ABSTRACT
To reduce morbidity and mortality associated with vaccine-preventable diseases (VPD), it is imperative that vaccination programs are implemented and prioritized throughout all stages of life across all populations. This study aimed to determine vaccine uptake and barriers to vaccination against VPDs among at-risk adult populations in the United States. We conducted a systematic literature review for articles published between January 2010 and June 2020 and identified 153 publications. The review identified 17 at-risk populations. Vaccine uptake was suboptimal among many populations, with factors including age, gender, and disease severity, associated with uptake. This review identified several barriers that impact vaccine uptake among at-risk populations, with concerns over safety, vaccine costs, lack of insurance, and lack of provider recommendation commonly reported across populations. Embracing a national life-course immunization framework that integrates developing policies, guidelines, and education would be a step to addressing these barriers.
Introduction
Vaccines are critical to the prevention and control of vaccine-preventable diseases (VPDs) and therefore underpin global health security.Citation1 Vaccination throughout life brings significant benefits at the individual, community, and socio-economic levels.Citation2–4 Despite the contribution of vaccination in reducing the burden of VPDs, these diseases continue to confer a significant burden on society and the healthcare system.Citation2,Citation5 Furthermore, not all individuals experience the same risk of VPDs, and those defined as at-risk populations in the Advisory Committee on Immunization Practices (ACIP) recommendations due to existing health conditions, occupational risks, or behavior are classified at increased risk.Citation3,Citation4,Citation6 This is especially relevant because herd immunity is not sufficient to prevent or reduce the substantial morbidity and mortality among these populations.Citation2,Citation7,Citation8 This is of particular importance given the risk of worse clinical outcomes, including more frequent hospitalizations and increased length of stay, experienced among at-risk groups compared to the general population.Citation2,Citation7,Citation8
To reduce morbidity and mortality associated with VPDs, it is imperative that vaccination programs are implemented and prioritized across all populations.Citation3–5 The concept of a life-course approach to immunization is to protect individuals against VPDs and provide health benefits including reducing hospitalizations and healthcare costs to individuals throughout their lives, at different ages and situations, including at-risk populations.Citation2 The World Health Organization (WHO) Immunization Agenda 2030 established life-course immunization as a strategic priority, with mobilizing support among at-risk groups as one of the key areas of focus.Citation1 The life-course immunization framework involves comprehensive immunization programs, with clear guidelines, vaccine access, data generation, and community partnerships.Citation2 Furthermore, these programs involve public demand for vaccination, engaged healthcare workers (HCWs), and robust surveillance and vaccine uptake data informing policies and programs.Citation1,Citation2 Understanding vaccine uptake and the impact of VPDs among at-risk populations is critical for informing a life-course immunization framework for immunization.Citation2,Citation7
Despite the widespread and marked impact VPDs have on individual health, the healthcare system and on the economy, to the authors’ knowledge, no review has thoroughly explored vaccine uptake and barriers to vaccination among at-risk populations in the United States (US). This knowledge is imperative to understand the benefits of a life-course approach to immunization among individuals in this group.
In order to support disease prevention among at-risk adults, similar to the achievement of successful vaccine recommendations and immunization frameworks among pediatric and older adult populations, this systematic literature review (SLR) sought to identify evidence related to VPDs among at-risk adult populations. The objectives of the overall SLR were therefore to (1) quantify the epidemiological burden of VPDs among the at-risk populations of interest; (2) determine the clinical, economic, and societal burden of VPDs among these populations; and (3) determine current vaccine uptake and barriers to vaccination. This review identified a substantial volume of data on disease burden and vaccine uptake and therefore focuses on determining current vaccine uptake and barriers to vaccination (objective 3). A companion article will explore the first and second objectives on quantifying the burden of VPD.
Methods
Search strategy
We searched Medline, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, and Cochrane Central Register of Controlled Trials for articles published between January 2010 and June 2020. The search comprised combinations of keywords and Medical Subject Headings (MeSH) terms pertaining to at-risk populations, VPDs, epidemiology, clinical, economic, and societal burden, and vaccine uptake (Table S1). We reviewed bibliographies of included publications to identify relevant publications not captured through the database searches. We performed a gray literature search of the Centers for Disease Control and Prevention (CDC), Office of Disease Prevention and Health Promotion, US Department of Health & Human Services, and Immunization Action Coalition websites.
Study selection
We reviewed all identified references for inclusion at the title and abstract level. Full-text screening was conducted by two reviewers using the population, comparators, outcomes, and study design (PICOS) framework (Table S2). Briefly, included articles were primary studies that met the following criteria: (1) individuals defined as at-risk by the ACIP recommendations and CDC adult immunization schedule; (2) epidemiology, economic, and social burden outcomes; (3) vaccination outcomes; (4) studies conducted with US adults (≥19 years). Following a double-blind review, all discrepancies identified were resolved by consensus with a senior researcher.
Data were extracted on the epidemiology, economic, social burden, and vaccination outcomes into a data extraction form by one reviewer and checked for accuracy by a second reviewer.
Results
Overview of results
The search, ran on July 31st, 2020, identified 6,076 articles from both database and gray literature searches, of which 458 were taken forward to full-text review. After screening against the eligibility criteria, 196 publications were included (). As this paper aims to evaluate vaccine uptake and barriers to vaccination of VPDs among at-risk populations, only studies reporting these outcomes were included (N = 153).
The SLR identified publications reporting vaccination uptake for 17 at-risk populations. The greatest number of publications were identified among pregnant women (N = 53) and individuals with occupational exposures (N = 31), followed by men who have sex with men (MSM; N = 19). The following sections present trends among populations where ≥5 publications reported on vaccine uptake or barriers to vaccination.
Vaccine uptake and barriers to vaccination
Vaccine uptake among at-risk populations was reported in 150 studies, with over a third reporting on pregnant women (N = 53). Barriers to vaccination among at-risk populations were reported in 107 studies; the greatest number reported on pregnant women (N = 46). There was variation in available data, with limited (<5 publications) or no data available for 13 at-risk populations (Table S2). Frequently reported barriers to vaccination included concerns regarding vaccine safety or efficacy, vaccine hesitancy, lack of insurance, cost of vaccination, perceived lack of risk, and lack of guidelines or education and HCW recommendation. The entire dataset for all populations is reported in Tables S4 and S5 in the appendices.
Occupational exposures
Vaccine uptake
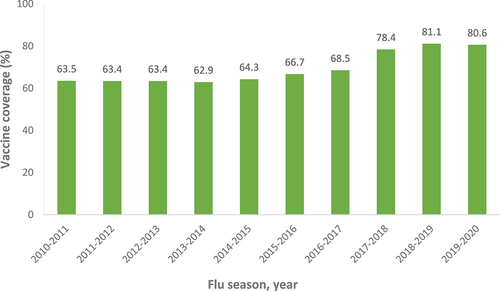
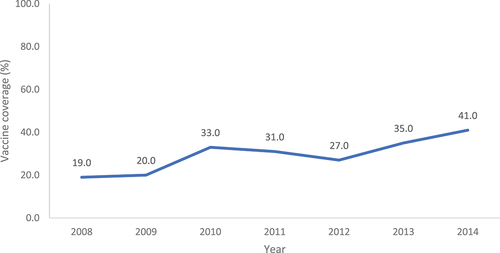
