ABSTRACT
The Victorian Specialist Immunization Services (VicSIS) was established in Victoria, Australia, in February 2021, aiming to enhance vaccine safety services for Coronavirus disease (COVID-19) vaccines. VicSIS supports practitioners and patients with complex vaccine safety questions, including those who experience adverse events following immunization (AEFI) after COVID-19 vaccines. VicSIS provides individual vaccination recommendations, allergy testing, vaccine challenges, and vaccination under supervision. VicSIS initially comprised of eight adult COVID-19 specialist vaccination clinics, subsequently, expanding to better support pediatric patients as the Australian vaccine roll-out extended to adolescents and children. Since their establishment to September 2021, the inaugural VicSIS clinics received a total of 26,401 referrals and reviewed 6,079 patients. Consults were initially predominantly for pre-vaccination reviews, later predominantly becoming post-vaccination AEFI reviews as the program progressed. Regardless of the type of consult, the most common consult outcome was a recommendation for routine vaccination (73% and 55% of consult outcomes respectively). VicSIS is an integral component of the COVID-19 vaccination program and supports confidence in COVID-19 vaccine safety by providing consistent advice across the state. VicSIS aims to strengthen the health system through the pandemic, bolstering specialist immunization services beyond COVID-19 vaccines, including training the next generation of vaccinology experts.
Introduction
With the emergence of SARS-CoV-2 variants such as the B.1.617.2 Delta and B.1.1.529 Omicron variants, high vaccine uptake is an even more crucial component of the global pathway out of the Coronavirus disease (COVID-19) pandemic, including in Australia, which did very well in minimizing COVID-19 morbidity and mortality through stringent public health measures.Citation1 Vaccine safety is an integral component to vaccine confidence and uptake both in Australia and globally.Citation2 This has been demonstrated by the impact of thrombosis with thrombocytopenia syndrome (TTS), a rare but serious condition associated with the Vaxzevria® (AstraZeneca, Cambridge, UK) vaccine, and myocarditis associated with mRNA vaccines, which became the most important safety signals of 2021.Citation3,Citation4
The process for vaccine pharmacovigilance varies globally depending on individual countries’ healthcare systems, with many countries favoring a spontaneous passive surveillance system due to the lower cost and resourcing necessary.Citation5 In Australia, adverse events following immunization (AEFI) surveillance occurs via jurisdictional based reporting systems, with regional differences in surveillance methodologies and reporting rates.Citation6 In the Australian jurisdiction of Victoria [population ~6.6 million], AEFI are spontaneously reported by patients or health-care providers to SAEFVIC, the state-wide vaccine safety service.Citation7,Citation8 SAEFVIC comprises a central reporting enhanced passive and active surveillance system integrated with clinical services. This follows World Health Organization (WHO) recommendations that spontaneous reporting alone does not allow for timely detection of new vaccine safety signals or prompt assessment of causality.Citation9 SAEFVIC forwards vaccine safety reports to the Therapeutic Goods Administration (TGA), the national medicine and therapeutic regulatory agency of the Australian Government, to support national signal investigation. The TGA is responsible for pharmacovigilance, national collation of vaccine adverse event reports and undertaking causality assessments.Citation10,Citation11
Prior to the COVID-19 pandemic, most AEFI reported were in children, with clinical management occurring at established pediatric specialist vaccination clinics.Citation12 Adult specialist vaccination services, however, were limited across the state. Given the initial phase of the COVID-19 vaccine roll-out was primarily focused on adults, a need was identified to rapidly expand and enhance adult vaccine safety services in Victoria to support immunizers and patients with complex safety questions. The Victorian Specialist Immunization Services (VicSIS) was therefore established by the Victorian Department of Health (DH) with the specific objectives to: (i) facilitate consistent approaches to the clinical response to serious AEFI following COVID-19 vaccination, (ii) standardize the approach regarding further vaccine doses following serious AEFI and adverse events of special interest (AESI), (iii) address vaccine safety concerns, and (iv) provide allergy assessment and vaccination under supervision where required. VicSIS also provides clinical input to local and national vaccine safety signal investigations and supports the development of guidelines and educational resources for the identification and management of significant AEFI.
