Cytological tests were assessed using the Bethesda System-2001.Citation10 HPV genotyping was conducted using the PCR-reverse dot blot kit (Yaneng BioSciences, Shenzhen, China) which can identify 23 HPV types including 6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82, and 83, Participants with any of the following abnormal findings would be referred for colposcopy: (1) Cytological abnormality: atypical squamous cells—undetermined significance (ASC-US), atypical squamous cells—cannot exclude HSIL (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), atypical glandular cells, adenocarcinoma in situ or cervical cancer; (2) High risk HPV detection in HC2 test; (3) HPV genotyping: infection of HPV 6, 11, 16, or 18.
In cases where abnormalities were detected in colposcopy, cervical, vaginal, or external genital biopsy was performed if necessary. The specimen was formalin-fixed and paraffin-embedded. Histology slides were prepared and reviewed by physicians in the Peking University People’s Hospital (Beijing, China). If precancerous or cancerous lesions were observed, the cervical tissue samples would be tested for HPV DNA genotyping.
Statistical analyses
Study endpoints include abnormal cervical cytological findings in the study population and histologically confirmed HPV-related diseases among those tested positive for TCT or HPV DNA. The latter endpoint is defined as pathological diagnosis of CIN1+, VIN1+, or VaIN1+ disease and the detection of HPV DNA for the same tissue block on a single cervical/LVPP biopsy. No statistical hypothesis is to be tested in this LTFU. All results are descriptive and reported by group, i.e., 4vHPV vaccine group and placebo group, per the initial allocation in the base study. Baseline characteristics were reported as mean (±standard deviation, SD) or number (%, percentage) as appropriate.
Results
Baseline characteristics and follow-up information
Of the 750 Chinese female participants who were invited for the LTFU study, 368 participants were included with 171 initially assigned to the vaccination group and 197 assigned to the placebo group. showed the baseline characteristics of the 368 participants before receiving the first dose. Participants’ age, cervical cytology, HPV DNA positivity, and HPV antibody serostatus were generally similar between the two groups.
Table 1. Baseline characteristics of the study population before receipt of the first vaccination
The median follow-up time from the completion of three doses of vaccine/placebo administration until the TCT and HPV test in the LTFU study was 94.4 months with the longest follow-up time of 125 months and the minimum interval of 92 months. The median follow-up time of the extended period in this LTFU study (from 78 months following the first dose of vaccine/placebo until the TCT test) was 22.7 months.
Among the 368 patients included in the LTFU study, 49 (vaccine vs. Placebo: 19 vs. 30) were referred for colposcopy due to abnormal TCT results (n = 16), positive HC2 test (n = 24), or detection of HPV DNA 6/11/16/18 in cervical cells (n = 12) (). These indications were not mutually exclusive. Numbers of participants with negative testing results were 152 and 167 in vaccine and placebo group, respectively.
Figure 1. Participant flow chart.
Results from TCT test
A total of 16 cases were abnormal (ASC-US, LSIL or worse) in TCT test with 4 (2.3%) in 4vHPV vaccine group and 12 (6.1%) in placebo group (). None of them tested positive for HPV DNA type 6, 11, 16, or 18.
Table 2. Findings in cervical cytology test
Pathologic findings for participants with abnormal cervical cytology or HPV infection
As shown in , there was a total of 49 participants referred for colposcopy. In the end, more than half of the eligible subjects (n = 27) underwent colposcopy and biopsy (). Eight of them were from the vaccine group and 19 were from the placebo group. Out of these 27 subjects who received colposcopy and biopsy, six were found to have HPV-associated precancerous lesions in cervix or vagina. There were no observed cases of HPV16/18-related CIN.
The TCT, HPV, and biopsy results of the six cases in both base study and LTFU were summarized in . The time to detection of cytological abnormalities and pathological lesions of the six cases were shown in The one subject in the vaccine group had CIN I lesion but its relationship with HPV 51 was undetermined as HPV 51 was not detected in the biopsied sample. Another 5 cases of CIN I or VaIN I/II were from the placebo group among which four were HPV-related. One subject had HPV-16 related VaIN; another one developed HPV52-related CIN1, the other two subjects received definitive therapy for HPV 16-related lesions in the base study and had tested negative for HPV 16 during LTFU. However, they developed HPV infection of other types (HPV 51, 52, 58) and intraepithelial lesions.
Figure 2. Time to the detection of cytological and pathological abnormalities for the seven cases of intraepithelial disease diagnosed during LTFU.