Thirty-three publications reported vaccine uptake in individuals with occupational exposures and notably, HCWs were the only population identified. There was large heterogeneity in the data, with 18 studies reporting influenza vaccine uptake; estimates ranged between 32.0% and 91.6% for overall vaccine uptake among HCWs.Citation9–23 Notably, the annual survey of HCW influenza uptake conducted by the CDC highlighted that uptake has been steadily increasing over the past 10 years ().Citation24–30 Uptake was highest among HCWs working in hospitals and lowest among HCWs in long-term care settings.Citation15 Furthermore, HCWs with health conditions, such as asthma, diabetes, or human immunodeficiency (HIV) infection, were more likely to be vaccinated than healthy controls.Citation23
Figure 2. Influenza vaccine uptake among HCWs, by year.Citation22–28
Several studies evaluated the impact of mandated vaccination among HCWs for influenza and Tdap vaccines.Citation10,Citation12,Citation14,Citation31,Citation32 A study of HCWs in a long-term acute care facility reported that the implementation of mandated influenza vaccination increased vaccination rates from 25% (N = 272) in 2008–2009 to 65% (N = 279) in the 2010–2011 influenza season (p < .05).Citation12 A further study of HCWs during a pertussis epidemic reported that mandated Tdap vaccination improved vaccination rates from 67% to 92%.Citation32 However, opposition to vaccine mandates was reported with HCWs stating that mandates are an infringement on workers’ rights and, in some instances, HCWs reported that they would seek employment elsewhere.Citation32
Among the three studies reporting on tetanus, diphtheria, and pertussis (Tdap) vaccine, uptake estimates among HCWs ranged between 39.0% and 97.0%.Citation31–33 Notably, a study of HCWs in California reported that Tdap uptake improved with increased influenza vaccination (2008–2009: 60%; 2009–2010: 63%).Citation31,Citation32
A study of Native Hawaiian and Pacific Islanders reported a hepatitis B virus (HBV) vaccine uptake of 65.6% among HCWs.Citation34 Furthermore, HBV vaccination for HCWs was 4.8 (95% CI; OR 1.7–14.0) times higher than for non-providers.Citation34 A second study reported suboptimal hepatitis A virus (HAV) vaccine uptake among HCWs in Texas (28.9%: N = 60/207), with only half receiving the full vaccination series.Citation35
Barriers to vaccination
Cost and perceived safety of vaccination were identified as barriers to vaccination among HCWs. Among HCWs who did not receive the pertussis vaccination (61%), 55% (N = 83) cited concerns about the safety of the vaccine, while 14% cited their physicians not recommending it, and 14% cited lack of awareness of current guidelines.Citation33
Furthermore, there is evidence indicating that, when cost is removed as a barrier, vaccine uptake among HCWs increases. Vaccine uptake among HCWs who worked in locations where their employer made vaccination available on-site at no cost for >1 day was 83.9%.Citation15 Uptake was 59.5% among HCWs who worked in locations where their employer did not freely provide influenza vaccination on-site, but actively promoted vaccination through other mechanisms.Citation15
Tourists/travelers
Vaccine uptake
Ten publications reported on vaccine uptake among tourists and travelers. Uptake rates among tourists/travelers differed by vaccine, with estimates ranging from 36.0% to 67.0% for influenza, 25.9% to 40.2% for HBV, 18.8% to 48.3% for HAV and 23.0% to 72.0% for MMR.Citation34,Citation36–42
Notably, age, receipt of influenza vaccine, education, and health insurance, among others, were all independently associated with vaccine uptake with regional variations noted.Citation37,Citation41
Barriers to vaccination
Limited awareness about the specific vaccines recommended for different global regions, provider decisions, lack of insurance and the costs of vaccination were identified as barriers to vaccination among tourists/travelers (Table S4). For example, in a 2017 study among travelers eligible for MMR, 48% (N = 1,689) travelers refused the vaccine either due to not being concerned about illness, or cost concerns.Citation37 Furthermore, 28% of travelers (N = 966) did not receive the vaccine due to provider decisions.Citation37
Migrants/immigrants
Vaccine uptake
Among the eight studies reporting on vaccine uptake among migrants/immigrants, suboptimal HBV uptake was identified (compared to the Healthy people 2020 goal of 60%), with estimates ranging between 8.4% and 54.7%.Citation43–49 Comparing vaccination between US- and foreign-born women of reproductive age, using NHIS data, lower HBV uptake was identified among foreign-born women (27.3% versus 40.9%).Citation45
Initiation of human papillomavirus (HPV) vaccination was also found to be low among immigrants where a 2019 study reported only 6.7% (N = 22) of foreign-born US patients had initiated a vaccination series.Citation50
Vaccine uptake also varied depending on country of birth. A 2014 study compared influenza and pneumococcal uptake between European-born, Arab-born and US-born individuals using NHIS data (N = 117,893).Citation51 Vaccine uptake was lower among European- and Arab-born individuals than US-born individuals for both pneumococcal (84% and 89% not vaccinated versus 78%, respectively) and influenza vaccination (71% and 74% not vaccinated versus 64%, respectively).Citation51
Barriers to vaccination
Access to healthcare and educational attainment level were identified as key barriers to vaccination among Latino and Asian migrants and immigrants.Citation45,Citation46,Citation49 A study of US- and foreign-born women reported that fewer foreign-born women were insured than US-born women (68.2% versus 86.4%, respectively) and fewer foreign-born women had visited a healthcare provider in the past year (74.4% versus 85.3%, respectively).Citation45 Similarly, a 2013 study of Laotian immigrants in Minnesota identified limited English fluency (12%) and not knowing where to go for vaccination (22.6%) as important barriers to vaccination.Citation49
Liver disease
Vaccine uptake
Six studies reported on vaccine uptake among patients with chronic liver disease (CLD). Uptake of HAV and HBV vaccination ranged from 6.7–40.5% and 8.2–53.8%, respectively, with rates increasing in recent years ().Citation34,Citation39,Citation52–54 Over the same period, the number of individuals receiving <2 recommended doses decreased from 46.7% to 9.3% for HAV, and from 17.9% to 7.0% for HBV.Citation53
Figure 3. Vaccine uptake among individuals with chronic liver disease, by year.Citation51
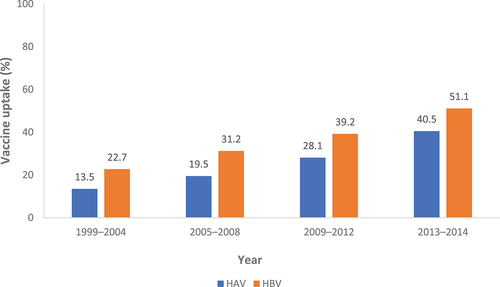
A 2012 survey study utilizing data from the 2007 to 2011 National Health and Wellness Survey reported uptake rates of 34.9% for HAV and 40.5% for HBV vaccination, both of which were found to be significantly greater among patients with CLD when compared with healthy individuals (p < .05).Citation54
However, uptake has been found to vary with age.Citation39 A 2016 study of 32,296 individuals using NHIS data reported higher uptake among patients with CLD aged 19–49 compared with those aged ≥50 years for both HAV (18.2% versus 12.3%) and HBV (41.6% versus 25.1%).Citation39
Barriers to vaccination
No publications reported on the barriers to vaccination among individuals with CLD.
People with diabetes
Vaccine uptake
Among the nine studies reporting on vaccine uptake among people with diabetes, uptake estimates ranged from 37.0% to 53.0% for pneumococcal, 9.4% to 30.7% for HAV and 13.5% to 39.0% for HBV, and 41.0% to 79.5% for influenza.Citation34,Citation39,Citation52–59 Notably, vaccine uptake was consistently lower among people with diabetes compared with the general population (15.4% versus 20.5% for HAV; 22.4% versus 34.3% for HBV in 2008).Citation52 A 2016 national survey of 32,296 individuals reported low HBV vaccine uptake among people with diabetes, with age negatively associated with vaccination (23.5% among those aged 19–59 years versus 13.5% for those ≥60 years).Citation39 Comparatively, a 2016 retrospective cohort study of 245,480 individuals reported that age was positively associated with influenza vaccination, with uptake increasing from 45.8% among those aged 18–49 to 56.6% among those aged 50–65 years with diabetes.Citation59
Barriers to vaccination
No publications reported on the barriers to vaccination among people with diabetes.
End-stage renal disease
Among the ten studies reporting on vaccine uptake among patients with end-stage renal disease (ESRD), uptake estimates ranged from 18.7% to 68.7% for influenza, 39.0% to 59.7% for pneumococcal, and 21.6% to 80.1% for HBV.Citation54,Citation56,Citation60–67 Within these estimates, variation was noted according to disease severity,Citation61 the presence of a comorbidityCitation61 and according to race.Citation56,Citation60,Citation62
Barriers to vaccination
No publications reported on the barriers to vaccination among individuals with ESRD.
People living with HIV
Fourteen publications reported vaccine uptake among people living with HIV (PLWH), among a range of vaccines, including HBV, meningococcal disease, influenza, pneumococcal disease, and HPV. Uptake of HBV vaccination ranged from 28% to 45%.Citation34,Citation46,Citation68–72 HBV uptake differed based on the location where patients received HIV care.Citation72 A significantly larger percentage of patients who received care at Ryan White HIV/AIDS Program (RWHAP)-funded facilities versus non-RWHAP-funded facilities were vaccinated (12.5% versus 3.7%; p < .001).Citation72 Conversely, fewer patients who received care at private practices (versus non-private) were vaccinated (5.6% versus 11.8%; p < .001).Citation72
Influenza uptake ranged from 14.2% to 57.0%.Citation73–75 In a 2011 study reporting influenza uptake using data from the HIV outpatient study (N = 5,365), annual uptake ranged from 25.8% to 43.3%.Citation74 Influenza uptake in this study was highest among individuals aged 30–39 years (40.0%) and lowest in those aged ≥50 years (14.2%).Citation74
Two studies reported similar rates of pneumococcal vaccination; 13.1% among PLWH in Texas and 13.8% among PLWH in California.Citation75,Citation76 A third study reported that individuals aged >65 years had the highest uptake (59.7%) and those aged 18–49 years had the lowest (16.7%).Citation56
Barriers
Concerns about the effectiveness of vaccination and lack of insurance or provider recommendation were identified as barriers to vaccination among PLWH.Citation68,Citation72,Citation73,Citation77,Citation78 A 2010 cohort study of 1,293 women living with HIV cited beliefs about the effectiveness of influenza vaccination as an important barrier to uptake.Citation73 Within this study, those who were vaccinated were less likely to report that they were “not at risk of influenza”, “flu is not a serious disease” and that “the flu shot made [them] sick”.Citation73
Further, lack of health insurance and the cost of vaccination have also been cited as prohibitors to vaccine uptake among PLWH.Citation68,Citation72,Citation77 A 2018 cross-sectional study of 18,089 individuals reported that lack of access to affordable vaccines for some PLWH contributed to low vaccine uptake.Citation72 A 2018 cross-sectional study (N = 18,089) reported that lack of clinical recommendation was an important barrier to vaccine uptake.Citation72 Similarly, inconsistency among guideline recommendations and reports of reduced immunogenicity among patients with HIV prevented physicians from regularly offering routine vaccination to this group.Citation72
Immunocompromised individuals
Among the 23 publications reporting on vaccine uptake among immunocompromised individuals, uptake estimates ranged from 27.1% to 63.7% for influenza, 7.6% to 59.7% for pneumococcal disease and 19.5% to 33.2% for Tdap.Citation40,Citation54,Citation57,Citation61,Citation62,Citation79–84 While vaccination rates among those with immunocompromising conditions have increased over time, uptake remains suboptimal against the 2020 Healthy People Goal (60%).Citation8,Citation61 A 2018 retrospective study of 35,696,718 individuals reported an increase in pneumococcal vaccine uptake from approximately 20% between 2012 and 2014, to 24% in 2016.Citation8 Similarly, a 2020 observational study of 91,520 individuals reported increased influenza vaccine uptake among those with reduced kidney function, from 53.3% to 63.7% between 2005 and 2015.Citation61 Notably, uptake was negatively associated with disease severity, with higher uptake observed among those with normal range kidney function (eGFR ≥60: 62.9% versus eGFR <30: 57.3%).Citation61
Similarly, a 2015 retrospective cohort of kidney transplant candidates (N = 363) in Philadelphia reported receipt of other vaccines (OR: 10.55) and dialysis (OR: 2.00) to be significantly associated with increased vaccine uptake (p < .05).Citation62 However, within this study, both Black (OR: 0.27; <0.001) and Hispanic ethnicity (OR: 0.35; p < .05) were significantly associated with a reduced likelihood of pneumococcal vaccination.Citation62
Barriers to recommendation
Missed opportunities for vaccination due to ongoing medical treatments were identified as barriers to vaccination.Citation40,Citation54,Citation57,Citation61,Citation80,Citation81 A 2016 review reporting on vaccination of special populations, including immunocompromised individuals, identified that patients with chronic medical problems may miss routine vaccinations because of frequent hospital admissions.Citation57 Furthermore, these patients are likely to receive care from a specialist physician rather than a primary care provider; therefore, vaccinations may be missed if specialists assume that vaccination is the responsibility of a general practitioner.Citation57
Injection drug users
Five studies reported on vaccine uptake among injection drug users (IDUs), of which four reported HBV vaccination rates, with estimates ranging from 21.6% to 45.0%.Citation66,Citation68,Citation77,Citation85 A 2012 national cross-sectional study reported that at-risk adults tested for HIV at a drug treatment facility were more likely to be vaccinated (OR: 1.73) than IDUs not being tested.Citation77 A fifth study reported lower uptake of the HPV vaccine (15.3%) among females in New Orleans.Citation78
Barriers to vaccination
No publications reported on the barriers to vaccination among IDUs.
Men who have sex with men
Seventeen studies reported vaccine uptake for HBV, HPV, and meningococcal vaccines among MSM. Among these, HBV vaccine uptake estimates ranged from 14.0% to 50.5%.Citation68,Citation77,Citation85–89 Conversely, HPV vaccine uptake ranged from 13.0% to 39.8% among individuals completing all three doses of the vaccination series in two separate 2015 national studies.Citation90,Citation91 However, a 2019 study of MSM in Philadelphia reported that only 7.0% of the study population (N = 5,329) received all three vaccine doses.Citation92 Furthermore, a 2016 study (N = 336) reported that HIV-positive MSM were more than twice as likely to be vaccinated compared to HIV-negative men.Citation93
A 2016 study evaluating both HPV and HBV vaccine uptake among MSM in New Orleans (N = 358) reported higher HBV uptake compared to HPV among males (28% versus 14.9%).Citation78 Among MSM, HPV vaccine uptake was associated with having been tested for a sexually transmitted disease (STD) in the past 12 months (p = .019) and having ever received a hepatitis vaccine (p = .004).Citation78 Other sociodemographic factors, such as education, sexual identity and having health insurance, were not significantly associated with HPV vaccine uptake, and only 22.9% of individuals received both HPV and HBV vaccines.Citation78
Meningococcal vaccine uptake estimates ranged from 17.0% to 51.3%.Citation94–96 A 2018 cross-sectional survey in California reported that vaccine uptake was higher among HIV-positive versus HIV-negative MSM overall, and in the previous 6 months (82.6% versus 51.3% in the previous 6 months).Citation96
Barriers to vaccination
Lack of insurance, education and provider recommendation were identified as barriers to vaccination.Citation66,Citation77,Citation91,Citation93,Citation97 A 2012 nationwide survey reported that individuals who reported being unable to see a doctor because of cost were less likely to be vaccinated against HBV (OR: 0.77, 95% CI: 0.60–0.98).Citation77 Data collected through the National Health and Nutrition Examination Survey (NHANES) survey also identified lack of insurance as a significant negative predictor for vaccination (p < .001).Citation66
In a 2016 study (N = 336), provider recommendation predicted vaccine uptake; individuals who received a recommendation for HPV vaccine were >40 times more likely to have been vaccinated than those without.Citation93
A lack of education regarding HPV vaccination may be a driver of low vaccine uptake among MSM.Citation93,Citation97 A 2013 study reported limited awareness as a reason for being unvaccinated: not knowing that males are allowed to get the HPV vaccination (17%), not having heard of the HPV vaccine (12%) and not knowing enough about the HPV vaccine yet (11%).Citation91
Importantly, many MSM did not disclose their sexuality to their insurance provider or at medical consultations, with one study reporting that 29% (N = 28) of unvaccinated MSM who were not planning on being vaccinated stated that their provider did not know their sexual identity.Citation93 Furthermore, 41% (N = 29) of men who were undecided about receiving vaccination stated that their provider was not aware of their sexuality, indicating an important proportion of at-risk individuals who are therefore not targeted for vaccination recommendation.Citation95
Pregnant women
Fifty-three studies reported on vaccine uptake among pregnant women, including influenza vaccination (N = 31), Tdap vaccination (N = 12) and both influenza and Tdap (N = 8). Influenza vaccine uptake ranged from 35.6% to 75.0%.Citation13,Citation40,Citation98–108 The highest overall uptake (75.0%) was reported in a 2018 study of expectant parents in Texas (N = 638).Citation98 Conversely, the lowest (35.6%) was reported in a 2017 nationwide study of pregnant women (N = 2,254).Citation99 Notably, vaccine uptake differed between self-reported and medical record reported uptake, with one study highlighting a clear disparity between the two (self-reported: 71% versus medical record uptake: 36.1%).Citation13
Studies investigating uptake over time typically reported that vaccine uptake has typically increased in recent years ( and ).Citation23,Citation58,Citation109–112 However, a 2016 study of pregnant women in Georgia (N = 8,300) reported that the proportion of women not receiving influenza vaccination had increased from 11.1% in 2004 to 35.8% in 2011.Citation113
Figure 4. Influenza vaccine uptake among pregnant women, by year.Citation97–99,Citation102,Citation107,Citation112,Citation113*
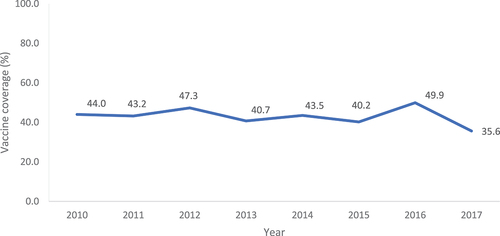
Figure 5. Tdap vaccine uptake among pregnant women, by year.Citation107*
The annual CDC reports on influenza and Tdap vaccine uptake consistently identified that older pregnant women had higher rates of influenza vaccine uptake compared to younger women.Citation99–102,Citation111
Overall, Tdap uptake ranged from 14.3% to 58.2%.Citation40,Citation114–121 Similarly to influenza, Tdap uptake among pregnant women has increased in recent years, with a 2017 study (N = 5,606) reporting an increase from <1% (2006–2009) to 54% in 2015.Citation109
Among the eight identified studies reporting on both influenza and Tdap vaccine uptake, Tdap uptake was typically higher than influenza uptake among pregnant women (Tdap: 44.0% to 65.0%; influenza 34.0% to 63.0%).Citation17,Citation102,Citation122–124
Of two studies reporting on hepatitis vaccine uptake in pregnant women,Citation40,Citation45 a 2013 study reported HAV vaccine uptake to be 29.2% among pregnant women in Boston.Citation40 A 2018 study evaluating the difference in HBV vaccine uptake reported this to be higher among US-born versus foreign-born women (45.1% versus 27.6%).Citation45
Barriers to vaccination
Concerns of vaccine safety and effectiveness and lack of provider recommendation and education/guidelines were identified as vaccination barriers.Citation13,Citation17,Citation23,Citation58,Citation99–102,Citation104–108,Citation110,Citation112,Citation113,Citation117,Citation118,Citation123–134 A yearly nationwide survey of influenza vaccination among pregnant women reported that concerns about becoming ill from the vaccine ranged from 10.4% to 21.3% between 2011–2017.Citation99–101,Citation104,Citation129,Citation130 Notably, results from the Pregnancy Risk Assessment Monitoring System (PRAMS) survey highlighted that women who had low perceptions of influenza vaccine safety were significantly less likely to intend to receive an influenza vaccine (48% vs. 20%; p < .001) or a Tdap vaccine (53% vs. 33%; p < .001) during their current pregnancy compared to women who perceived the vaccine as safe.Citation127 Furthermore, a 2020 survey identified that first-time pregnant women were also more likely to be uncertain about maternal vaccines compared to women with prior children (8% vs. 19%, p < .01).Citation124
Concerns over the safety of the vaccine for both the mother and their baby was a commonly cited barrier against vaccination. During a study of the 2009–2010 vaccine season by the CDC, among those who did not get vaccinated for influenza (N = 2,994), 45.2% women cited they were worried about side effects for themselves and 47.7% were worried the vaccine would harm the baby.Citation125 Similarly, of those who did not get the H1N1 vaccination, the majority were worried the vaccine would harm themselves (61.4%) or their baby (63.6%).Citation125
Lack of provider recommendation was particularly evident among pregnant women, with a 2010 CDC study using PRAMS data reporting that 31.4%/24.8% of pregnant women did not receive the seasonal influenza vaccination/H1N1 vaccination, respectively, because their physician did not recommend it.Citation125 In a 2012 study investigating PH1N1 vaccine uptake among women during pregnancy (N = 4,205), 9.6% of individuals were not vaccinated due to a lack of recommendation from their provider.Citation128 Conversely, influenza vaccine uptake among pregnant women was highest among women who reported their doctor or other medical professional recommended and offered the vaccination compared to a recommendation but no offer and no recommendation or offer at all.Citation99,Citation100,Citation113 A medical provider’s recommendation also increased the likelihood of accepting the vaccine by 2.6 (p < .01).Citation13
In a 2018 nationwide internet survey (N = 2,236), the most common reason for not receiving Tdap during pregnancy was a lack of knowledge about the need to be vaccinated during every pregnancy (45.1%): 31.6% of women who did not receive vaccine during pregnancy reported having been vaccinated previously, and 13.5% reported not knowing they were supposed to receive Tdap during their recent pregnancy.Citation123
Discussion
This review identified that, overall, vaccine uptake has increased across at-risk populations over the past 10 years. Despite this, uptake among at-risk adults was typically below the Healthy People 2020 goal of 60% uptake.Citation135 Several barriers to vaccination were commonly reported across populations; including, structural barriers such as cost of vaccination and lack of health insurance, and patient-centered barriers such as concerns regarding vaccine safety and lack of provider recommendation. However, there were limited data reporting on barriers to vaccination among several at-risk populations, including individuals with CLD, ESRD, and IDUs.
Studies have demonstrated that a lack of health insurance coverage and high costs can be an obstacle to vaccination, resulting in a high burden of VPDs among at-risk populations.Citation68,Citation72,Citation77 There is evidence that vaccine uptake increases when cost is removed as a barrier; therefore, it is important that there is support from State or Federal policymakers to remove these financial barriers.Citation15,Citation136,Citation137 The Affordable Care Act (ACA) mandates that ACIP-recommended vaccines are covered by private insurance plans without a copay, therefore reducing the financial barrier to insured at-risk adults receiving vaccines.Citation138 However, the ACA does not require State Medicaid agencies to cover all ACIP recommended vaccines for individuals on Medicaid, resulting in a disparity of coverage compared to individuals with private insurance.Citation138 This implies that the cost of vaccination may not be the main barrier to vaccination; however, vaccination cost barriers need to be considered in the context of the potential lack of private insurance among at-risk individuals and a reliance on Medicaid coverage.Citation15,Citation136–138
In order to support effective life-course immunization, it is imperative that HCWs are equipped with the guidelines, training, and tools to provide individualized, patient-centered care. This includes open discussions with patients about the availability of vaccination and the eligibility to receive vaccination.Citation139 Additionally, these data emphasize the importance of educating individuals about their increased risk of VPDs and publicly promoting the availability of vaccines to contribute in mitigating this risk.Citation139 Integrating vaccination with other primary healthcare systems, health registers, and notification systems will minimize missed vaccination encounters.Citation1 Moreover, telephone and digital reminders to vaccine-eligible adults have proven effective in reducing missed opportunities for vaccination, whilst also benefiting pediatric vaccine coverage.Citation1,Citation140
An important element of life-course immunization is continued education of the public and HCWs to highlight the risk of VPDs and emphasize the important role of vaccination.Citation2 In order to overcome barriers related to vaccine safety and effectiveness, wider vaccine education needs to be implemented throughout an individual’s lifetime to reduce vaccine complacency.Citation2,Citation7 The wider literature supports the need for more vaccine education materials, outlining facts about vaccines and the diseases they prevent, common misconceptions, and the risks of not getting vaccinated, as key education topics for individuals.Citation2–4,Citation7,Citation135 Studies have indicated that sessions delivering educational interventions greatly increased vaccine knowledge among target groups, such as HCWs, resulting in increased support for vaccination.Citation141 This review has identified the need for clear and consistent guidelines for HCWs, since HCW attitude toward vaccination has been recognized as one of the strongest influences on the decision-making of patients.Citation7 This is particularly important since there were mixed perceptions and a lack of knowledge around vaccination among HCWs, with some citing safety concerns as a reason for not recommending vaccines for pregnant women.Citation13
Life-course immunization policies, such as the WHO Immunization Agenda 2030, focusing on increasing awareness regarding the health benefits of vaccination, together with cross-sector collaboration to prioritize vaccination, will improve vaccine uptake and reduce the burden of VPDs.Citation1,Citation2,Citation4,Citation142 Focused polices and integrated vaccination in public and private payer access programs will promote increased uptake of adult vaccination. It is critical that key stakeholders, including HCWs, are engaged with vaccination policies and guidelines to ensure successful uptake of life-course immunization and reduce missed opportunities for vaccination.Citation1,Citation2,Citation4,Citation142
Strengths and limitations
This comprehensive review applied a wide timeframe to ensure all key publications and any key updates in this research area were captured, such as updates to ACIP recommendations and changes to vaccination receipt.
There are several limitations to consider. Many (N = 44) of the identified studies reporting on vaccine uptake were surveys that relied on self-reported individuals’ vaccination history, which may have led to recall bias and a potential over- or underestimation of vaccine uptake. Despite potential biases in the estimated vaccine uptake, this literature review identified vaccine uptake data among several at-risk populations. Additionally, it should be noted that direct comparisons cannot be made between vaccine uptake rates due to the heterogeneity in sampling methods and study populations. Finally, the search was limited to the English Language; it may be possible that further evidence is published in other languages. However, this is a minor limitation, given the review was US-focused.
Conclusions
Despite the substantial burden that VPDs confer on both individuals and healthcare systems, vaccine uptake is suboptimal and remains below the Healthy People 2020 goals across many adult at-risk populations. This suboptimal vaccine uptake facilitates the continued burden of VPDs and therefore reinforces the importance of life-course immunization.
This literature review identified several key barriers to vaccination, including concerns regarding perceived vaccine safety and effectiveness, cost of vaccination, and lack of insurance and provider recommendation. These barriers represent missed opportunities for vaccination of individuals in at-risk populations and emphasize the importance of continued education, clear guidelines, and policies to promote higher vaccine uptake. Embracing a national life-course immunization program provides opportunities to improve immunization policies and increases attention to the importance of vaccines throughout life.
Future research needs to focus on identifying the barriers to vaccination among these populations, in order to appropriately target individuals for vaccination and improve suboptimal vaccine uptake.
Author contributions
IK, MKN, MOB, AK, DB, and IF contributed to study conception and study design. All authors contributed to the writing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Supplemental Material
Download Zip (944.3 KB)Disclosure statement
IK, MKN and MOB are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may own stocks and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. AK, DB, and IF are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ was compensated by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA for the conduct of the study and development of the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2055422.
Additional information
Funding
References
- World Health Organization. Immunization agenda 2030: a global strategy to leave no one behind. 2020.
- Health Policy Partnership. A life-course approach to vaccination: adapting European policies. 2018.
- Tate J, Aguado T, Belie JD, Holt D, Karafillakis E, Larson HJ, Nye S, Salisbury D, Votta M, Wait S, et al. The life-course approach to vaccination: harnessing the benefits of vaccination throughout life. Vaccine. 2019;37(44):6581–13. doi:10.1016/j.vaccine.2019.09.016.
- Global Coalition on Aging. Life-course immunization: a driver of healthy aging. 2018.
- Rémy V, Zöllner Y, Heckmann U. Vaccination: the cornerstone of an efficient healthcare system. J Mark Access Health Policy. 2015:3. doi:10.3402/jmahp.v3403.27041.