Materials and methods
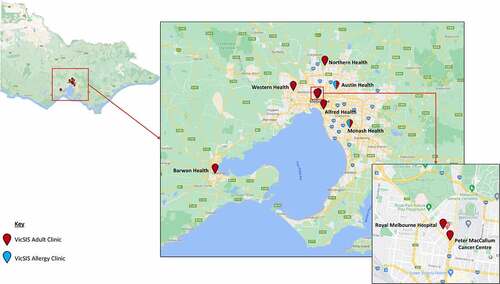
VicSIS was established in an attempt to coordinate and formalize adult vaccine safety services for COVID-19 vaccines. VicSIS clinic locations were selected primarily in the Victorian capital city of Melbourne due to its population density (81% of the total population) with a single regional clinic ().Citation13 Clinic sites were initially established at seven tertiary hospitals, including two sites with dedicated allergy services (). One quaternary center, Peter MacCallum Cancer Center, provides specialized advice to individuals who are immunocompromised with underlying cancer and its associated therapies.
Each VicSIS clinic is coordinated by a medical lead (infectious diseases or allergy/immunology physician), in conjunction with whom terms of reference, referral criteria and reporting mechanism were drafted. VicSIS clinics undertake two types of consultations
Pre-vaccination
Persons at-risk of an AEFI with a COVID-19 vaccinefor example, those with a history of anaphylaxis to a COVID-19 vaccine component, previous cerebral venous sinus thrombosis or recent acute inflammatory cardiac disease; or those in special risk groups with vaccination concerns e.g., pregnant women, previous Guillain Barre Syndrome (GBS).
Post-vaccination
Persons who experienced an AEFI with a COVID-19 vaccine and require clinical review prior to a second dose or ongoing follow-up and management of their AEFI
Consults are conducted via telehealth/telephone or face to face. High-risk allergy patients are seen by the two specialist vaccine allergy services that undertake allergy testing and supervised vaccination where required. An on-call clinician roster provided clinical expertise supporting the vaccination program rollout in risk assessment and programmatic public health response to serious AEFI.
Weekly clinical meetings (to discuss adverse events of special interest (AESI) and standardize clinical guidance and management) and regular operations meetings (to discuss any operational issues within the network) were commenced. In addition, an allergy subgroup was formed to review suspected allergy cases to COVID-19 vaccines, contribute to state and national guidelines, provide key reporting on allergy to the department and SAEFVIC, as well as offer clinical advice and management guidance. A nursing subgroup was also established to connect nurses from across the VicSIS clinic sites, to facilitate standardization and offer an opportunity to share experiences, undertake capacity building, and collaborate between clinics. VicSIS clinicians assist SAEFVIC with collating clinical information and assessing clinical case definitions for vaccine safety signal investigations for key AESI, such as myocarditis, TTS, immune thrombocytopenia and GBS.Citation14,Citation15 This information is crucial for the TGA to undertake rapid causality assessments where indicated. In addition to information gathering, VicSIS clinicians play the vital role of providing clinical assessment and management of these patients, and advise on subsequent doses of vaccines. This supports individuals to be up-to-date with COVID19 vaccines and addresses vaccination concerns with an equity lens.
The VicSIS network is supported by subject matter experts (SME) from various subspecialties, including allergy/immunology, hematology, neurology, cardiology, geriatrics, and obstetrics. SME provides expert advice on serious AEFI and have assisted with the development of state and national clinical guidance on significant AEFI and special risk groups. VicSIS also collaborates with the Melbourne Vaccine Education Center (MVEC) to provide expertise for the development of vaccine safety materials directed at health professionals and community, such as the creation and review of COVID-19 frequently asked questions (FAQs).Citation16
Results
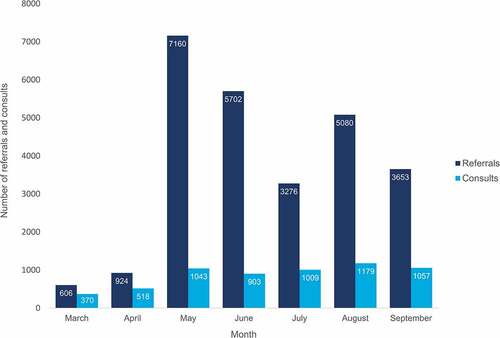
Since they began to receive referrals in March 2021 to September 2021, the inaugural 8 VicSIS clinics received a total of 26,401 referrals and reviewed a total of 6,079 patients. Most of these referrals did not meet criteria for review in a specialist vaccination clinic. Referral numbers increased exponentially in the initial weeks of the program with a peak referral volume of 7,160 in May 2021, outstripping the consult capacity and requiring refinement of the referral process to manage the volume and focus referrals appropriately to the specialist service objectives, described in more detail below. Total number of referrals and consults can be seen in Of the total consults seen, 5362 (88%) were reviewed via telehealth/telephone and 717 (12%) were reviewed face to face. 4174 (69%) of the consults were completed by a medical specialist, 1378 (23%) by a registered nurse, and 527 (9%) by a nurse practitioner.
