ABSTRACT
Life-course immunization holds significant benefit for population health by reducing the burden of vaccine-preventable diseases (VPD) through vaccinating individuals at different stages and circumstances in life. The study aimed to determine the epidemiologic, clinical, economic, and societal burden of VPDs among at-risk adult subpopulations in the United States. A systematic literature review was conducted for articles published between January 2010 and June 2020, which identified 72 publications. There was heterogeneity in available epidemiology data, with the prevalence of VPDs ranging from 1.1% to 68.7%. Where the disease burden was described, outcomes were typically worse among high-risk subpopulations than in the general population. Several VPDs, including herpes zoster, meningococcal, and pneumococcal infections were associated with increased costs. This review suggests that subpopulations may not frequently interact with the healthcare system, or their risk factors may not be recognized by healthcare providers, and therefore individuals may not be appropriately targeted for vaccination.
Introduction
Vaccine-preventable diseases (VPD) continue to confer a substantial economic and clinical burden among individuals and healthcare providers, with United States (US) expenditure estimated at nearly $27 billion annually for adult VPDs treatment.Citation1–3 However, this burden is not equally distributed across populations, with some at-risk subpopulations experiencing an increased burden compared to the general population, i.e. individuals with higher exposure risks to VPDs or at risk of severe clinical outcomes due to comorbidities.Citation4 This increased burden intensifies the inequalities in health outcomes among at-risk subpopulations who already face preexisting health inequalities due to their circumstances and access to health care. Thus, the preventable burden of VPD has substantial downstream effects on healthcare utilization among these subpopulations.Citation5,Citation6
Life-course immunization is the concept of providing protection and health benefits to people throughout their lives, at different stages and circumstances, with vaccination.Citation4 The Centers for Disease Control and Prevention (CDC) recommends that individuals receive additional vaccination throughout their adult life due to various reasons, including occupation, travel, comorbidities, circumstance (e.g. homelessness) and behavior (e.g. unsafe sex or drug use).Citation4,Citation6
Vaccination throughout life holds significant benefit at an individual, population, and socioeconomic level.Citation7 For example, vaccination results in the decreased incidence of VPDs and associated morbidity and mortality, resulting in socioeconomic benefits such as reduced treatment courses, shorter hospital stays, and reduced productivity losses.Citation5,Citation7,Citation8
The life-course immunization framework involves comprehensive immunization programs, public demand for immunization, engaged HCPs, multidisciplinary coordination, and robust data informing policies and programs.Citation4 Australia and several European countries, including Austria, Germany, and the United Kingdom, have immunization programs that cover most stages of life.Citation5,Citation9 Notably, German studies have demonstrated that increased adult vaccination resulted in decreased hospital admission rates, length of hospital stay, and mortality.Citation10,Citation11 Understanding the impact of VPDs among at-risk subpopulations in the US is important for developing similar policies to support a life-course immunization framework.Citation4,Citation7
Despite the widespread and marked impact of VPDs within the adult population, to the authors’ knowledge, no review has thoroughly explored the burden of diseases and vaccine uptake in at-risk subpopulations in the US. Without this understanding, the benefits of life-course immunization programs cannot be fully understood.
In order to support disease prevention among at-risk adults, similar to the achievement of successful vaccine recommendations and immunization frameworks among pediatric and older adults, this systematic literature review (SLR) sought to identify evidence related to VPDs among at-risk adult populations. The objectives included in this study were therefore to (1) quantify the epidemiologic burden of VPDs among the at-risk subpopulations of interest; (2) determine the clinical, economic, and societal burden of VPDs among the at-risk subpopulations of interest; and (3) determine current vaccine uptake. This review identified a substantial volume of data on burden and vaccine uptake; therefore, this paper focuses on quantifying the burden of VPDs (objectives 1 and 2). A companion article will explore the third objective on vaccine uptake and barriers to vaccination.
Methods
Search strategy
Databases including Medline, Embase, Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), and Cochrane Central Register of Controlled Trials (CENTRAL) were searched for articles published between January 2010 and June 2020. The search comprised of combinations of keywords and Medical Subject Headings (MeSH) terms pertaining to at-risk subpopulations, VPDs, epidemiology, clinical, economic, and societal burden, and vaccination uptake (Table S1). Bibliographies of included publications were reviewed to identify relevant publications not captured through the database searches. A gray literature search of the Centers for Disease Control and Prevention (CDC), Office of Disease Prevention and Health Promotion, US Department of Health & Human Services (HHS), and Immunization Action Coalition (IAC) websites was performed.
Study selection
Eligible studies met the following inclusion criteria: (1) individuals defined as at-risk by the Advisory Committee on Immunization Practices (ACIP) recommendations and CDC adult immunization schedule; (2) epidemiology, economic and social burden outcomes; (3) vaccination outcomes; (4) studies conducted in the US adults (≥19 years). Full PICOS criteria are reported in Table S2.
Results
Overview of results
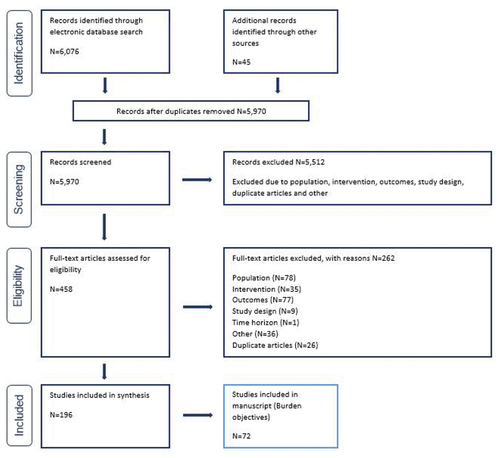
The search ran on July 31, 2020 identified 6,076 articles, of which 458 were taken forward to full-text review. After screening against the eligibility criteria, 196 publications were included () for the SLR. As this paper aims to characterize the burden of VPDs among at-risk subpopulations, only studies reporting these outcomes have been included in this manuscript (N = 72).
The SLR identified publications reporting on burden outcomes for 17 at-risk subpopulations; however, no publications were identified for caregivers, adoptees, homeless persons, or prisoners (Table S3). Conversely, the greatest number of publications is identified for individuals with HIV infections (N = 22) and immunocompromised individuals (N = 15).