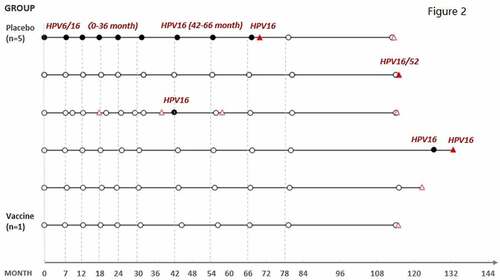
Table 3. The TCT, HPV, and biopsy results for the six cases of intraepithelial disease detected during long-term follow-up
Discussion
The 4vHPV vaccine has been proved to be efficacious globally in preventing persistent HPV infection, genital warts, pre-cancerous lesions, and genital cancers associated with HPV 6/11/16/18 in clinical studies as well as real-world experience.Citation11–13 The US has seen a decline of 56% in the incidence of cervical precancer among 18–20 year-olds and a decline of 39% among 21–24 year-olds from 2008 to 2015 which indicates a population-level impact of HPV vaccine.Citation14 Recent data in Europe suggested that 4vHPV vaccine provided sustained protection against HPV 16/18-related high-grade cervical dysplasia for >12 years.Citation9 In China, the 4vHPV vaccine was approved in 2017 for women 20–45 years of age after 100% efficacy was demonstrated in prevention for low-and-high grade genital precancerous lesions among Chinese females.Citation7 Our study followed the trial participants and provided the first-hand evidence about the long-lasting effect of 4vHPV in Chinese population.
Our preliminary results showed that 4vHPV vaccine could provide a protection against cytology abnormality and cervical, vaginal, or external genital lesions for a median 7.8 years in a subset of participants vaccinated with 4vHPV vaccine. Participants with no abnormal findings in all three tests include 152 of 171 in vaccinated group and 167 of 197 in placebo group. A total of 16 cases with abnormal cytologic results were observed with 4 in 4vHPV vaccine group and 12 in placebo group. Results of HPV genotyping showed that none of these cases tested positive for vaccine HPV types, thus we cannot assess the effectiveness of this 4vHPV vaccine against HPV6/11/16/18-related abnormal cytology. As in the base study where 4vHPV vaccine had demonstrated 94% efficacy against HPV 6/11/16/18-related abnormal cervical cytology, no HPV 6/11/16/18-related abnormal cytology in the vaccine group in the LTFU study may suggest a long-term persistent protection provided by the 4vHPV vaccine.
High efficacy against both HPV 6/11/16/18-related CIN2+ and CIN1+ have been observed in China local trial and global trials. This LTFU discloses a potential trend for long-term effectiveness. Only one case (CIN1) in the vaccine group tested positive for HPV 51 in TCT sample but negative in biopsied sample, indicating no breakthrough case in the subset of participants from the vaccine group during a median 94-month follow-up post vaccination. In contrast, two cases (VaIN 1, CIN1) in the placebo group tested positive for HPV 16 for the biopsied sample, indicating an HPV 16 associated lesion. The development of non-vaccine HPV type related lesions in the placebo group might suggest a potential for cross-protection, but it may also result from preexisting infection of non-vaccine HPV types during the base study. The accrued cases for HPV 6/11/16/18-related CIN2+ and CIN1+ in the base study was 7 and 14, respectively, in 2575 subjects in PPE population,Citation7 indicating a low incidence rate of the related CIN1+ cases and in this LTFU only one 4vHPV type related CIN1+ case was observed which seems to be consistent with the trend in the base study.
Our study has several strengths. It is the first to assess the long-term effectiveness of 4vHPV vaccine among Chinese women aged 20–45 years. Although the 4vHPV vaccine has been mostly replaced by 9vHPV in the US and Europe, in China, the 9vHPV has not been approved for women aged 27–45 years yet. As the efficacy study for 9vHPV in Chinese women of this age group is still in progress, a large number of Chinese women will be continuing to receive 4vHPV vaccine to prevent HPV infection and its related genital precancerous diseases. We also acknowledged that long-term effectiveness of 4vHPV has been demonstrated in previous studies conducted in European population, however, the variation in age-specific HPV prevalence and genotype distribution across countries might lead to geographical difference in vaccine efficacy, which makes local studies imperative. Hence our study has provided important evidence to support continued use of 4vHPV in Chinese women aged 20–45 years in anticipation of long-term protection. Furthermore, our study is based on the pivotal clinical trial in China, and we are continuing to follow these participants for a longer period of time. Besides, participants in the control arm have not received 4vHPV vaccine, which makes the comparison legitimate. We also provided catch-up 4vHPV vaccine for those who would like to get vaccinated after the TCT sampling or biopsy. Last but not least, we performed cervical cytology test and HPV test (both HC2 and HPV genotyping) for the entire study population and recommended colposcopy for subjects with any abnormal findings. This ensures us to capture all possible HPV-related diseases.
The study is not without limitations. First, we were not able to include all the trial participants from the base study. Considering the potential impact of loss to follow-up and relatively small sample size, we did not calculate the vaccine efficacy. Instead, we reported the results descriptively which still shows distinct outcomes between the vaccinated and non-vaccinated females. Second, the study methods and endpoints were not exactly the same as those in the base study. The HPV genotyping method is different from the INNO-LiPA HPV v2 Genotyping PCR assay (Innogenetics, Ghent, Belgium) used in the base study, which may cause difference in the ability of HPV detection. Positive results of genotyping were generated from one-time testing in the LTFU study instead of initial testing plus a confirmative testing in clinical study procedure. Third, the follow-up period is relatively short, and we had only one chance for sampling, making our ability to detect cases somewhat compromised. As the participants in this cohort are still being followed, we will be able to address these limitations in future studies.
In conclusion, our study is the first to suggest that the 4vHPV vaccine provided persistent protection against HPV-16/18-related genital precancerous and cancer cases for a median follow-up of 94 months in Chinese women aged 20–45 years with a trend of continued protection up to 11 years. The vaccine has also shown potential in reducing cervical cytology abnormalities which could benefit the health system by decreasing the number of colposcopy examinations and biopsy.
Acknowledgements
We would like to thank each of the participants for their invaluable contributions to this study.
Disclosure statement
Chao Zhao, Yun Zhao, Jingran Li, Mingzhu Li, Danhua Shen, and Lihui Wei have received grants/research support from MSD R&D (China).
Additional information
Funding
References
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018. Lancet Glob Health. 2020;8(2):e180–6. doi:10.1016/S2214-109X(19)30488-7.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi:10.1002/ijc.30716.
- Food and Drug Administration. Summary basis for regulatory action - Gardasil 9. 2018 [accessed 2021 Sep 1]. https://www.fda.gov/media/117054/download.
- European Medicines Agency. Gardasil 9 - Human papillomavirus 9-valent vaccine (recombinant, adsorbed) (EMA/192711/2016). 2016 [accessed 2021 Sep 1]. https://www.ema.europa.eu/en/documents/overview/gardasil-9-epar-summary-public_en.pdf.
- Chinese vaccine for HPV approved. 2020 [accessed 2021 Sep 1]. https://www.chinadaily.com.cn/a/202001/02/WS5e0d4067a310cf3e35581efd.html.
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt R, Joura E. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–14. doi:10.1086/597071.
- Wei LH, Xie X, Liu JH, Zhao Y, Chen W, Zhao C, Wang S, Liao X, Shou Q, Qiu Y, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up in Chinese women. Vaccine. 2019;37(27):3617–24. doi:10.1016/j.vaccine.2018.08.009.
- Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, Wang S, Liao X, Shou Q, Zheng M, et al. Safety of a quadrivalent human papillomavirus vaccine in a Phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine. 2019;37(6):889–97. doi:10.1016/j.vaccine.2018.12.030.
- Kjaer SK, Nygard M, Sundstrom K, Dillner J, Tryggvadottir L, Munk C, Berger S, Enerly E, Hortlund M, Ágústsson ÁI, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four nordic countries. E Clin Med. 2020;23:100401.
- Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Jama. 2002;287(16):2114–19. doi:10.1001/jama.287.16.2114.
- Muñoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–57. doi:10.1016/S0140-6736(09)60691-7.
- Yoshikawa H, Ebihara K, Tanaka Y, Noda K. Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (GARDASIL) in Japanese women aged 18-26 years. Cancer Sci. 2013;104(4):465–72. doi:10.1111/cas.12106.
- Garland SM, Kjaer SK, Munoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;63(4):519–27. doi:10.1093/cid/ciw354.
- Gargano JW, Park IU, Griffin MR, Niccolai LM, Powell M, Bennett NM, Johnson Jones ML, Whitney E, Pemmaraju M, Brackney M, et al. Trends in high-grade cervical lesions and cervical cancer screening in 5 states, 2008–2015. Clin Infect Dis. 2019;68(8):1282–91. doi:10.1093/cid/ciy707.