Figure 2. Total VicSIS referrals (n = 26,401) and consults (n = 6,079) by month, March to September 2021.
Of the consults undertaken, the majority were initially pre-vaccination due to concerns mostly pertaining to previous history of allergy or clotting history. This changed across the program to favor post-vaccination referrals for AEFI as can be seen in Most post-vaccination consults were for AEFI following dose 1 vaccination. This was in the setting of an initial recommended dose interval of 3 and 2 months for Vaxzevria® and Corminaty BNT Pfizer-BioNTech vaccines, respectively, and the majority of the population being vaccinated in quarters 3 and 4. Of the pre-vaccination consults undertaken, 24% (n = 752) were for special risk groups, 22% (n = 680) for clotting concerns relating to the Vaxzevria® vaccine, 21% (n = 660) for history of allergy, 16% (n = 518) for previous AEFI to a non-COVID-19 vaccine, and 17% (n = 550) due to other reasons such as severe needle phobia, pregnancy, dose interval queries, and cardiac history. Outcomes for VicSIS consults divided by pre- and post-vaccination consults can be seen in . The predominant outcome for both consult categories was a recommendation for routine vaccination, with an as expected higher proportion of this for pre- (73%) rather than post-vaccination (55%) consults. Only a small number of consults resulted in a recommendation for an alternate vaccine brand (6% and 14% for pre- and post-vaccination consults respectively).
Figure 3. VicSIS clinic consultations, by type and month, March to September 2021 (n = 6,047)*.
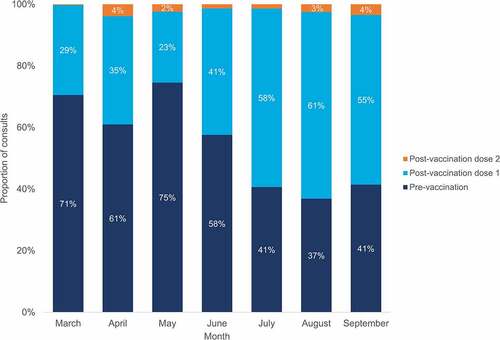
Table 1. Pre- and post-vaccination consults by outcome, March to September 2021 (n = 5,815)*
Discussion
VicSIS was resourced and funded based on modeling from previously established specialist pediatric vaccination clinics, as well as AEFI data from post-implementation studies in Israel and the United States.Citation17 However, unanticipated issues led to unexpectedly high activity levels at VicSIS clinics. These included concerns around allergy in those not identified as being at higher risk of allergy to a COVID-19 vaccine (e.g. food allergy), with an unmet need for review of historical vaccine reactions (including from childhood) being identified. In addition, the unexpected safety signal of TTS and corresponding changes in vaccine prioritization by the Australian and other governments led to reduction in community confidence in the Vaxzevria® vaccine and vaccine hesitancy. This corresponded with a notable increase in individuals and their treating clinicians preferentially requesting the Corminaty BNT Pfizer-BioNTech vaccine due to perceived risk factors for receiving the Vaxzevria® vaccine. VicSIS clinics were inappropriately seen as the gatekeepers to Corminaty BNT Pfizer-BioNTech vaccine access. Resultantly, while VicSIS was established with a predominant focus on assessment and management of AEFI with COVID-19 vaccines, the service was initially overwhelmed by pre-vaccination referrals. This was compounded by an increase in vaccine demand following a COVID-19 outbreak in Victoria in May/June 2021.Citation18
To manage this surge in clinical demand for VicSIS services, several mitigating strategies were put into place, including more stringent referral criteria, automated e-mail responses and standardized rejection letters, additional staffing, increased resources for vaccine providers and patients, and a state-based communication campaign. This led to a decrease in referral volume, and particularly inappropriate referrals, after the peak seen in May 2021. Emerging evidence suggesting lower-than-expected rates of significant allergic reactions to COVID-19 vaccines, coupled with an increase in supply in Corminaty BNT162b2 vaccine locally, likely also contributed to the observed decrease in referrals. In October 2021, a VicSIS eReferral hub was established at SAEFVIC to assist with the centralized triaging and management of referrals and improving standardization and reporting of the service.Citation19
VicSIS was developed rapidly in response to the COVID-19 vaccination program in Victoria and based on a presumption of need. The establishment and implementation of this service has taught us several useful learnings to be taken forward. In retrospect, planning should have considered the possibility of large numbers of pre-vaccination consults, particularly given heightened anxiety around COVID-19 vaccines. Strategies for addressing referral volumes could have been implemented early to avoid the system becoming overwhelmed, acknowledging the thrombosis with thrombocytopenia (TTS) safety signal and vaccine supply issues compounded the issue. Moving into the future, better resourcing is required to support vaccine safety in the context of the COVID-19 vaccination program, including improved regional coverage through targeted expansion of the VicSIS network.