Epidemiology of vaccine-preventable diseases
The epidemiologic burden of VPDs was reported in 59 studies, of which the greatest number was reported on individuals with HIV (N = 22; Table S4). There was variation in the available epidemiologic data, with the prevalence of VPDs ranging from 1.1% to 68.7% among at-risk subpopulations reported by ≥ 3 publications. Conversely, limited (<3 publications) or no data were identified for 13 populations, with the prevalence of VPDs ranging from 1.1% to 75.0% (Table 3). The following sections present trends among populations where ≥3 publications reported on epidemiology outcomes for VPDs.
Occupational exposures
Five studies reported epidemiologic data in individuals with occupational exposures to VPDs (influenza, measles, varicella).Citation12–16 Of these, four reported on HCPs and one on crew members on maritime vessels. Bhadelia et al. (2013) and Jones et al. (2018) reported on the prevalence of influenza among HCPs.Citation12,Citation13 A retrospective analysis of employee health records in New York City, investigating the impact of the H1N1 pandemic on healthcare workers (HCWs), reported that only 40% of HCWs tested positive with influenza after presenting with flu-like symptoms (N = 393).Citation12 Furthermore, modeling of occupationally acquired influenza infections in emergency departments and hospitals estimated that among 1.62 million HCPs, the incidence of occupationally acquired influenza was 9.3% and 2.1%, respectively, during one influenza season.Citation13
A surveillance study by Fiebelkorn et al. (2015) of imported and non-imported measles cases in the US reported that, of 1,822 measles cases reported between 2001 and 2014, 1,318 were non-imported, with 78 (5.9%) transmitted within US healthcare facilities. Of these, only 29 cases were among HCPs who were infected as a result of occupational exposure.Citation14
Comparatively, the literature suggests that the prevalence of VPDs was higher among professions that experienced higher exposure to VPDs, such as maritime crew. A retrospective analysis by Rice et al. (2018) of varicella outbreaks on board maritime vessels reported that 92% of all cases were among crew members.Citation16 Moreover, index-case patients were identified for all reported outbreaks of which 79.8% (N = 79) were crew members, with only 20.2% identified among passengers.Citation16
Migrants or immigrants
Six publications reported on the epidemiologic burden of hepatitis B virus (HBV) among migrants and immigrants.Citation17–22 Notably, five of these studies reported a higher prevalence of HBV infection among Asian migrants/immigrants compared with other migrants and US-born individuals.Citation17–21 Ha et al. (2016) reported a higher prevalence of HBV infection among Asian immigrants (5.8%) than African immigrants (4.6%) in the Baltimore-Washington metropolitan area (statistical significance not reported).Citation17 Similarly, a study of HBV seroprevalence in California by Levy et al. (2010) reported that Asian immigrants had the highest prior HBV exposure when compared to Latino immigrants and US-born men (15.1% versus 2.0% and 5.1%, respectively; P < .01).Citation18 Moreover, chronic HBV infection was also most prevalent among Asian immigrants (3.8%) compared to Latino immigrants and US-born men (<1%; P = .03).Citation18
These studies also identified that the prevalence of HBV was higher among immigrants from East and Southeast Asia compared to individuals from other regions.Citation17,Citation19,Citation21 First-generation Chinese and Southeast Asian immigrants in Ohio had substantially higher rates of chronic HBV infection than other Asian subgroups and those born in the US (China and Southeast Asia 7.7%, respectively, versus .8% for US-born individuals).Citation19 Rossi et al. (2012) also reported that HBV seroprevalence among individuals from these regions differed substantially, with migrants from East Asia and sub-Saharan Africa at highest risk of infection.Citation21 Similarly, Ha et al. (2016) reported that prevalence was highest among immigrants from Vietnam (9.0%).Citation17 Furthermore, in a study of 4,301 individuals, Pollack et al. (2014) reported that Chinese-born individuals had the highest HBV seroprevalence rates (23.2%) among all migrant groups.Citation20 Moreover, seroprevalence varied significantly with age, gender, education, birthplace, and family history of infection among Asian ethnic subgroups in New York City (P < .001 for each).Citation20
Wasley et al. (2010) reported that the overall prevalence of HBV infection among foreign-born persons had reduced from 14.4% in 1988–1994 to 10.3% in 1999–2006.22 In both periods, HBV prevalence was highest among Black non-Hispanic individuals (28.1% and 25.9%) compared to White non-Hispanic (7.5% and 8.1%), and Mexican American individuals (4.3% and 2.2%).Citation22
Refugees
Four studies reported on the epidemiologic burden of HBV among refugees or asylees within the US.Citation23–26 In a retrospective cohort study of 210 asylum seekers from various countries, Bertelsen et al. (2018) reported an HBV prevalence of 9.4% among the sample (N = 9).Citation23 The authors noted that none of the asylees or refugees could have received a routine childhood HBV vaccination based on their ages and year of vaccine introduction into their countries of origin.Citation23
In a cross-sectional study of refugees (N = 36,896), Mitruka et al. (2019) reported that region of birth emerged as the strongest correlate of infection after adjustment for age, gender, and state of refugee health assessment.Citation25 Compared with individuals born in the Eastern Mediterranean region, those born in the Western Pacific, South-East Asian or African regions had 4.8 (95% CI: 2.9–7.9), 3.3 (95%: 1.8–6.1), and 3.1 (95% CI: 1.6–6.0) times higher prevalence of HBV infection, respectively.Citation25
People living with HIV
Twenty-two studies reported on the epidemiologic burden of HBV, human papillomavirus (HPV), pneumococcal infections, meningococcal infections, and herpes zoster (HZ), among PLWH.Citation27–48 There was a considerable epidemiologic burden associated with HBV infections among people living with HIV (PLWH). In a retrospective cohort study including 24,490 participants, Buskin et al. (2011) reported HBV prevalence rates of 8.0% for chronic and 3.0% for acute HBV from 1998 to 2004.Citation28 In this period, HBV infection had a mortality rate of 68.9 per 1,000 person-years.Citation28 Similarly, Abara et al. (2017), presenting screening results from 2005 to 2017, reported HBV prevalence rates of 4.0–17.0% in PLWH compared to 7.0% (95% CI: 6–8%) in IDUs co-infected with HIV.Citation27 Furthermore, Chun et al. (2010) reported that the prevalence of HBV among 2,536 individuals included in the US military HIV natural history study was 6.0% for chronic HBV.Citation31 Chun et al. (2010) previously reported that 24.2% of study participants (N = 2,769) were infected with HBV at the time of HIV diagnosis, of whom 7.2% had chronic HBV infection.Citation30 Overall, the prevalence of HBV was 38.9% during the study period.Citation30