- Centers for Disease Control and Prevention. Immunization schedules: recommended adult immunization schedule for ages 19 years or older, United States, 2021. Published 2021 [ accessed 2022 Jan 28th]. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
- Philip RK, Attwell K, Breuer T, Di Pasquale A, Lopalco PL. Life-Course immunization as a gateway to health. Expert Rev Vaccines. 2018;17(10):851–64. doi:10.1080/14760584.2018.1527690.
- Zhang D, Petigara T, Yang X. Clinical and economic burden of pneumococcal disease in US adults aged 19-64 years with chronic or immunocompromising diseases: an observational database study. BMC Infect Dis. 2018;18(1):436. doi:10.1186/s12879-018-3326-z.
- Caban-Martinez AJ, Lee DJ, Davila EP, LeBlanc WG, Arheart KL, McCollister KE, Christ SL, Clarke T, Fleming LE. Sustained low influenza vaccination rates in US healthcare workers. Prev Med. 2010;50(4):210–12. doi:10.1016/j.ypmed.2010.01.001.
- Calabria CL, Vignari M, Yamshchikov A. Impact of patient safety and legislative standards on healthcare worker vaccination rates: a single center experience. Am J Infect Control. 2014;42(6 SUPPL. 1):S164. doi:10.1016/j.ajic.2014.03.348.
- Control CfD, Prevention. Influenza vaccination coverage among health-care personnel: 2011-12 influenza season, United States. MMWR Morb Mortal Wkly Rep 2012;61:753.
- Jegede O, Escamilla A, James F, Patton M, Kaye K, Chopra T. Implementing mandatory influenza vaccination policy for health care workers at a long term acute care facility. Am J Infect Control. 2012;40(5):e102–e103. doi:10.1016/j.ajic.2012.04.176.
- Stark LM, Power ML, Turrentine M, Samelson R, Siddiqui MM, Paglia MJ, Strassberg ER, Kelly E, Murtough KL, Schulkin J, et al. Influenza vaccination among pregnant women: patient beliefs and medical provider practices. Infect Dis Obstet Gynecol. 2016;2016:3281975. doi:10.1155/2016/3281975.
- Wagner S, Winston L, Chan SB. Health care workers and the mandated H1N1 vaccine: a survey to assess employee perception. Acad Emerg Med. 2011;18:S220–S221.
- Black CL, Yue X, Ball SW, Donahue SMA, Izrael D, de Perio MA, Laney AS, Williams WW, Lindley MC, Graitcer SB, et al. Influenza vaccination coverage among health care personnel — United States, 2014–15 influenza season. Morb Mortal Wkly Rep. 2015;64(36):993–99. doi:10.15585/mmwr.mm6436a1.
- Prevention CfDCa. Influenza vaccination coverage among health care personnel — United States, 2019–20 influenza season. 2020.
- Lindley MC, Kahn KE, Bardenheier BH, D’-Angelo DV, Dawood FS, Fink RV, Havers F, Skoff TH. Vital signs : burden and prevention of influenza and pertussis among pregnant women and infants — United States. Morb Mortal Wkly Rep. 2019;68(40):885. doi:10.15585/mmwr.mm6840e1.
- Black CL, Xin Yue M, Ball SW, Fink R, de Perio MA, Laney AS, Williams WW, Lindley MC, Graitcer SB, Lu P-J, et al. Influenza vaccination coverage among health care personnel — United States, 2016–17 influenza season. MMWR Morb Mortal Wkly Rep. 2017;66(38):1009. doi:10.15585/mmwr.mm6638a1.
- Black CL, Yue X, Ball SW, Donahue SMA, Izrael D, de Perio MA, Laney AS, Williams WW, Lindley MC, Graitcer SB, et al. Influenza vaccination coverage among health care personnel — United States, 2015–16 influenza season. Morb Mortal Wkly Rep. 2016;65(38):1026–31. doi:10.15585/mmwr.mm6538a2.
- Black CL, Yue X, Ball SW, et al. Influenza vaccination coverage among health care personnel—United States, 2013–14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(37):805.
- Zhang L, Wang Y, Peng M, She Q, Xiang Q, Chen Q, Liu Z, Zhang W, Tao N, Qiu L, et al. Prevalence and type distribution of high-risk human papillomavirus infections among women in Wufeng County, China. Arch Gynecol Obstet. 2012;286(3):695–99. doi:10.1007/s00404-012-2344-0.
- Lindley MC, Zhang J, Euler GL. Health care personnel flu vaccination–Internet panel survey, United States, November 2011. Health Care (Don Mills). 2008;2007:2006–07.
- Panda B, Stiller R, Panda A. Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Maternal-Fetal Neonatal Med. 2011;24(3):402–06. doi:10.3109/14767058.2010.497882.
- Centers for Disease Control and Prevention. Influenza vaccination coverage among Health care personnel — United States, 2018–19 influenza season. 2019. Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Health care personnel and flu vaccination, Internet panel survey, United States, November 2017. 2018. Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Health care personnel and flu vaccination, Internet panel survey, United States, November 2016. 2017. Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Health care personnel and flu vaccination, Internet panel survey, United States, November 2015. 2016. Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Health care personnel and flu vaccination, Internet panel survey, United States, November 2014. 2015. Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Health care personnel and flu vaccination Internet panel survey, United States, November 2013. 2014. Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Health Care Personnel Flu Vaccination Internet Panel Survey, United States, November 2012. 2013. Centers for Disease Control and Prevention.
- Gornick W, Singh J, Santos N. Does tdap (Tetanus, diphtheria acellular pertussis) vaccine compliance increase with “mandatory” healthcare worker (HCW) influenza immunization (FluImm) program? Am J Infect Control. 2010;38:E59–E60.
- Gornick W, Santos N, Patel B, Singh J. Healthcare worker (HCW) pertussis (Tdap) vaccine compliance improves during a statewide pertussis epidemic. Am J Infect Control. 2012;40(5):e73. doi:10.1016/j.ajic.2012.04.130.
- Gollapudi LA, Jolly G, Kar K, Dhand A. Knowledge, attitute and practice survey regarding pertussis vaccination among transplant health care workers. Open Forum Infect Dis. 2017;4(Supplement 1):S517–S518. doi:10.1093/ofid/ofx163.1345.
- Elsaid MI, Moyer RN, Narayanan N, Li Y, John T, Rustgi VK. Hepatitis B vaccination coverage among native Hawaiian and pacific islanders: a population study of United States (US) adults. Hepatology. 2019;70(Suppl 1):583A–4A.
- Guturu P, Cicalese L, Duchini A. Hepatitis a vaccination in healthcare personnel. Ann Hepatol. 2012;11(3):326–29. doi:10.1016/S1665-2681(19)30927-5.
- Yanni EA, Marano N, Han P, Edelson PJ, Blumensaadt S, Becker M, Dwyer S, Crocker K, Daley T, Davis X, et al. Knowledge, attitudes, and practices of US travelers to Asia regarding seasonal influenza and H5N1 avian influenza prevention measures. J Travel Med. 2010;17(6):374–81. doi:10.1111/j.1708-8305.2010.00458.x.
- Hyle EP, Rao SR, Jentes ES, Parker Fiebelkorn A, Hagmann SHF, Taylor Walker A, Walensky RP, Ryan ET, LaRocque RC. Missed opportunities for measles, mumps, rubella vaccination among departing U.S. adult travelers receiving pretravel health consultations. Ann Intern Med. 2017;167(2):77–84. doi:10.7326/M16-2249.
- Hyle EP, Fields NF, Fiebelkorn AP, Walker AT, Gastañaduy P, Rao SR, Ryan ET, LaRocque RC, Walensky RP. The clinical impact and cost-effectiveness of measles-mumps-Rubella vaccination to prevent measles importations among international travelers from the United States. Clin Infect Dis. 2019;69(2):306–15. doi:10.1093/cid/ciy861.
- Williams WW, Lu P-J, O’-Halloran A, et al. Surveillance of vaccination coverage among adult populations—United States, 2014. Morb Mortal Wkly Rep. 2016;65(1):1–36.
- Hochberg NS, Barnett ED, Chen LH, Wilson ME, Iyer H, MacLeod WB, Yanni E, Jentes ES, Karchmer AW, Ooi W, et al. International travel by persons with medical comorbidities: Understanding risks and providing advice. Mayo Clin Proc. 2013;88(11):1231–40.
- Tian C, Ding X, Wang H, Wang W, Luo X. Characteristics associated with hepatitis B vaccination initiation and completion among adults traveling to a country of high or intermediate endemicity. Am J Infect Control. 2019;47(8):883–88. doi:10.1016/j.ajic.2019.02.014.
- Lu P-J, Byrd KK, Murphy TV. Hepatitis a vaccination coverage among adults 18-49 years traveling to a country of high or intermediate endemicity, United States. Vaccine. 2013;31(19):2348–57. doi:10.1016/j.vaccine.2013.03.011.
- Ha E, Kim F, Pan J, Hong DTP, Juon HS. Prevalence of viral hepatitis B and C infection among immigrants in the Baltimore-Washington metropolitan area screened from 2009-2014. Cancer Epidemiol Biomark Prev. 2016;25(Supplement 3):B37.
- Mishra AC. Emerging viral infections in 21st century and their implications on health system. Indian J Virol. 2013;24:99–100.
- Kilmer GA, Barker vK, Ly KN, Jiles RB. Hepatitis B vaccination and screening among foreign-born women of reproductive age in the United States: 2013-2015. Clin Infect Dis. 2019;68(2):256–65. doi:10.1093/cid/ciy479.
- Levy V, Yuan J, Ruiz J, Morrow S, Reardon J, Facer M, Molitor F, Allen B, Ajufo BG, Bell-Sanford G, et al. Hepatitis B sero-prevalence and risk behaviors among immigrant men in a population-based household survey in low-income neighborhoods of northern California. J Immigr Minor Health/center Minor Public Health. 2010;12(6):828–33. doi:10.1007/s10903-009-9239-6.
- Ogunwobi OO, Dibba O, Zhu L, Ilboudo A, Tan Y, Fraser MA, Ma GX. Hepatitis B virus screening and vaccination in first-generation African immigrants: a pilot study. J Community Health. 2019;44(6):1037–43. doi:10.1007/s10900-019-00668-z.
- Shaleh HM, Giama NH, Mohamed EA, et al. A cross-sectional assessment of knowledge, attitudes, and behaviors about viral hepatitis and hepatocellular carcinoma among Kenyan and Liberian immigrants living in Minnesota. Cancer Epidemiol Biomark Prev. 2016;25(Supplement 3):A46.
- Xiong M, Nguyen RHN, Strayer L, Chanthanouvong S, Yuan J-M. Knowledge and behaviors toward hepatitis B and the hepatitis B vaccine in the Laotian community in Minnesota. J Immigr Minor Health. 2013;15(4):771–78. doi:10.1007/s10903-012-9768-2.
- Bhattacharya M, Reiter PL, McRee AL. Nativity status and genital HPV infection among adults in the U.S. Hum Vaccin Immunother. 2019;15(7–8):1897–903. doi:10.1080/21645515.2019.1578592.
- Dallo FJ, Kindratt TB. Disparities in vaccinations and cancer screening among U.S.- and foreign-born Arab and European American non-Hispanic White women. Women’s Health Issues. 2015;25(1):56–62. doi:10.1016/j.whi.2014.10.002.
- Younossi ZM, Stepanova M. Changes in hepatitis a and B vaccination rates in adult patients with chronic liver diseases and diabetes in the U.S. population. Hepatology (Baltimore, Md). 2011;54(4):1167–78. doi:10.1002/hep.24510.
- Koenig A, Stepanova M, Felix S, Kalwaney S, Clement S, Younossi ZM. Vaccination against hepatitis a and B in patients with chronic liver disease and type 2 diabetes: has anything changed? Liver Int. 2016;36(8):1096–100. doi:10.1111/liv.13164.
- Annunziata K, Rak A, Del Buono H, DiBonaventura M, Krishnarajah G, Xu J. Vaccination rates among the general adult population and high-risk groups in the United States. PLoS ONE. 2012;7(11):e50553. doi:10.1371/journal.pone.0050553.
- Bluml BM, Watson LL, Skelton JB, Manolakis PG, Brock KA. Improving outcomes for diverse populations disproportionately affected by diabetes: final results of project IMPACT: diabetes. J Am Pharm Assoc. 2014;54(5):477–85. doi:10.1331/JAPhA.2014.13240.
- Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-Effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21. doi:10.1016/j.vaccine.2013.10.024.
- Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: Protecting the vulnerable. Vaccine. 2016;34(52):6681–90. doi:10.1016/j.vaccine.2016.11.015.
- Lu P, Santibanez TA, Williams WW, et al. Surveillance of influenza vaccination coverage–United States, 2007-08 through 2011-12 influenza seasons. Morb Mortal Wkly Rep Surveill Summ (Washington, DC : 2002). 2013;62(4):1–28.
- O’-Halloran AC, Lu PJ, Williams WW, Bridges CB, Singleton JA. Influenza vaccination coverage among people with high-risk conditions in the U.S. Am J Prev Med. 2016;50(1):e15–e26. doi:10.1016/j.amepre.2015.06.008.
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol. 2011;6(5):1192–97. doi:10.2215/CJN.05430610.
- Ishigami J, Sang Y, Grams ME, Coresh J, Chang A, Matsushita K. Effectiveness of influenza vaccination among older adults across kidney function: pooled analysis of 2005-2006 through 2014-2015 influenza seasons. Am J Kidney Dis. 2020;75(6):887–96. doi:10.1053/j.ajkd.2019.09.008.
- Lee DH, Boyle SM, Malat G, Sharma A, Bias T, Doyle AM. Low rates of vaccination in listed kidney transplant candidates. Transpl Infect Dis. 2016;18(1):155–59. doi:10.1111/tid.12473.
- Gilbertson DT, Guo H, Arneson TJ, Collins AJ. The association of pneumococcal vaccination with hospitalization and mortality in hemodialysis patients. Nephrol Dial Transplant. 2011;26(9):2934–39. doi:10.1093/ndt/gfq853.
- McGrath LJ, Layton JB, Krueger WS, Kshirsagar AV, Butler AM. High-Dose influenza vaccine use among patients receiving hemodialysis in the United States, 2010-2013. Vaccine. 2018;36(41):6087–94. doi:10.1016/j.vaccine.2018.08.079.
- Bond TC, Spaulding AC, Krisher J, McClellan W. Mortality of dialysis patients according to influenza and pneumococcal vaccination status. Am J Kidney Dis. 2012;60(6):959–65. doi:10.1053/j.ajkd.2012.04.018.
- Devaki P, Wong R, Nguyen LH, et al. Suboptimal vaccination rates for hepatitis B virus (HBV) among U.S. adults at high risk for HBV infection in National Health and Nutrition Examination Survey (NHANES) from 2001-2010. Hepatology. 2013;58(4 SUPPL. 1):611A–2A.
- Le AK, Toy M, Yang HI, et al. Age and gender-specific disease progression rates to cirrhosis and hepatocellular carcinoma (HCC) in treated and untreated patients with chronic hepatitis B. Hepatology. 2017;66(Supplement 1):994A.
- Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794–804. doi:10.7326/M17-1106.
- Buskin SE, Barash EA, Scott JD, Aboulafa DM, Wood RW. Hepatitis B and C infection and liver disease trends among human immunodeficiency virus-infected individuals. World J Gastroenterol. 2011;17(14):1807–16. doi:10.3748/wjg.v17.i14.1807.
- Chun HM, Mesner O, Thio CL, Bebu I, Macalino G, Agan BK, Bradley WP, Malia J, Peel SA, Jagodzinski LL, et al. HIV outcomes in hepatitis B virus coinfected individuals on HAART. JAIDS J Acquir Immune Defic Syndr. 2014;66(2):197–205. doi:10.1097/QAI.0000000000000142.
- Landrum ML, Hullsiek KH, Chun HM, Crum-Cianflone NF, Ganesan A, Weintrob AC, Barthel RV, O’Connell RJ, Agan BK. The timing of hepatitis B virus (HBV) immunization relative to human immunodeficiency virus (HIV) diagnosis and the risk of HBV infection following HIV diagnosis. Am J Epidemiol. 2011;173(1):84–93. doi:10.1093/aje/kwq326.
- Weiser J, Perez A, Bradley H, King H, Shouse RL. Low prevalence of hepatitis B vaccination among patients receiving medical care for HIV infection in the United States, 2009 to 2012. Ann Intern Med. 2018;168(4):245–54. doi:10.7326/M17-1689.
- Althoff KN, Anastos K, Nelson KE, Celentano DD, Sharp GB, Greenblatt RM, French AL, Diamond DJ, Holman S, Young M, et al. Predictors of reported influenza vaccination in HIV-infected women in the United States, 2006–2007 and 2007–2008 seasons. Prev Med. 2010;50(5–6):223–29. doi:10.1016/j.ypmed.2010.03.007.
- Durham MD, Buchacz K, Armon C, Patel P, Wood K, Brooks JT. Rates and correlates of influenza vaccination among HIV-infected adults in the HIV outpatient study (HOPS), USA, 1999-2008. Prev Med. 2011;53(1–2):89–94. doi:10.1016/j.ypmed.2011.04.015.
- Nduaguba SO, Barner JC, Ford KH, Lawson KA, Barnes JN, Wilson JP. Association between quality-of-care indicators for HIV infection and healthcare resource utilization and costs. AIDS. 2020;34(2):291–300. doi:10.1097/QAD.0000000000002418.
- Marcus JL, Baxter R, Leyden WA, Muthulingam D, Yee A, Horberg MA, Klein DB, Towner WJ, Chao CR, Quesenberry CP, et al. Invasive pneumococcal disease among HIV-infected and HIV-uninfected adults in a large integrated healthcare system. AIDS Patient Care STDS. 2016;30(10):463–70. doi:10.1089/apc.2016.0165.
- Ladak F, Gjelsvik A, Feller E, Rosenthal S, Montague BT. Hepatitis B in the United States: ongoing missed opportunities for hepatitis B vaccination, evidence from the behavioral risk factor surveillance survey, 2007. Infection. 2012;40(4):405–13. doi:10.1007/s15010-011-0241-2.
- Fisher KA, Cahill L, Tseng T-S, Robinson WT. HPV vaccination coverage and disparities among three populations at increased risk for HIV. Transl Cancer Res. 2016;5(S5):S1000–S1006. doi:10.21037/tcr.2016.10.66.
- Akivis Y, Leong J, Rosenstein I, Myrie A, Moy M, Markell M. Vaccination rates and relation to health beliefs in inner-city kidney transplant recipients (KTRS). Am J Transpl. 2019;19:390.
- Dhar JP, Essenmacher L, Dhar R, Ragina N, Sokol RJ. Lack of uptake of prophylactic human Papilloma virus vaccine among women with systemic Lupus Erythematosus seen at a regional medical center. J Clin Rheumatol. 2019;25(8):348–50. doi:10.1097/RHU.0000000000000866.
- Lu P-J, Gonzalez-Feliciano A, Ding H, Bryan LN, Yankey D, Monsell EA, Greby SM, Euler GL. Influenza a (H1N1) 2009 monovalent and seasonal influenza vaccination among adults 25 to 64 years of age with high-risk conditions—United States, 2010. Am J Infect Control. 2013;41(8):702–09. doi:10.1016/j.ajic.2012.10.027.
- Lu PJ, O’-Halloran A, Ding H, Srivastav A, Williams WW. Uptake of influenza vaccination and missed opportunities among adults with high-risk conditions, United States, 2013. Am J Med. 2016;129(6). 636e631-636e611. doi:10.1016/j.amjmed.2015.10.031.
- Vietri J, DiBonaventura MD, Chilson E, Malhotra D, Emir B. Lack of recommended pneumococcal conjugate vaccine uptake among gastroenterology patients at high risk. Gastroenterology. 2019;156(6 Supplement 1). S-253. doi:10.1016/S0016-5085(19)37437-2.
- Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16:182. doi:10.1186/s12913-016-1432-4.
- Le MH, Toy M, Gane E, So S, Nguyen MH. Trends in prevalence and predictors of hepatitis B virus vaccination in the general United States population from 1999-2014. Gastroenterology. 2017;152(5 Supplement 1):S1052. doi:10.1016/S0016-5085(17)33557-6.
- Yin S, Barker L, Ly KN, Kilmer G, Foster MA, Drobeniuc J, Jiles RB. Susceptibility to hepatitis A virus infection in the United States, 2007–2016. Clin Infect Dis. 2020;71(10):e571–e579. doi:10.1093/cid/ciaa298.
- Metheny N, Stephenson R. Disclosure of sexual orientation and uptake of HIV testing and hepatitis vaccination for rural men who have sex with men. Ann Fam Med. 2016;14(2):155–58. doi:10.1370/afm.1907.
- Hoover KW, Butler M, Workowski KA, Follansbee S, Gratzer B, Hare CB, Johnston B, Theodore JL, Tao G, Smith BD, et al. Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2012;39(5):349–53. doi:10.1097/OLQ.0b013e318244a923.
- Meites E, Markowitz LE, Paz-Bailey G, Oster AM, Group NS. HPV vaccine coverage among men who have sex with men - National HIV behavioral surveillance system, United States, 2011. Vaccine. 2014;32(48):6356–59. doi:10.1016/j.vaccine.2014.09.033.
- Cummings T, Kasting ML, Rosenberger JG, Rosenthal SL, Zimet GD, Stupiansky NW. Catching up or missing out? Human Papillomavirus vaccine acceptability among 18- to 26-year-old men who have sex with men in a US National Sample. Sex Transm Dis. 2015;42(11):601–06. doi:10.1097/OLQ.0000000000000358.
- Reiter PL, Katz ML, Ruffin MT, Hade EM, DeGraffenreid CR, Patel DA, Paskett ED, Unger ER. HPV prevalence among women from Appalachia: results from the CARE project. PLoS ONE. 2013;8(8):e74276. doi:10.1371/journal.pone.0074276.
- Goldstein ND, LeVasseur MT, Tran NK, Purtle J, Welles SL, Eppes SC. Modeling HPV vaccination scale-up among urban young men who have sex with men in the context of HIV. Vaccine. 2019;37(29):3883–91. doi:10.1016/j.vaccine.2019.05.047.
- Gerend MA, Madkins K, Phillips G 2nd, Mustanski B. Predictors of human papillomavirus vaccination among young men who have sex with men. Sex Transm Dis. 2016;43(3):185–91. doi:10.1097/OLQ.0000000000000408.
- Simon MS, Weiss D, Geevarughese A, Kratz MM, Cutler B, Gulick RM, Zucker JR, Varma JK, Schackman BR. Cost-Effectiveness of meningococcal vaccination among men who have sex with men in New York City. J Acquir Immune Defic Syndr. 2016;71(2):146–54. doi:10.1097/QAI.0000000000000822.
- Frew PM, Holloway IW, Goldbeck C, Tan D, Wu E, Jauregui J, Fenimore VL, Randall LA, Lutz CS, Mendel J, et al. Development of a measure to assess vaccine confidence among men who have sex with men. Expert Rev Vaccines. 2018;17(11):1053–61. doi:10.1080/14760584.2018.1541405.
- Holloway IW, Wu ESC, Gildner J, Fenimore VL, Tan D, Randall L, Frew PM. Quadrivalent meningococcal vaccine uptake among men who have sex with men during a meningococcal outbreak in Los angeles County, California, 2016-2017. Public Health Rep. 2018;133(5):559–69. doi:10.1177/0033354918781085.
- Halkitis PN, Valera P, LoSchiavo CE, et al. Human papillomavirus vaccination and infection in young sexual minority men: the P18 cohort study. AIDS Patient Care STDS. 2019;33(4):149–56. doi:10.1089/apc.2018.0276.
- Cunningham RM, Minard CG, Guffey D, Swaim LS, Opel DJ, Boom JA. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad Pediatr. 2018;18(2):154–60. doi:10.1016/j.acap.2017.08.003.
- Ding H, Black CL, Ball S, Fink RV, Williams WW, Fiebelkorn AP, Lu P-J, Kahn KE, D’-Angelo DV, Devlin R, et al. Influenza vaccination coverage among pregnant women — United States, 2016–17 influenza season. MMWR Morb Mortal Wkly Rep. 2017;66(38):1016–22. doi:10.15585/mmwr.mm6638a2.
- Ding H. Pregnant Women and Flu Vaccination, Internet Panel Survey, United States, November 2016. CDC. 2016.
- Ding H, Black CL, Ball S, Donahue S, Fink RV, Williams WW, Kennedy ED, Bridges CB, Lu P-J, Kahn KE, et al. Influenza Vaccination Coverage Among Pregnant Women — United States, 2014–15 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64(36):1000–05. doi:10.15585/mmwr.mm6436a2.
- Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, Cortés M, Cota P, Whitney EAS, Flowers LC, et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: a Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. PLoS Curr. 2015;7. doi:10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819.
- Scheminske M, Henninger M, Irving SA, Thompson M, Williams J, Shifflett P, Ball SW, Avalos LA, Naleway AL. The association between influenza vaccination and other preventative health behaviors in a cohort of pregnant women. Health Educ Behav. 2015;42(3):402–08. doi:10.1177/1090198114560021.
- Ding H. Pregnant women and flu vaccination, Internet panel survey, United States, November 2013. CDC; 2013.
- Drees M, Johnson O, Wong E, Stewart A, Ferisin S, Silverman P, Ehrenthal D. Acceptance of 2009 H1N1 influenza vaccine among pregnant women in Delaware. Am J Perinatol. 2012;29(4):289–94. doi:10.1055/s-0031-1295660.
- Memoli MJ, Harvey H, Morens DM, Taubenberger JK. Influenza in pregnancy. Influenza Other Respi Viruses. 2013;7(6):1033–39. doi:10.1111/irv.12055.
- Fisher BM, Scott J, Hart J, Winn VD, Gibbs RS, Lynch AM. Behaviors and perceptions regarding seasonal and H1N1 influenza vaccination during pregnancy. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S107–111. doi:10.1016/j.ajog.2011.02.041.
- Centers for Disease C. Prevention. Influenza vaccination coverage among pregnant women–United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62(38):787–92.
- Kerr S, Van Bennekom CM, Mitchell AA. Influenza vaccination coverage during pregnancy - selected sites, United States, 2005-06 through 2013-14 influenza vaccine seasons. MMWR Morb Mortal Wkly Rep. 2016;65(48):1370–73. doi:10.15585/mmwr.mm6548a3.
- Ding H, Kahn KE, Black CL, O’-Halloran A, Lu P-J, Williams WW. Influenza vaccination coverage among pregnant women in the U.S., 2012-2015. Am J Prev Med. 2019;56(4):477–86. doi:10.1016/j.amepre.2018.11.020.
- Groom HC, Henninger ML, Smith N, et al. Influenza vaccination during pregnancy: influenza seasons 2002-2012, vaccine safety datalink. Am J Prev Med. 2016;50(4):480–88.
- Drees M, Tambourelli B, Denstman A, Zhang W, Zent R, McGraw P, Ehrenthal DB. Sustained high influenza vaccination rates and decreased safety concerns among pregnant women during the 2010–2011 influenza season. Vaccine. 2013;31(2):362–66. doi:10.1016/j.vaccine.2012.10.112.
- Chamberlain AT, Berkelman RL, Ault KA, Rosenberg ES, Orenstein WA, Omer SB. Trends in reasons for non-receipt of influenza vaccination during pregnancy in Georgia, 2004-2011. Vaccine. 2016;34(13):1597–603. doi:10.1016/j.vaccine.2016.01.058.
- Barber A, Muscoplat MH, Fedorowicz A. Coverage with Tetanus, Diphtheria, and Acellular Pertussis vaccine and influenza vaccine among pregnant women - Minnesota, March 2013-December 2014. MMWR Morb Mortal Wkly Rep. 2017;66(2):56–59. doi:10.15585/mmwr.mm6602a4.
- Beel ER, Rench MA, Montesinos DP, Mayes B, Healy CM. Knowledge and attitudes of postpartum women toward immunization during pregnancy and the peripartum period. Hum Vaccin Immunother. 2013;9(9):1926–31. doi:10.4161/hv.25096.
- Butler AM, Layton JB, Li D, Hudgens MG, Boggess KA, McGrath LJ, Weber DJ, Becker-Dreps S. Predictors of low uptake of Prenatal Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis immunization in privately insured women in the United States. Obstet Gynecol. 2017;129(4):629–37. doi:10.1097/AOG.0000000000001927.
- Dempsey AF, Brewer SE, Sevick C, Pyrzanowski J, Mazzoni S, O’-Leary ST. Tdap vaccine attitudes and utilization among pregnant women from a high-risk population. Hum Vaccines Immunother. 2016;12(4):872–78. doi:10.1080/21645515.2015.1094594.
- Kriss JL, Albert AP, Carter VM, Jiles AJ, Liang JL, Mullen J, Rodriguez L, Howards PP, Orenstein WA, Omer SB, et al. Disparities in Tdap vaccination and vaccine information needs among pregnant women in the United States. Matern Child Health J. 2019;23(2):201–11. doi:10.1007/s10995-018-2633-8.
- Payakachat N, Hadden KB, Ragland D. Promoting Tdap immunization in pregnancy: associations between maternal perceptions and vaccination rates. Vaccine. 2016;34(1):179–86. doi:10.1016/j.vaccine.2015.09.062.
- Ahluwalia IB, Ding H, D’-Angelo D, et al. Tetanus, diphtheria, pertussis vaccination coverage before, during, and after pregnancy - 16. States and New York City, 2011. States and New York City, 2011. MMWR Morb Mortal Wkly Rep. 2015;64(19):522–26.
- Housey M, Zhang F, Miller C, Lyon-Callo S, McFadden J, Garcia E, Potter R. Vaccination with tetanus, diphtheria, and acellular pertussis vaccine of pregnant women enrolled in Medicaid--Michigan, 2011-2013. MMWR Morb Mortal Wkly Rep. 2014;63:839–42.
- Merritt TA, Rasmussen SA, Bright MA, Roussos-Ross D, Sims SM, Gurka MJ, Thompson LA. Variation in Tdap and influenza vaccination coverage among pregnant women by insurance type — Florida, 2016–2018. MMWR Morb Mortal Wkly Rep. 2020;69(3):72–76. doi:10.15585/mmwr.mm6903a4.
- Kahn KE, Black CL, Ding H, Williams WW, Lu P-J, Fiebelkorn AP, Havers F, D’-Angelo DV, Ball S, Fink RV, et al. Influenza and Tdap vaccination coverage among pregnant women — United States, April 2018. MMWR Morb Mortal Wkly Rep. 2018;67(38):1055–59. doi:10.15585/mmwr.mm6738a3.
- Dudley MZ, Limaye RJ, Omer SB, O’-Leary ST, Ellingson MK, Spina CI, Brewer SE, Chamberlain AT, Bednarczyk RA, Malik F, et al. Characterizing the vaccine knowledge, attitudes, beliefs, and intentions of pregnant women in Georgia and Colorado. Hum Vaccin Immunother. 2020;16(5):1109–17. doi:10.1080/21645515.2020.1717130.
- Centers for Disease C, Prevention. Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women–10 states, 2009-10 influenza season. MMWR Morb Mortal Wkly Rep. 2010;59(47):1541–45.
- Chan HJ, Chang JY, Erickson SR, Wang CC. Influenza vaccination among pregnant women in the United States: findings from the 2012-2016 National Health Interview Survey. J Women’s Health (2002). 2019;28(7):965–75. doi:10.1089/jwh.2018.7139.
- Chamberlain AT, Seib K, Ault KA, et al. Factors associated with intention to receive influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) vaccines during pregnancy: a focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLoS Curr. 2015;7. doi:10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819.
- Kay MK, Koelemay KG, Kwan-Gett TS, Cadwell BL, Duchin JS. 2009 pandemic influenza a vaccination of pregnant women–king County, Washington State, 2009-2010. Am J Public Health. 2012;102(Suppl 3):S368–374. doi:10.2105/AJPH.2012.300676.
- Ding H, Euler GL, Kennedy ED, Greby SM. Pregnant women and flu shots, Internet panel survey, United States, November 2012. CDC. 2012.
- Ding H, Euler GL, Singleton JA. Pregnant women and flu shots, Internet panel survey, United States November 2011. CDC. 2011.
- Ahluwalia IB, Singleton JA, Jamieson DJ, Rasmussen SA, Harrison L. Seasonal influenza vaccine coverage among pregnant women: pregnancy risk assessment monitoring system. J Women’s Health (2002). 2011;20(5):649–51. doi:10.1089/jwh.2011.2794.
- Goldfarb I, Panda B, Wylie B, Riley L. Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S112–115. doi:10.1016/j.ajog.2011.01.007.
- New S, Winter K, Boyte R, Harriman K, Gutman A, Christiansen A, Royce S. Barriers to receipt of Prenatal Tetanus Toxoid, reduced Diphtheria Toxoid, and Acellular Pertussis vaccine among mothers of infants aged <4 months pertussis — California. MMWR Morb Mortal Wkly Rep. 2018;67(38):1068–71. doi:10.15585/mmwr.mm6738a6.
- Razzaghi H, Kahn KE, Black CL, Lindley MC, Jatlaoui TC, Fiebelkorn AP, Havers FP, D’-Angelo DV, Cheung A, Ruther NA. Influenza and Tdap vaccination coverage among pregnant women — United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1391–97. doi:10.15585/mmwr.mm6939a2.
- HealthyPeople.gov. Immunization and infectious diseases. Published 2020. Accessed. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases
- Kaplan-Weisman L, Waltermaurer E, Crump C. Assessing and improving Zoster vaccine uptake in a homeless population. J Community Health. 2018;43(6):1019–27. doi:10.1007/s10900-018-0517-x.
- Cooper White P, Baum DL, Ross H, Falletta L, Reed MD. Cocooning: influenza vaccine for parents and caregivers in an urban, pediatric medical home. Clin Pediatr (Phila). 2010;49(12):1123–28. doi:10.1177/0009922810374353.
- Hurley LP, Lindley MC, Allison MA, Crane LA, Brtnikova M, Beaty BL, Snow M, Bridges CB, Kempe A. Primary care physicians’ perspective on financial issues and adult immunization in the era of the Affordable Care Act. Vaccine. 2017;35(4):647–54. doi:10.1016/j.vaccine.2016.12.007.
- Anderson EL. Recommended solutions to the barriers to immunization in children and adults. Mo Med. 2014;111:344–48.
- Kepka D, Christini K, McGough E, Wagner A, Del Fiol G, Gibson B, Ayres S, Brandt HM, Mann S, Petrik AF, et al. Successful multi-level HPV vaccination intervention at a rural healthcare center in the era of COVID-19. Front Digital Health. 2021;3. doi:10.3389/fdgth.2021.719138
- Reiter PL, Stubbs B, Panozzo CA, Whitesell D, Brewer NT. HPV and HPV vaccine education intervention: effects on Parents, Healthcare Staff, and School Staff. Cancer Epidemiol Biomark Prev. 2011;20(11):2354. doi:10.1158/1055-9965.EPI-11-0562.
- Orenstein WA, Gellin BG, Beigi RH, Buck T, Despres S, LaRussa PS, Lynfield R, Maldonado Y, Morita J, Mouton C. National Vaccine Advisory C. Protecting the public’s health: critical functions of the Section 317 immunization program—A report of the National Vaccine Advisory Committee. Public Health Rep. 2013;128(2):78–95. doi:10.1177/003335491312800203.