VicSIS has developed to become much more than just a network of specialist vaccination clinics. It is an integral support to the COVID-19 vaccination program in terms of review and management of serious AEFI and development of state guidelines to support vaccination providers and other clinicians. VicSIS has supported confidence in the safety of COVID-19 vaccines by providing consistent advice on vaccine safety across the state, with 73% of pre-vaccination and 55% of post vaccination consults resulting in a recommendation for routine vaccination.
The VicSIS network has secured funding for the next financial year ending 30 June 2022, to continue to support vaccination activities of the COVID-19 vaccine program. This will ensure strong systems are in place to provide ongoing provision of vaccination rollout and safety guidelines, as well as flexibility associated with expansion of programs and vaccination recommendations. Looking beyond 2021, VicSIS aims to have better coverage of vaccine safety services across regional Victoria to ensure equitable access to specialist vaccination advice and assessment, with a new regional clinic recently set up at Bendigo Health, the principal hospital serving one of Victoria’s largest regional catchment areas. In October 2021, VicSIS began to leverage off our successful model and existing pediatric immunization services to expand to support pediatric patients aged 12 and over as the roll-out moved into the adolescent population.Citation20 The next challenge for the service will be preparing for the program in those primary school aged (5–11 years), due to commence in January 2022, as this cohort is not part of the routine Australian National Immunization Program and have a unique set of needs.Citation21,Citation22 VicSIS is an example of health system strengthening in a pandemic. In the future the aim is for this service to not only address COVID19 vaccines but expand to include high level clinical advice and support for all vaccines administered across the life course.
Acknowledgements
Department of Health: Samantha Axford, Miriam Brown, Carly McLelland, Anita Ona, Anna Power, Verni Ananthanathan, Shaun Coutts, Naomi Bromley.
VicSIS clinic sites: Sarah Bullen, Danielle Kennedy, Kayleigh Malone, Ciara Burke, Brian Price, Jason Trubiano, Kerryn McInnes, Jamie Rotin, Catriona Fleming, Elyse Stevens, Julie Carlilse, Loretta Mithen, Katrina Bellamy, Caroline Poynder, Susan Cirillo, Jane Standish, Katherine Gibney, Samantha Chan, Janet Pasricha, Elise Wang, Karen Bellamy, Jo Hickman, Elizabeth Leahy, Antje Luten, Sara Pitts, Jeremy Carr, Jenna Paterson, Hayley Gray, Jade Mertens, Luma Gashi, Aurelie Abasolo, David Tran, Cindy Yuen, Marion Kainer, Claire Sanguinetti, Kayleen Kraal, Yoko Asakawa, Lianne Cox, Teresa Lazzaro, Sonja Elia, Andrew Mahoney, Kong Kong.
All other medical, nursing and administrative staff that support the VicSIS clinics.
SAEFVIC: Josh Osowicki, Daryl Cheng, Priya Shenton, Linny Phuong, Emma Roney, Mel Addison, Louise Dempsey, Adele Harris, Georgie Lewis, Bianca Penak, Laura Voss, Jaimee Craft, Victoria Scott, Lois Tham, Maaki Dusanovic, Klara Glavacevic, Kylie Richter, Alexandra Walker.
MVEC: Daryl Cheng, Rachel McGuire, Francesca Machingaifa, Courtney De Ambrosis
Subject matter experts: Belinda Cruse, Belinda Hibble, Bryn Jones, Chitherangee Arumugananthan, Clare White, Elspeth Hutton, Erica Wood, Fran Milat, Huyen Tran, Izanne Roos, Jeff Szer, John Kanellis, Kirsten Palmar, Kirsten Perrett, Kymble Spriggs, Lyn Kiers, Michael Murray, Mya Cubitt, Nicholas Cox, Paxton Loke, Peter Archer, Samar Ojami, Tissa Wijeratne, Jason Fok, Paul Monagle, Sanjeev Chunilal.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Giles ML, Wallace EM, Alpren C, Brady N, Crouch S, Romanes F, Sutton B, Cheng A. Suppression of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after a second wave in Victoria, Australia. Clin Infect Dis. 2021;73(3):e808–6. doi:10.1093/cid/ciaa1882 .