There is evidence that the incidence of HBV has increased among PLWH.Citation28 An aforementioned study by Chun et al. (2010) reported HBV incidence rates of 9.7 per 100 person-years in PLWH, compared to 4.0 per 100 person-years before 1996 (pre-HAART era).Citation28
Despite the burden of HBV among PLWH, two studies reported that the incidence of pneumococcal and meningococcal infections is decreasing. Marcus et al. (2016) estimated that the incidence of pneumococcal infections in 1996–1999 was 305 per 100,000, compared to 88 per 100,000 in 2010–2011.Citation45 Similarly, in an observational study, Miller et al. (2014) reported that the incidence of meningococcal infections had reduced from 4.7 per 100,000 in 2000–2002 to 1.9 per 100,000 in 2009–2011.Citation44 Although there is evidence that the incidence of pneumococcal infections may be decreasing among PLWH, Zhang et al. (2018) reported that the incidence of both all-cause pneumonia (ACP) and invasive pneumococcal disease (IPD) was substantially higher among PLWH than healthy individuals.Citation42 For instance, the incidence risk ratio was 4.1 (95% CI: 3.89–4.26) and 17.4 (95% CI: 12.48–24.29) times higher among PLWH compared to healthy individuals for ACP and IPD, respectively.Citation42
Conversely, there was heterogeneity among the identified publications reporting on the epidemiologic burden of HPV among PLWH, with the prevalence of anal HPV ranging from 12% to 90%.Citation33–36–Citation40–47 Moreover, Kojic et al. (2018 and 2019) reported that the prevalence of cervical HPV among women with HIV was lower than the prevalence of anal HPV (6–83%).Citation37,Citation38
Furthermore, Guignard et al. (2013) reported an annual incidence rate of HZ among PLWH of 15 per 1,000 person-years (95% CI: 13.56–16.62).Citation34 Overall, the incidence of HZ among PLWH has decreased from 1998 to 2004.Citation34
Immunocompromised individuals
Eleven publications reported on the epidemiologic burden of pneumococcal disease, HPV, and HZ among patients with immunocompromising conditions.Citation29–42–Citation46–49–Citation56 The incidence of VPDs was higher among immunocompromised compared with immunocompetent populations.Citation42,Citation49 In a retrospective cohort study (N = 35,696,718), Zhang et al. (2018) reported that rates of ACP and IPD were approximately 3.7 and 4.7 times higher for immunocompromised than immunocompetent adults, respectively.Citation42 Additionally, rates of ACP and IPD were found to be positively associated with the presence of additional at-risk conditions.Citation42 For instance, ACP and IPD rates for adults with one at-risk condition were 2.9 and 3.7 times higher than immunocompetent adults, yet 8.1 and 10.6 times higher for adults with ≥2 at-risk conditions.Citation42 Similarly, in an observational study, Dhar et al. (2019) reported that the proportion of women with lupus (N = 1,349) testing positive for high-risk HPV was significantly higher compared with the general population (.169 versus .088, p < .001).Citation49
In addition to the presence of comorbidities, the prevalence of HZ in immunocompromised individuals differed depending on tumor/transplant type, with heterogeneity among the literature.Citation46–51–Citation53 Li et al. (2016) found the annual incidence of HZ was higher among patients with solid organ transplants compared to bone-marrow or stem cell transplants (42.9 versus 16.7 per 1,000 person-years, respectively).Citation46 Conversely, in a retrospective cohort of 14,670 individuals, Habel et al. (2013) reported that the incidence of HZ was higher among patients with hematologic malignancies compared to solid tumors (31.2 versus 12.3 per 1,000 person-years, respectively).Citation52 Furthermore, Mao et al. (2016) reported that the incidence of HZ among patients who underwent autologous hematopoietic stem cell transplant was 62.18 per 1,000 person-years.Citation51 A systematic review (McKay et al. 2019) investigating the risk of HZ among immunocompromised adults, including patients with hematopoietic cell transplants, and solid organ transplants, observed an incidence ranging from 9 to 95 per 1,000 person-years.Citation53
Injection drug users
Six publications reported on the epidemiologic burden of HBV and HPV among injection drug users (IDUs).Citation27–40–Citation47–57–Citation59 Injection drug use has previously been identified as an independent predictor for HBV infection.Citation58 In a case-control study of 23,585 individuals from Michigan, Tsai et al. (2019) reported a higher incidence of HBV among IDU compared with non-IDU (.52% versus .03%) from 2005 to 2018.Citation58 Furthermore, only 78.8% of intravenous drug users were found to be free of viral infection (HBV, hepatitis C virus and HIV), compared with 98.3% of non-IDU.Citation58 Conversely, a systematic review (Robbins et al. 2015) reported that none of the types of drug use examined, including IDU (OR: 1.2%), were significantly associated with oral HPV prevalence.Citation40 Further, a retrospective cohort study of 18,486 individuals by Yin et al. (2020) reported that susceptibility to HBV among individuals who inject drugs remained largely unchanged between 2007–2010 and 2011–2016.Citation57
Men who have sex with men
Six studies reported on the epidemiologic burden of hepatitis and HPV in men who have sex with men (MSM); two reported on hepatitis and five on HPV.Citation27,Citation33,Citation47,Citation57,Citation60,Citation61 Of the two studies reporting on the epidemiology of hepatitis, a systematic review by Abara et al. (2017) reported an HBV antibody prevalence of 1.1–2.3% among MSM aged <30 years.Citation27 Furthermore, an aforementioned study (Yin et al. 2020) of the National Health and Nutrition Examination Survey (NHANES) data identified that the prevalence of hepatitis A susceptibility was unchanged between 2007 and 2016 (2007–2010: 68.2% [95% CI: 56.1–80.3%] versus 68.2% [95% CI: 59.7–76.7%] in 2011–2016).Citation57
Four studies reported on the prevalence of HPV infection among MSM, however there was heterogeneity among the reported prevalence (32–65%), likely as a result of the variation in the methods used to collect data.Citation33,Citation47,Citation60,Citation61 Two studies reported on the prevalence of oral and anal HPV specifically. In a prospective study in Philadelphia (N = 5,329), Goldstein et al. (2019) reported anal HPV prevalence of 63% and oral HPV prevalence of 8%.Citation33 Similarly, a survey (Halkitis et al. 2019) of MSM in New York reported the prevalence of anal infection as 56.0% and oral HPV infection of 8.8%.Citation61
Clinical burden of vaccine-preventable diseases
The review identified 18 publications reporting on the clinical burden of VPDs among at-risk subpopulations (Table S5). Despite the available evidence, the clinical burden was poorly described, with limited (≤3 publications) identified for 18 at-risk subpopulations. No data were available describing the burden among caregivers, adoptees, homeless persons, migrants or immigrants, prisoners, refugees, individuals with alcohol dependency, or people practicing unsafe sex (Table S3).
Among populations where the disease burden was described, at-risk subpopulations typically demonstrated worse outcomes than the general population. Pneumococcal infections in at-risk patients were associated with a greater number of medical visits, hospitalizations, and increased length of hospital stay compared to healthy controls or at-risk patients without VPDs. In an observational study, Zhang et al. (2018) reported that resource use per ACP episode was significantly higher for adults with at-risk conditions compared to healthy individuals, with the exception of medical office visits, which were significantly lower (all P < .001).Citation42 Comparatively, the same study reported that resource use per IPD episode was similar among at-risk and healthy individuals (P > .05).Citation42 The exception to this was individuals with end-stage renal disease (ESRD) who had a significantly lower number of ED visits, and adults with HIV who had a significantly higher number of inpatient hospitalizations (both P < .05).Citation42
Variable differences in length of hospital stay between IDU and non-IDU have been observed.Citation58,Citation62
A retrospective cohort of 29,455 individuals (Vazquez De Lara et al. 2018) reported no significant difference in length of pneumonia-related hospital stay between IDU and non-IDU patients (−.042 days, P = .825).Citation62 However, an increased likelihood of ventilation was found among IDU patients (OR: 1.45, P < .05).Citation62 Conversely, in a case-control study of 47,281 individuals, Tsai et al. (2019) reported a significant increase in both cost and length of hospital stay among patients with pneumonia who injected drugs ($30,471 versus $16,020, P < .001; 5.7 days versus 3.7 days; P < .001).Citation58 IDU was also more likely to require ventilation (OR: 1.45, P < .05).Citation58
Similarly, in an observational database study of patients with HIV, Li et al. (2016) reported statistically significant differences in healthcare use between matched HZ and non-HZ cohorts (6.3%; P < .05).Citation46 Patients with HIV and HZ also had more hospitalizations, longer inpatient stays, more ED visits and more outpatient visits, on average, than non-HZ patients (P < .05).Citation46 A 2017 study (Folaranmi et al.) characterizing the risk of meningococcal disease among MSM (N = 74) reported that HIV-infected MSM had 10.1 times (95% CI: 6.1–16.6%) the risk of HIV-uninfected MSM.Citation63
Furthermore, an observational study by Ishigami et al. (2020) of 91,520 individuals with reduced kidney function reported increased resource use among those not vaccinated against influenza.Citation50 Within this study, influenza vaccination was found to be associated with a reduction in hospitalization for pneumonia/influenza (OR: .86; 95% CI: .79–09.93), coronary heart disease (OR: .93; 95% CI: .88–.97), and heart failure (OR: .92; 95% CI: .86–.99).Citation50 Based on the observed increase in hospitalization among those not vaccinated, there is evidence that improved adherence to vaccination is important to avoid unnecessary healthcare resource use.Citation50
Economic burden of vaccine-preventable diseases
A total of 14 studies reported on the economic burden of VPDs among at-risk subpopulations (Table S6). No data were identified for caregivers, adoptees, homeless persons, migrants or immigrants, prisoners, injection drug users, individuals with alcohol dependency, and people practicing unsafe sex (Table S3). The greatest number of publications reported on individuals with immunocompromising conditions (N = 7). Several VPDs, including HZ, meningococcal and pneumococcal infections were associated with increased costs among individuals with VPDs versus healthy comparators.
Among several at-risk subpopulations, the presence of pneumococcal infections was associated with increased treatment costs, particularly with an increased mean cost per episode compared with healthy individuals.Citation42 An aforementioned study of 35,696,718 individuals reported substantially higher costs per episode of ACP in adults in at-risk subpopulations, with costs up to $9,168 compared to $4,725 for healthy adults.Citation42 Zhang et al. (2018) evaluated the costs per ACP and IPD episode among individuals with chronic liver disease (CLD), diabetes, heart or lung disease (i.e. at-risk conditions), and among individuals with HIV, asplenia, and immunocompromised individuals including patients with cancer and organ transplants (i.e. high-risk conditions).Citation42 Average costs per ACP episode in at-risk adults overall ($6,534) were significantly higher than in healthy adults ($4,725) (P < .0001), with the highest costs seen in adults with CLD ($8,729), followed by chronic lung disease ($7,688), and chronic heart disease ($7,425). For IPD, the average cost per IPD episode in adults with at-risk conditions was similar to healthy adults across all conditions (P > .05).Citation42 Furthermore, costs per ACP episode in at-risk and high-risk adults overall ($9,168) were significantly higher than in healthy adults ($4,725) (P < .0001), with the highest costs seen in adults with asplenia ($11,847), followed by cancer ($9,577), and chronic renal disease ($9,098).Citation42 The findings by Zhang et al. (2018) are supported by Weycker et al. (2016), who reported that healthcare costs among two million adults included in the Truven commercial claims database were higher among both at-risk and at-risk subpopulations compared to healthy individuals.Citation64 The at-risk group, including CLD, heart/lung disease, and diabetes, was associated with healthcare costs of $21.7 million per 100,000 at-risk person-years, which were 8.7 (8.5–8.8) times higher than healthy individuals.Citation64 Among the high-risk group, including immunocompromised individuals, and HIV, costs totaled $58.5 million per 100,000 at-risk years person-years, which were 23.4 (22.9–23.8) times higher than corresponding costs among healthy individuals.Citation64
Similarly, HZ infections were associated with increased costs per case among individuals with immunocompromising conditions (e.g., HIV, cancer, and transplants), particularly compared to healthy individuals. In a retrospective cohort of 438,817 individuals, Li et al. (2016) reported that, during the first quarter after diagnosis, patients with immunocompromising conditions and HZ had higher healthcare costs than their matched controls.Citation46 For instance, increased costs for patients with HIV (+$3,056), solid organ transplant (+$2,649), bone-marrow/stem cell transplant (+$13,332) and cancer (18–64 years of age: +$2,549; ≥65: +$3,108) diagnosed with HZ, compared to those without HZ.Citation46 Furthermore, a retrospective cohort study of health insurance claims (N = 143,892; Meyers et al. 2018) reported an increase in all-cause healthcare costs among immunocompromised individuals diagnosed with HZ (HZ=$13,471 versus non-HZ=$11,192).Citation65
A high economic burden of VPDs was also identified among MSM; notably, the high costs of acute meningococcal infections, with substantial costs associated with the long-term impact of infections. A 2017 study (Folaranmi et al.) reported that meningococcal infections were associated with hospitalization costs of $51,627 for acute invasive meningococcal disease (IMD).Citation63
Societal burden of vaccine-preventable diseases
There is a paucity of data on the societal burden of VPDs among at-risk subpopulations. No publications were identified reporting on quality of life (QoL) outcomes in any of the at-risk subpopulations included. The lack of humanistic data identified among the included publications suggests that there is limited research among these at-risk subpopulations in the US.
Discussion
To our knowledge, this study represents the first systematic review investigating the burden of VPDs among the adult at-risk subpopulations defined by the CDC. Overall, this review identified 72 publications on the epidemiology and disease burden for VPDs among adult at-risk subpopulations.
There was heterogeneity in the available epidemiology data among different at-risk subpopulations, with limited (<3 publications) or no data available for many groups. This suggests the burden of VPDs in at-risk subpopulations is not fully understood and these populations are unlikely to be appropriately targeted for vaccination, further contributing to the burden of VPDs. Notably, there was a lack of data for several populations, including homeless persons, refugees, people with alcohol dependency and people practicing unsafe sex. These at-risk subpopulations often have limited contact with HCPs due to a lack of access and complexities in reimbursement, or may be less likely to discuss their lifestyle at medical visits, thus demonstrating the need for a multifaceted approach to vaccination.Citation66–68 To exemplify this likely additional burden, where comparative data between at-risk subpopulations and healthy individuals were available, the prevalence of VPDs was higher in at-risk subpopulations, demonstrating the importance of quantifying the burden of VPDs for all special population groups.
In addition to an increased prevalence of VPDs among at-risk subpopulations, VPDs were also associated with worse clinical outcomes compared to healthy individuals, e.g. ACP and IPD in individuals with asplenia, chronic heart disease, CLD, diabetes, and HIV.Citation42 Among those with at-risk conditions, infection with VPDs resulted in a greater number of hospitalizations and an increased length of hospital stay compared to other at-risk patients without VPDs or healthy individuals.Citation42,Citation43,Citation46 These data indicate that VPDs cause a substantial clinical burden for both patients and HCPs and subsequently the healthcare system, which could be prevented by life-course immunization.
Further, VPDs are associated with a substantial economic burden to both individuals and the healthcare system, however this review was unable to quantify this burden among many at-risk subpopulations. Several studies reported an increased mean cost per episode compared with healthy individuals.Citation42,Citation46,Citation64 However, the cost per episode varied by at-risk subpopulation and VPDs and these data were not identified for all subpopulations.Citation42,Citation46,Citation64 While the evidence suggests an increased burden among certain groups, further research is required to elucidate this burden, particularly among at-risk subpopulations, to support the positive impact of vaccination.
An absence of societal burden data among at-risk subpopulations was also identified. However, a literature search may not accurately characterize the humanistic burden of VPDs in these populations, as this review has highlighted that publications in this area focus on epidemiology and economic outcomes. This type of evidence also tends to be lacking from database studies or larger retrospective studies where QoL outcomes are often not explicitly captured.Citation69
Adult vaccines, as a part of life-course immunization, are a cost-effective solution to promoting health in the adult population. Vaccination leads to the decreased incidence of VPDs and associated mortality, resulting in cost savings for both direct and indirect costs associated with treatment and lost productivity.Citation7,Citation8 However, lack of health insurance coverage and high costs can be an obstacle to life-course vaccination, resulting in a high burden of VPDs among at-risk subpopulations.Citation4,Citation6,Citation70 Medicaid coverage varies between states, while Medicare payment for vaccination is often complex and not all recommended vaccines are covered by Medicare Part B (which covers outpatient medical costs), often resulting in prohibitive costs for many adults.Citation71 The burden of VPDs for at-risk subpopulations, highlights the necessity for adequate funding for adult vaccination across providers to reduce this burden.Citation72
Life-course immunization policies, focusing on increasing awareness of the health benefits of vaccination and improving vaccination coverage, will reduce the burden of VPDs among the US population.Citation4,Citation6 In order to implement successful life-course immunization policies in the US, there must be collaboration between national and state-level policymakers to ensure that the importance of the life-course approach is prioritized and well understood.Citation4–6 It is also critical that HCPs are engaged with these policies and are equipped with sufficient knowledge regarding the risk-factors for VPDs to provide appropriate vaccination.Citation4,Citation6
Strengths and limitations
This comprehensive review identified publications reporting on a breadth of outcomes among at-risk subpopulations. Furthermore, the review applied a wide timeframe to ensure all key publications and any key progressions in this research area were captured, such as updates to ACIP recommendations.
As with all literature reviews, it is important to recognize that there are methodological limitations; the most notable being that a literature search may not accurately characterize the burden of VPDs in these populations due to the categorization of populations or publication bias limiting peer-reviewed evidence in certain populations. For example, adults are infrequently classified as adoptees in studies and the emphasis in the wider literature for prisoners focuses on screening for infectious diseases, rather than vaccinating against VPDs. However, despite heterogeneity within the literature, this review has identified that VPDs confer a substantial burden to at-risk subpopulations. Furthermore, certain populations may not disclose their lifestyle choices to healthcare professionals/insurance companies, e.g., MSM, people practicing unsafe sex, and IDUs. Therefore, while there have been data identified in this literature review, further research may be needed to ascertain the true burden of VPDs for these groups. Moreover, this review identified that the measurement of the burden of VPDs is highly heterogeneous between at-risk subpopulations, which limits the comparison between VPDs and subpopulations. Finally, the search was limited to the English language; it may be possible that further evidence is published in other languages. However, this is a minor limitation, given the review was US-focused.
Conclusions
There is a paucity of epidemiologic data for VPDs, which represent a substantial clinical and economic burden in certain populations (i.e., immunocompromised individuals). Moreover, consistent burden data are lacking for several populations. Therefore, this review confirms that further research is required to accurately characterize the burden in at-risk subpopulations and target them for vaccination. Embracing a life-course immunization framework, where providers and systems prioritize the recommended vaccinations, would reduce the disparities in VPDs faced by these at-risk subpopulations.
While further research is necessary, the current review suggests that at-risk subpopulations are likely consistently under-vaccinated. There are challenges within the US healthcare system that must be overcome to address this subpar vaccination rate: challenges with improved patient access, expansion of service providers, tracking infrastructure, and increased funding, to ensure that all adults are appropriately targeted for vaccination. Improved vaccination policies, together with integrated vaccination in public and private payer access programs, will promote higher uptake of vaccination across all the population, including at-risk subpopulations.
Author contributions
IK, MKN, MOB, AK, DB, and IF contributed to study conception and study design. All authors contributed to the writing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Supplemental Material
Download MS Word (173.4 KB)Disclosure statement
IK, MKN, and MOB are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may own stocks and/or stock options in Merck & Co, Inc., Kenilworth, NJ, USA. AK, DB, and IF are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ was compensated by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA for the conduct of the study and development of the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2054602
Additional information
Funding
References
- AJMC Peer Exchange. Assessing the cost of vaccine-preventable diseases; 2021 [accessed 2021 Mar 28]. https://www.ajmc.com/journals/supplement/2020/new-directions-immunization-awareness-engagement/assessing-the-cost-of-vaccinepreventable-diseases
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–9. doi:10.1016/j.vaccine.2007.03.046.
- McLaughlin J, McGinnis J, Tan LJ, Mercatante A, Fortuna J. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–73. doi:10.1007/s10935-015-0394-3.
- Health Policy Partnership. A life-course approach to vaccination: adapting European policies; 2018.
- Tate J, Aguado T, Belie JD, Holt D, Karafillakis E, Larson HJ, Nye S, Salisbury D, Votta M, Wait S, et al. The life-course approach to vaccination: harnessing the benefits of vaccination throughout life. Vaccine. 2019;37(44):6581–83. doi:10.1016/j.vaccine.2019.09.016.
- Global Coalition on Aging. Life-course immunization: a driver of healthy aging; 2018.
- Philip RK, Attwell K, Breuer T, Di Pasquale A, Lopalco PL. Life-Course immunization as a gateway to health. Expert Rev Vaccines. 2018;17(10):851–64. doi:10.1080/14760584.2018.1527690.
- Rémy V, Zöllner Y, Heckmann U. Vaccination: the cornerstone of an efficient healthcare system. J Mark Access Health Policy. 2015;3: doi:10.3402/jmahp.v3403.27041.
- Morris T, Tate J, Wait S, Scrutton J. Implementing a life-course approach to immunization. The Health Policy Partnership; 2019.
- Alphons M. Influencing policy to improve adult vaccination in Germany: expert meeting report; 2020.
- Haas J, Braun S, Wutzler P. Burden of influenza in Germany: a retrospective claims database analysis for the influenza season 2012/2013. Eur J Health Econ. 2016;17(6):669–79. doi:10.1007/s10198-015-0708-7.
- Bhadelia N, Sonti R, McCarthy JW, Vorenkamp J, Jia H, Saiman L, Furuya EY. Impact of the 2009 influenza a (H1N1) pandemic on healthcare workers at a tertiary care center in New York City. Infect Control Hosp Epidemiol. 2013;34(8):825–31. doi:10.1086/671271.
- Jones RM, Xia Y. Annual burden of occupationally-acquired influenza infections in hospitals and emergency departments in the United States. Risk Anal: An Off Publ Soc Risk Anal. 2018;38(3):442–53. doi:10.1111/risa.12854.
- Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the Postelimination Era, 2001-2014. Clin Infect Dis. 2015;61(4):615–18. doi:10.1093/cid/civ387.
- Guturu P, Cicalese L, Duchini A. Hepatitis a vaccination in healthcare personnel. Ann Hepatol. 2012;11(3):326–29. doi:10.1016/S1665-2681(19)30927-5.
- Rice ME, Bannerman M, Marin M, Lopez AS, Lewis MM, Stamatakis CE, Regan JJ. Maritime varicella illness and death reporting, U.S., 2010–2015. Travel Med Infect Dis. 2018;23:27–33. doi:10.1016/j.tmaid.2018.04.001.
- Ha E, Kim F, Pan J, Hong DTP, Juon HS. Prevalence of viral hepatitis B and C infection among immigrants in the Baltimore-Washington metropolitan area screened from 2009-2014. Cancer Epidemiol Biomarkers Prev. 2016;25(3).
- Levy V, Yuan J, Ruiz J, Morrow S, Reardon J, Facer M, Molitor F, Allen B, Ajufo BG, Bell-Sanford G, et al. Hepatitis B sero-prevalence and risk behaviors among immigrant men in a population-based household survey in low-income neighborhoods of northern California. J Immigrant Minority Health/Center Minority Public Health. 2010;12(6):828–33. doi:10.1007/s10903-009-9239-6.
- Mishra AC. Emerging viral infections in 21st century and their implications on health system. Indian J Virol. 2013;24:99–100.
- Pollack HJ, Kwon SC, Wang SH, Wyatt LC, Trinh-Shevrin C, Coalition A. Chronic hepatitis B and liver cancer risks among Asian immigrants in New York City: Results from a large, community-based screening, evaluation, and treatment program. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2229–39. doi:10.1158/1055-9965.EPI-14-0491.
- Rossi C, Shrier I, Marshall L, Cnossen S, Schwartzman K, Klein MB, Schwarzer G, Greenaway C. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PloS One. 2012;7(9):e44611. doi:10.1371/journal.pone.0044611.
- Wasley A, Kruszon-Moran D, Kuhnert W, Simard E, Finelli L, McQuillan G, Bell B. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202(2):192–201. doi:10.1086/653622.
- Bertelsen NS, Selden E, Krass P, Keatley ES, Keller A. Primary care screening methods and outcomes for Asylum Seekers in New York City. J Immigrant Minority Health. 2018;20(1):171–77. doi:10.1007/s10903-016-0507-y.
- Chai SJ, Davies-Cole J, Cookson ST. Infectious disease burden and vaccination needs among asylees versus refugees, district of Columbia. Clin Infect Dis. 2013;56(5):652–58. doi:10.1093/cid/cis927.
- Mitruka K, Pezzi C, Baack B, Burke H, Cochran J, Matheson J, Urban K, Ramos M, Byrd K. Evaluation of Hepatitis B virus screening, vaccination, and linkage to care among newly arrived refugees in four states, 2009–2011. J Immigrant Minority Health. 2019;21(1):39–46. doi:10.1007/s10903-018-0705-x.
- Museru OI, Vargas M, Kinyua M, Alexander KT, Franco-Paredes C, Oladele A. Recent immigrants and the use of cervical cancer screening test in Canada. J Immigrant Minority Health. 2010;12(1):1–5. doi:10.1007/s10903-009-9237-8.
- Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American college of physicians and the centers for disease control and prevention. Ann Intern Med. 2017;167(11):794–804. doi:10.7326/M17-1106.
- Buskin SE, Barash EA, Scott JD, Aboulafa DM, Wood RW. Hepatitis B and C infection and liver disease trends among human immunodeficiency virus-infected individuals. World J Gastroenterol. 2011;17(14):1807–16. doi:10.3748/wjg.v17.i14.1807.
- Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-Effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21. doi:10.1016/j.vaccine.2013.10.024.
- Chun HM, Fieberg AM, Hullsiek KH, Lifson A, Crum‐cianflone N, Weintrob A, Ganesan A, Barthel R, Bradley W, Agan B, et al. Epidemiology of Hepatitis B virus infection in a US Cohort of HIV-Infected individuals during the past 20 years. Clin Infect Dis. 2010;50(3):426–36. doi:10.1086/649885.
- Chun HM, Mesner O, Thio CL, Bebu I, Macalino G, Agan BK, Bradley WP, Malia J, Peel SA, Jagodzinski LL, et al. HIV outcomes in hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr. 2014;66(2):197–205. doi:10.1097/QAI.0000000000000142.
- Fong S, Tokman S, Jarlsberg L, et al. Prevalence and outcomes of HIV-associated opportunistic pneumonias in the ERA of combination antiretroviral therapy. Am J Respir Crit Care Med. 2012;185(MeetingAbstracts).
- Goldstein ND, LeVasseur MT, Tran NK, Purtle J, Welles SL, Eppes SC. Modeling HPV vaccination scale-up among urban young men who have sex with men in the context of HIV. Vaccine. 2019;37(29):3883–91. doi:10.1016/j.vaccine.2019.05.047.
- Guignard AP, Haguined F, St Laurent S, Vannappagari V. Incidence of, and risk factors for herpes zoster in the haart era: an analysis of the chorus cohort. Pharmacoepidemiol Drug Saf. 2013;22:266–67.
- Jolley SE, Alkhafaf Q, Hough C, Welsh DA. Presence of an alcohol use disorder is associated with greater Pneumonia severity in hospitalized HIV-Infected patients. Lung. 2016;194(5):755–62. doi:10.1007/s00408-016-9920-1.
- Keller MJ, Burk RD, Xie X, Anastos K, Massad LS, Minkoff H, Xue X, D’-Souza G, Watts DH, Levine AM, et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. Jama. 2012;308(4):362–69. doi:10.1001/jama.2012.5664.
- Kojic EM, Conley L, Bush T, Cu-Uvin S, Unger ER, Henry K, Hammer J, Escota G, Darragh TM, Palefsky JM, et al. Prevalence and incidence of anal and cervical high-risk Human Papillomavirus (HPV) types covered by current HPV vaccines among HIV-Infected women in the SUN study. J Infect Dis. 2018;217(10):1544–52. doi:10.1093/infdis/jiy087.
- Kojic EM, Cu-Uvin S, Conley L, Bush T, Onyekwuluje J, Swan DC, Unger ER, Henry K, Hammer JH, Overton ET, et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study). Sex Transm Dis. 2011;38(4):253–59. doi:10.1097/OLQ.0b013e3181f70253.
- Ortiz AP, Tamayo V, Scorsone A, Soto-Salgado M, Febo I, Piovanetti P, Venegas-Ríos HL, Yamamura Y, Zorrilla C. Prevalence and correlates of cervical HPV infection in a clinic-based sample of HIV-positive hispanic women. Papillomavirus Res. 2017;4:39–44. doi:10.1016/j.pvr.2017.06.006.
- Robbins HA, Fennell CE, Gillison M, Xiao W, Guo Y, Wentz A, Kirk GD, Mehta SH, D’-Souza G, et al. Prevalence of and risk factors for oral human papillomavirus infection among HIV-Positive and HIV-Negative people who inject drugs. PloS One. 2015;10(11):e0143698. doi:10.1371/journal.pone.0143698.
- Weiser J, Perez A, Bradley H, King H, Shouse RL. Low prevalence of hepatitis B vaccination among patients receiving medical care for HIV infection in the United States, 2009 to 2012. Ann Intern Med. 2018;168(4):245–54. doi:10.7326/M17-1689.
- Zhang D, Petigara T, Yang X. Clinical and economic burden of pneumococcal disease in US adults aged 19-64 years with chronic or immunocompromising diseases: an observational database study. BMC Infect Dis. 2018;18(1):436. doi:10.1186/s12879-018-3326-z.
- Simon MS, Weiss D, Geevarughese A, Kratz MM, Cutler B, Gulick RM, Zucker JR, Varma JK, Schackman BR. Cost-effectiveness of meningococcal vaccination among men who have sex with men in New York City. J Acquir Immune Defic Syndr. 2016;71(2):146–54. doi:10.1097/QAI.0000000000000822.
- Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, Kennedy J, Steiner-Sichel L, Ngai S, Shepard C, Weiss D, et al. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med. 2014;160(1):30–37. doi:10.7326/0003-4819-160-1-201401070-00731.
- Marcus JL, Baxter R, Leyden WA, Muthulingam D, Yee A, Horberg MA, Klein DB, Towner WJ, Chao CR, Quesenberry CP, et al. Invasive pneumococcal disease among HIV-Infected and HIV-Uninfected adults in a large integrated healthcare system. AIDS Patient Care STDS. 2016;30(10):463–70. doi:10.1089/apc.2016.0165.
- Li Q, Chen SY, Burstin SJ, Levin MJ, Suaya JA. Cost of herpes zoster in patients with selected immune-compromised conditions in the United States. Open Forum Infect Dis. 2016;3(2):ofw067. doi:10.1093/ofid/ofw067.
- Fisher KA, Cahill L, Tseng T-S, Robinson WT. HPV vaccination coverage and disparities among three populations at increased risk for HIV. Transl Cancer Res. 2016;5(S5):S1000–S1006. doi:10.21037/tcr.2016.10.66.
- Leung J, Bialek SR, Marin M. Trends in varicella mortality in the United States: data from vital statistics and the national surveillance system. Hum Vaccines Immunotherapeutics. 2015;11(3):662–68. doi:10.1080/21645515.2015.1008880.
- Dhar JP, Essenmacher L, Dhar R, Ragina N, Sokol RJ. Lack of uptake of prophylactic human papilloma virus vaccine among women with systemic lupus erythematosus seen at a regional medical center. J Clin Rheumatol. 2019;25(8):348–50. doi:10.1097/RHU.0000000000000866.
- Ishigami J, Sang Y, Grams ME, Coresh J, Chang A, Matsushita K. Effectiveness of influenza vaccination among older adults across kidney function: pooled analysis of 2005-2006 through 2014-2015 influenza seasons. Am J Kidney Dis: The Off J National Kidney Found. 2020;75(6):887–96. doi:10.1053/j.ajkd.2019.09.008.
- Mao J, McPheeters J, Zhang D, Acosta C, Finelli L. Herpes zoster incidence and disease burden among patients underwent autologous hematopoietic stem cell transplant 2006-2011 in a large, insured us population. Open Forum Infect Dis. 2016;3(suppl_1). doi:10.1093/ofid/ofw172.1892.
- Habel LA, Ray GT, Silverberg MJ, Horberg MA, Yawn BP, Castillo AL, Quesenberry CP, Li Y, Sadier P, Tran TN, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(1):82–90. doi:10.1158/1055-9965.EPI-12-0815.
- McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis: An Off Publ Infect Dis Soc Am. 2019.
- P-J L, Gonzalez-Feliciano A, Ding H, Bryan LN, Yankey D, Monsell EA, Greby SM, Euler GL. Influenza a (H1N1) 2009 monovalent and seasonal influenza vaccination among adults 25 to 64 years of age with high-risk conditions—United States, 2010. Am J Infect Control. 2013;41(8):702–09. doi:10.1016/j.ajic.2012.10.027.
- Lu PJ, O’-Halloran A, Ding H, Srivastav A, Williams WW. Uptake of influenza vaccination and missed opportunities among adults with high-risk conditions, United States, 2013. Am J Med. 2016;129(6):636e611–636e631. doi:10.1016/j.amjmed.2015.10.031.
- Lee DH, Boyle SM, Malat G, Sharma A, Bias T, Doyle AM. Low rates of vaccination in listed kidney transplant candidates. Transplant Infect Dis. 2016;18(1):155–59. doi:10.1111/tid.12473.
- Yin S, Barker L, Ly KN, Kilmer G, Foster MA, Drobeniuc J, Jiles RB. Susceptibility to hepatitis a virus infection in the United States, 2007–2016. Clin Infect Dis: An Off Publ Infect Dis Soc Am. 2020;71(10):e571–e579. doi:10.1093/cid/ciaa298.
- Tsai J, Suh L, Annie F, Richmond BK. Substance abuse–related soft tissue infections: a uniquely complicated subset of a common problem. Am Surg. 2019;85(7):781–87. doi:10.1177/000313481908500744.
- US Department of Health Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care, and treatment of viral hepatitis. Washington, DC: US Department of Health and Human Services; 2011.
- Gerend MA, Madkins K, Phillips G 2nd, Mustanski B. Predictors of human papillomavirus vaccination among young men who have sex with men. Sex Transm Dis. 2016;43(3):185–91. doi:10.1097/OLQ.0000000000000408.
- Halkitis PN, Valera P, LoSchiavo CE, Goldstone SE, Kanztanou M, Maiolatesi AJ, Ompad DC, Greene RE, Kapadia F. Human papillomavirus vaccination and infection in young sexual minority men: the P18 Cohort study. AIDS Patient Care STDS. 2019;33(4):149–56. doi:10.1089/apc.2018.0276.
- Vazquez De Lara F, Bolivar F, Pan D, Santibanez V, Mathew JP. Effect of opioid abuse and dependence on outcomes of patients hospitalized with pneumonia: a 5-year propensity score matched analysis. Am J Respir Crit Care Med. 2018;197(MeetingAbstracts).
- Folaranmi TA, Kretz CB, Kamiya H, MacNeil JR, Whaley MJ, Blain A, Antwi M, Dorsinville M, Pacilli M, Smith S, et al. Increased risk for meningococcal disease among men who have sex with men in the United States, 2012–2015. Clin Infect Dis: An Off Publ Infect Dis Soc Am. 2017;65(5):756–63. doi:10.1093/cid/cix438.
- Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16:182. doi:10.1186/s12913-016-1432-4.
- Meyers JL, Candrilli SD, Rausch DA, Yan S, Patterson BJ, Levin MJ. Cost of herpes zoster and herpes zoster-related complications among immunocompromised individuals. Vaccine. 2018;36(45):6810–18. doi:10.1016/j.vaccine.2018.08.080.
- Omerov P, Åg C, Mattsson E, Klarare A. Homeless persons’ experiences of health- and social care: a systematic integrative review. Health Social Care Community. 2020;28(1):1–11. doi:10.1111/hsc.12857.
- Robertshaw L, Dhesi S, Jones LL. Challenges and facilitators for health professionals providing primary healthcare for refugees and asylum seekers in high-income countries: a systematic review and thematic synthesis of qualitative research. BMJ Open. 2017;7(8):e015981. doi:10.1136/bmjopen-2017-015981.
- Zhang X, Sherman L, Foster M. Patients’ and providers’ perspectives on sexual health discussion in the United States: a scoping review. Patient Educ Couns. 2020;103(11):2205–13. doi:10.1016/j.pec.2020.06.019.
- Dn GR, Leavy MB. Registries for evaluating patient outcomes: a user’s guide [Internet]. In: Gliklich R; Dreyer N, Leavy M, editors. AHRQ methods for effective health care. Vol. 13(14). Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. p. 1–426.
- Infectious Diseases Society of America. Infectious diseases society of America’s policy on state immunization mandates; 2012.
- Adult Immunization: Shots to Save Lives [press release]; 2010.
- Centers for Disease Control and Prevention. Recommended adult immunization schedule for ages 19 years or older; 2021 [accessed 2021 Sep 21]. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html