- World Health Organization (WHO). Data for action: achieving high uptake of COVID-19 vaccines: gathering and using data on the behavioural and social drivers of vaccination: a guidebook for immunization programmes and implementing partners: interim guidance, 3 February 2021. 2021 [accessed 2021 Nov 18]. https://apps.who.int/iris/handle/10665/339452 .
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101. doi:10.1056/NEJMoa2104840 .
- Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, Edwards K, Soslow JH, Dendy JM, Schlaudecker E, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–40. doi:10.1001/jama.2021.24110 .
- World Health Organization (WHO). Global vaccine safety blueprint 1.0. 2012 [accessed 2022 Feb 17]. https://apps.who.int/iris/handle/10665/70919 .
- Horvath J. Review of the management of adverse effects associated with Panvax and Fluvax. 2011 [accessed 2022 Feb 20]. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/53AE51CDFBC05086CA257D7A001A758A/$File/adverse-event-march-2011.pdf .
- Austalian Bureau of Statistics. National, state and territory population. 2020 [accessed 2021 Dec 17]. https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release#states-and-territories .
- Clothier HJ, Crawford NW, Kempe A, Buttery JP. Surveillance of adverse events following immunisation: the model of SAEFVIC, Victoria. Commun Dis Intell Q Rep. 2011;35:294–98 .
- World Health Organization (WHO). Global vaccine safety blueprint 2.0. 2021 [accessed 2022 Feb 20]. https://www.who.int/publications/i/item/10665348966_9789240036963 .
- Therapeutic Goods Administration (TGA). COVID-19 vaccine weekly safety report - 08-07-2021. 2021 [accessed 2021 Dec 17]. https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-08-07-2021 .
- Therapeutic Goods Administration (TGA). Reporting suspected side effects associated with a COVID-19 vaccine. 2021 [accessed 2021 Dec 18]. https://www.tga.gov.au/reporting-suspected-side-effects-associated-covid-19-vaccine .
- Clothier HJ, Lawrie J, Lewis G, Russell M, Crawford NW, Buttery JP. SAEFVIC: surveillance of adverse events following immunisation (AEFI) in Victoria, Australia, 2018. Commun Dis Intell. 2018;2020:44.
- Austalian Bureau of Statistics. 2016 census QuickStats: greater Melbourne. 2016 [accessed 2021 Dec 17]. https://www.abs.gov.au .
- Gordon SF, Clothier HP, Morgan H, Buttery JP, Phuong LK, Monagle P, Chunilal S, Wood EM, Tran H, Szer J, et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine. 2021;39:7052–57. doi:10.1016/j.vaccine.2021.10.030 .
- Osowicki J, Morgan H, Harris A, Crawford NW, Buttery JP, Kiers L. Guillain-Barré Syndrome in an Australian state using both mRNA and adenovirus-vector SARS-CoV-2 vaccines. Ann Neurol. 2021;90(5):856–58. doi:10.1002/ana.26218 .
- Melbourne Vaccine Education Centre (MVEC). COVID-19 vaccine safety information. 2021 [accessed 2021 Nov 18]. https://mvec.mcri.edu.au/covid-19/ .
- Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNtech COVID-19 vaccine. JAMA. 2021;325(8):780–81. doi:10.1001/jama.2021.0600.
- Victorian Department of Health. Victoria COVID-19 data. 2021 [accessed 2021 Dec 11]. https://www.coronavirus.vic.gov.au/victorian-coronavirus-covid-19-data .
- Melbourne Vaccine Education Centre. Victorian Specialist Immunisation Services (VicSIS). 2021 [accessed 2021 Nov 11]. https://mvec.mcri.edu.au/references/the-vicsis-victorian-specialist-immunisation-services-network/ .
- Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI statement regarding vaccination of adolescents aged 12-15 years. 2021 [accessed 2021 Nov 18]. https://www.health.gov.au/news/atagi-statement-regarding-vaccination-of-adolescents-aged-12-15-years .
- Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI recommendations on Pfizer COVID-19 vaccine use in children aged 5 to 11 years. 2021 [accessed 2021 Dec 12]. https://www.health.gov.au/resources/publications/atagi-recommendations-on-pfizer-covid-19-vaccine-use-in-children-aged-5-to-11-years .
- Australian Department of Health. National immunisation program schedule. 2021 [accessed 2021 Dec 12]. https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule .