ABSTRACT
Cervical cancer is one of the most common malignancies among females. As a virus-related cancer, cervical cancer has attracted a lot of attention to develop virus-targeted immune therapy, including vaccine and adoptive immune cell therapy (ACT). Adoptive tumor infiltrating lymphocytes (TILs) cell therapy has been found to be able to control advanced disease progression in some cervical cancer patients who have received several lines of treatment in a pilot clinical trial. In addition, sustainable therapeutic effect has been identified in some cases. The safety risks of TIL therapy for patients are minimal or at least manageable. In this review, we focused on the versatility of TILs and tried to summarize potential strategies to improve the therapeutic effect of TILs and discuss related perspectives.
Introduction
Cervical cancer ranks fourth with regard to both incidence and mortality among the female population globally.Citation1 With the clinical application of vaccine to avoid human papillomavirus (HPV) infection, the incidence of cervical cancer is declining throughout the worldwide scope, gradually. However, the occurrence of cervical cancer is still in a rising trend, and as a consequence, it ranks seventh among all malignant tumors of women in China. In 2015, proximately 98,900 new cervical cancer cases were reported in China, which accounted for 18.7% of the global incidence. In the particular group of young women (<45 years old), the incidence of cervical cancer is even higher than those common cancers like lung cancer, stomach cancer, and colorectal cancer.Citation2 Patients with early-stage cervical cancer can be cured by surgery, radiotherapy and adjuvant chemotherapy. However, patients with advanced cervical cancers can only be treated with chemotherapy or radiotherapy, but the outcomes are poor, with a median survival rate of only 16.8 months. During the recent 10 years, anti-angiogenesis therapy based on bevacizumab has been approved for clinical treatment of metastatic cervical cancers in combination with chemotherapy. Nevertheless, a small improvement in overall survival was observed in bevacizumab-treated patients.Citation3 Clearly, for patients with metastatic cervical cancers, alternatives and more effective treatments are urgently needed.
Immunotherapy, which aims to enhance host immunity or downregulate the negative regulators of the immune system to restore host immunity against cancers, is currently revolutionizing modern cancer therapy. The US Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab in the treatment of recurrent or metastatic cervical cancer in 2018 considering the favorable outcomes from the phase 2 KEYNOTE-158 trial. Results from the double-blind, phase 3 KEYNOTE-826 trial of immune checkpoint inhibitors (ICI) which indicated the potential role of first-line treatment of ICI in cervical cancer were published online in The New England Journal of Medicine in September of 2021. The phase 3 EMPOWER CERVICAL 1/GOG 3016/ENGOT c×9 trial provided the evidence for the application of another PD-1 antibody, Cemiplimab, in cervical cancer. Thus, cervical cancer patients can benefit from immunotherapy.
Adoptive T-cell therapy obtained a large amount of highly potent tumor-reactive T cells by isolation and expansion from a small number of T cells, which can recognize tumor cells with great efficiency. To date, three most accepted T-cell therapies have shown great potential in clinical application, including tumor infiltrating lymphocytes (TILs) cell therapy, T-cell receptors engineered T (TCR-T) cells and chimeric antigen receptors T (CAR-T) cells. Exciting achievements and outcomes of adoptive immune cell therapy (ACT) have been obtained in clinical practice, although it faces challenges of specificity and efficacy.Citation4–7 In the treatment of solid tumors, no adoptive T-cell therapy has been approved in clinic yet, even for the treatment of melanoma that has been paid most attention among solid tumors. Among the three ACT therapies, TIL therapy has the most promising evidence for further application in advanced cervical cancer. Comprehensive treatment strategy containing immunotherapy is considered as the most potential treatment. In this review, we summarized the current advances in the research and application of TILs for cervical cancer to provide a guide toward future development of TIL therapy for this cancer.
HPV in carcinogenesis of cervical cancer
HPV is a critical pathogenic factor in many cancers, including cervical cancer and vaginal cancer. High-risk HPV infection can be detected in up to 99.7% of cervical cancer specimens.Citation8 HPV16 and HPV18 are two most common carcinogenic genotypes referring to squamous carcinoma and adenocarcinoma of cervical cancer.Citation9 HPV replication is highly dependent on oncoproteins, E6 and E7 proteins coded by the viruses. E6 and E7 oncoproteins are critical both in the productive life cycle and in the carcinogenesis progress. E6 protein can recruit the cellular ubiquitin ligase E6-associated protein into a protein complex with p53, resulting in the disability of p53.Citation10 Besides, E6 proteins bind to and degrade PSD95/DLG/ZO-1(PDZ)-domain containing proteins.Citation11 In the process of cell differentiation,E7 proteins promote S phase reentry via the ability of binding to and inactivating the pocket family proteins. These interactions lead to the release of the transcription factor E2F, causing cell cycle progression in cells that would normally undergo differentiation.Citation12 The down regulation of major histocompatibility complex (MHC) class I prompts the escape of tumor cells from immune surveillance which was observed to be involved in the expression of E7. E7 protein could also inhibit Interferon-γ (IFN-γ)-mediated MHC class I antigen presentation.Citation13
Due to the distinct immunogenicity, HPV proteins are considered as the natural optimal targets for immunotherapy.Citation14 The L1 and L2 proteins composing of HPV viral capsid structure have been widely researched, and great achievements have been made in the development of preventive vaccines. Viral proteins, like E6/E7, which account for malignancy development, could serve as potential targets for immunotherapy. The emerging HPV-associated genetic immunization strategies have been developed to induce immune responses of T cells against E6 and E7 antigens and other neoantigen in cervical cancer.Citation15 The great advantage in taking viral protein as the target of immunotherapy is the impossibility to have cross reaction with normal proteins, which may greatly reduce the possibility of off-tumor effect. Various efforts have been made to develop HPV targeted vaccines, adoptive T-cell therapy and monoclonal antibodies therapy. However, few strategies have exposed clinical applicational potential.
TIL therapy in cervical cancer
Researchers have been looking for more specific immune cells for ACT. In 1986, RosenbergCitation16,Citation17proposed that tumor-infiltrating lymphocytes (TILs) are special immune cells that show outstanding abilities to inhibit tumor growth. TILs express natural T-cell receptors (TCR) that can recognize antigens expressed by a patient’s tumor in an MHC-restricted manner. Comparing to TCR-T and CAR-T cells which may completely rely on limited number of antigens to target tumor cells, TILs developed naturally from the microenvironment of cancer may act with diverse phenotypic profiles.Citation18
Back in 1986, Rosenberg et al.Citation17 found that infiltrating lymphocytes were composed of a group of tumor antigen-specific immune cells with potential persistent killing tumor cells. The first clinical trial to investigate TIL therapy in the treatment of metastatic melanoma in 1994, the current data suggested an overall objective response rate as high as 50%, and about 10–30% of patients achieved complete remission. A portion of patients treated with TILs even achieved a disease-free survival for more than 10 years.Citation19,Citation20 Comparatively, the most excellent TCR-T cells adoptive therapy may have a comparable or less clinical efficacy, and the CAR-T cell therapy has achieved a response rate of even less than 5%.Citation21 TIL therapy has great potential in treating cancer with successful cases not only in melanoma, but also in other cancer types such as lung cancer.Citation22 Due to the rapid development of technology such as image-navigation, the acquisition of tumor tissue through biopsy in patients with advanced malignant tumor has been easy to access.
The first reported clinical trial of TIL therapy in cervical cancer was published in 2015. Rosenberg et al.Citation23 demonstrated the outcome of cervical cancer patients receiving adoptive TIL therapy. A total of 9 patients with refractory metastatic cervical cancer patients were included. Two patients had complete remission diseases and 1 patient had a partial remission disease. It was the first reported positive result from TIL cells therapy in cervical cancer, providing an alternative for advanced cervical cancer patients who have received long courses of chemotherapy with limited effectiveness. The objective response of the TIL therapy model established by Rosenberg’s laboratory is almost 33.3%, indicating a potential need for further improvement in TIL therapy of cervical cancer. The same team published the results of a phase II one-armed clinical research for in 2017. A total of 18 HPV-positive cervical cancer patients receiving TIL adoptive therapy, and 3 patients had PR, 2 cases with CR with an objective effect rate of 27.8% (NCT01585428). In March 2018, the lead researcher of the American Cancer Institute reported that two cervical cancer patients treated with TIL therapy had obtained a complete remission who had a survival period of over 5 years.Citation24 Later in 2018, a further trial of TILs in treating HPV-related tumor was published with an ORR of 28% (NCT01585428).Citation25 Two of 29 patients had a complete response after TILs infusion. Up to now, there are five registered trials on the web of clinical trial ().
Table 1. Clinical trials studying TIL therapy in cervical cancers.
The toxicity observed with ACT can broadly be divided into three groups: toxicity due to the lymphodepleting preparative regimen, immune-related toxicity and cytokine-related toxicity.Citation26 During the treatment with TIL therapy, toxicity or side effect is light or moderate, and predominantly being caused by the lymphodepleting preparative regimens, resulting in pancytopenia and febrile neutropenia, and IL-2 administration including chills, high fever, hypotension, oliguria, and edema due to the systemic inflammatory and capillary leak syndrome effects. Similarly, the adverse effect was mild in cervical cancer. The milestone study by Stevanović et al.Citation25 reported TIL therapy in patients diagnosed with metastatic cervical cancer who had previously received platinum-based chemotherapy or chemoradiotherapy. There were no acute infusion-related toxicities and no autoimmune adverse events. The toxicity profile was consistent with the chemotherapy conditioning regimen and aldesleukin.
The heterogeneity of TILs promises the versatility of treatment
Tumor heterogeneity greatly contributes to the difficulty and complexity of clinical management in antitumor therapy, including immune therapy. The tumor ecosystem is a heterogeneous symbiotic ecosystem composed of tumor clones with different spatial functions and genomic characteristics. Besides, heterogenous stromal cells and immune cells also contribute to the diversity of tumor microenvironment. A single therapy with limited targets will promote tumor clonal as well as microenvironment evolution, ultimately leading to uncontrollable diseases.Citation27 According to this “tumor ecosystem” theory, therapeutics, which target different malignant clones, stromal cells and immune cells at multiple layers, may represent an optional approach with great potential for individualized cancer treatment, emphasizing the importance of dissecting the multi-cell tumor ecosystem from the perspective of malignant-stromal-immune system.Citation28
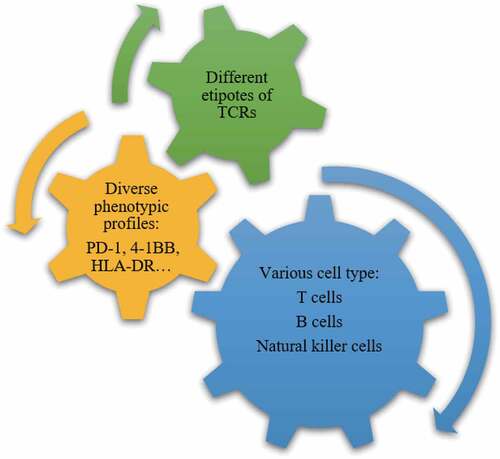
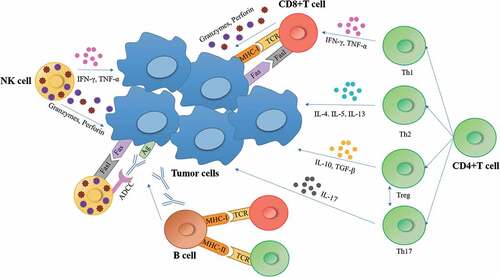
Tumor-reactive T cells appear to heavily infiltrate into the tumor microenvironment of patients and direct contact can promote antigen presentation. More and more evidences have indicated that tumor-mutation-derived neoantigen-specific T cells play a role in tumor control.Citation29,Citation30 Cultured tumor-infiltrating lymphocytes is a heterogeneous cell product roughly with three levels of diversity. It is commonly accepted that tumor antigen-specific cytotoxic CD8+ TILs expressing T-cell receptors account for the antitumor effect of immune system. TILs composed of not only T cells, but also B cells and NK cells may contribute to the immunity reaction. Secondly, the diversity of lymphocytes for the part of specific cell type of CD8+ effector T cell also accounts for heterogeneity, including subpopulations of CD8+PD-1+, CD8 + 4-1BB+, and CD8+PD-1 + 4-1BB+ cells, etc. These cells exist in varied frequencies, ranging from less than 1% to greater than 80%, depending on the marker and marker combination tested.Citation31,Citation32 Third, the diversity of TCRs makes the most complicated heterogeneity of T cells ().
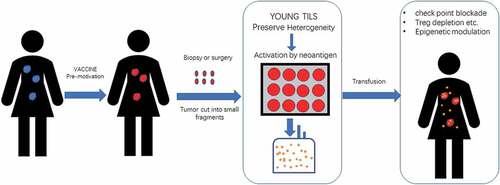
Distinguished correlating changes were observed between tumor burden after TIL infusion and the frequency and number of CD8+ TILs in the cell product.Citation33 However, transfusion of purified central memory CD8+ T cells has shown minimal clinical response even these cells could be detected for a long time.Citation34 Actually, RosenbergCitation35 and colleagues established a “Young TILs” method in 2008. This approach utilizes the entire resected tumor to rapidly expand TIL for administration without in vitro testing for tumor recognition. Young TILs can confer a higher objective effectiveness comparing to purified CD8+ T cells from TILs (35% vs 20%) in patients with malignant melanoma, and a trend of superiority was observed in patient survival for “Young TILs” treatmentCitation36 (NCT00513604). Thus, it was preferred that to preserve the beneficial interactions between different immune cells, that may contribute to a better clinical response.
For a relative long time, the major role of CD4+ T cells is supposed to support the development and function of CD8+ T cells by licensing antigen presenting cells for efficient antigen presentation and producing cytokines. It has been a source of controversy because in some cases (approximately 5% of patients), TIL products are composed predominantly of CD4+ effector-memory, and effector cells mediate strong and durable responses, many of which were complete remissions lasting for years.Citation33,Citation37 It was proposed that tumor-specific CD4+ T cells may also be able to exert anti-tumor activity through direct cell killing and cytokine release. CD4+ T cells exhibit tremendous plasticity as a lineage, much more than CD8+ T cells with effector cells being able to switch to regulatory phenotypes and regulatory phenotypes mutually being able to switch to cytolytic cells expressing granzyme and perforin.Citation38,Citation39 CD4+ T cells may migrate to tumors and produce chemokine and other mediators facilitating the migration of CD8+ T cells into the tumorCitation40 in addition to playing a direct tumor cytostatic and T-cell helper function. Adoptive transfer of CD4+ T cells recognizing one neoantigen alone was found to be able to induce clinical antitumor response in a single patient with cholangiocarcinoma.Citation41 Veatch et al. reported a patient with stage IV acral melanoma who obtained a complete response after adoptive tumor infiltrating lymphocytes from metastatic tumor and further analysis identified that TILs were mainly composed of CD4+ T cells specific for BRAF V600E and diverse CD8 T cells reactive to non-mutated self-antigens.Citation42 Another important role was proposed of TILs that have the ability to present tumor antigen acting as “tumor vaccine”, which was mainly due to the activity of CD4+ T cells.Citation23 To sum up, though the specific role of CD4+ T cells played in TIL products is still uncovered, current data supported the preservation of CD4+ T cells in the process of TILs culture.
Even among the same types of T cells, different TCRs targeting different antigen also contribute to the diversity and polyfunctionality of TILs.Citation43 Although some tumor have up to 100s or even 1000s of nonsynonymous mutations, there are multiple immunological processes dictating the successful presentation and recognition of a neoepitope by the immune system. It has become apparent that less than 1% of mutations found in a tumor are so-called immunologically “actionable” in that they successfully induce a mutanome-specific clonal T-cell response. Subclones of malignancy has their specific driver mutations which may be recognizable by immune system. The liquidity due to the open access of TILs to the peripheral blood makes the diversity of TCRs, helping to recognize different tumor antigens. Therefore, TILs that have been exposed to the diverse neoantigens of malignant cells have great capacity to identify as many targets as possible. The diversity of TIL cells makes it possible an ideal tool to recognize and kill the tumor cell subclones with great heterogeneity.Citation44 The last but not the least, T cell clones with the same TCR may be composed of cells at various states of differentiation that may exert different function. All elements of the heterogeneity make TILs to possess great capacity to identify different clones of tumor cells, which is the first critical step of antitumor immunotherapy. The heterogeneity of microenvironment also contributed to malignant cell invasion and expansion, which led to the shaping of clonal evolution of tumor cells.Citation45 We can assume that the rational activation of highly effective TIL cell therapy may be one of the most likely strategies to overcome the heterogeneity of tumor cells.
Potential strategies to promote therapeutic effect of TIL therapy against cervical cancer
TIL therapy targeting HPV antigen is a personalized treatment and the adoptively transferred T cells may exert antitumor effect and serve as a mechanism-driven biomarker to predict treatment response, then to guide patient selection. There have been evidences showing that magnitude of HPV reactivity of the infused TILs was associated with clinical response, indicating a causal relationship between the targeting of HPV antigens and tumor regression.Citation25
The in vivo anticancer response mediated by T cells involves three basic steps. First, antigen presenting cells (APC) capture cancer antigens and process them into antigenic peptides, which are then presented in combination with human leukocyte antigen (HLA) molecules for TCR recognition. The second step is T cell activation after recognizing tumor antigen, which requires co-stimulation of surface molecules B7 and CD28 to bind to antigen-presenting cells and T cells. Finally, after activation, T cells are transported to tumor microenvironment where they recognize and eliminate tumor cells (). A persistent memory response would play a role in both preventing disease recurrence and in taking precautions against the evolution of anti-therapeutic malignant cell clones. The precise implications of immunological memory formation are still undefined, but evidence for extremely durable remissions of neoantigen has been achieved.
There is a great potential demand to develop next-generation TIL products in which TILs are isolated or enriched during expansion facilitating improvements both in persistence and antitumor activity of T-cells. In addition, the growing consensus is that TIL therapy will have its real impact when combined concurrently or in sequence with other immunomodulatory therapies capitalizing on the idea that these responses will occur over protracted periods of time invoking multiple immunological compartments. There are a lot of report of parameters greatly correlated with the treatment response, like the number of TILs infused, the number and percentage of CD8+ TILs, CD8+ phenotype, and telomere length, et al.Citation31,Citation32,Citation46 Accordingly, we tried to present possible strategies to improve the antitumor effect of TIL therapy.
Culture model that can preserve the heterogeneity of TILs
The first issue for TIL culture is how to obtain a group of high-quality T cells, which is of great importance for the successful treatment of TIL cells due to the heterogeneity of cancer.Citation47 Actually, TILs are a cluster of cells with great heterogeneity demonstrating the great genomic differences of lymphocytes among different patients.Citation48 Both of cell variety and TCR variety make the heterogeneity of TILs among different tumor lesions in the same patient. In most cases, it is difficult to identify the neoantigen specificities of tumor infiltrating T cells with diverse phenotypes. A culture model that can preserve the heterogeneity and evolution ability of TILs will be the fundamental step. Excessive cell sorting and induction may do harm to the clinical response of cell product. Different types of cells,Citation49 like CD4+ T helper cells contributed to the antitumor activity of adoptive TIL therapy.Citation41,Citation42 Even expansion of bystander T-cell clones unrelated to tumor antigen recognition may also contribute to the final clinical response of TILs.Citation18,Citation50 “Young TILs” was a method to keep heterogeneity of TILs as mentioned above. Young TILs can have an undetermined but high level of antigen reactivity, and other advantageous attributes such as long telomeres and high levels of CD27 and CD28. Simplified culture maintaining the heterogeneity of TIL cells could not only makes the process feasible but also can overcome the difficulties in cancer treatment due to the heterogeneity of cancer. However, to reduce the activity of inhibitory cells is another option to supplemental treatment.
Promotion of antigen presentation and selective expansion during the TIL culture
The efficacy of adoptive T cell therapy depends on the ratio of tumor antigen-specific killer T cells. CD8+T cell activity is highly dependent on the active antigen presenting cell activation. TILs are fully exposed to tumor cells, and some of the T cells have received the persistent stimulation of tumor antigens, but only a small part of CD8+T cells are activated by tumor antigen. The advantage of in vitro culture may come from the artificial tumor antigen presentation. The proportion of CD8+T cells targeting tumor antigens can be further improved by continuous and effective antigen presenting cells. As mentioned above, TILs contain T cells reactive to cervical cancer cells or HPV+ cells. These cells or clones can be selectively expanded for enriched TIL therapy. For example, the special selection of HPV E6/E7-specific can increase the efficacy of TIL therapy.Citation23 In addition, there is a very important population of TILs that recognizes tumor neoantigens that should be considered for selective expansion. Since TILs derived from tumor environment, they are supposed to respond quickly and are sensitive to neoantigens. For cervical and other viral-induced cancers, viral antigens are considered as neoantigens.Citation51 Regardless of whether the viral antigens are tumor-specific antigens or neoantigens, targeting them is a better strategy, as they are foreign and selective expansion of TILs reactive to viral antigens or variants will increase the efficacy of TIL therapy with increased safety.Citation52
Another important issue is to protect the stability of the antigen specificity during TIL expansion. Tumor-infiltrating lymphocyte expansion is a stochastic process with different T-cell clones increasing or decreasing in frequency unpredictably during the rapid expansion process. Given this inter-clonal competition, the more tumor-specific clones going into the rapid expansion process for infusion, the greater chance that more tumor-specific clones (some of which can be more differentiated T cells) can compete and be maintained at an appreciable frequency in the final product.
Efficient trafficking of lymphocytes
It is well known that the limitation of TIL therapy efficiency is related to the poor infiltration of activated lymphocytes into tumors. A few of research demonstrated that “cold tumor” represents a fundamental factor limiting the therapeutic effect of immunotherapy. It can be expected that sufficient functional T cell infiltration may indicate favorable prognosis, which has been proved by pilot pre-clinical and clinical studies. The truth is less than 2% of transferred T cells actually infiltrate malignant solid tumors.Citation53 Thus, strategies to make adoptive T cells entering into the tumor microenvironment is of great importance. Intrinsic oncogenic pathway inhibitors, epigenetic modification inhibitors, antiangiogenic therapies, TGFβ inhibitors, and CXCR4 inhibitors promote T-cell trafficking and enable T cells to infiltrate the tumor more effectively.Citation54 Our effort has been made to modify the T cells with iRGD, a tumor-penetrating peptide, and significant promotion of tumor-specific lymphocyte infiltration has been observed, which leads to a better tumor control.Citation55 A deeper understanding of these mechanisms opens new possibilities for the development and improvement of TIL effectiveness.
Issues concerning to tumor microenvironment
Inhibitory immune microenvironment is an important reason why T cells cannot kill malignant tumor cells. Regardless of the extent of tumor specificity (or neoantigen specificity), infused TILs will still be subject to all the immunosuppressive mechanisms (e.g., PD-L1, IDO, low pH, high lactate, hypoxia, low glucose, glutamine deprivation, etc.) that any other T cell will face when migrating into the tumor microenvironment.Citation56 Data on tumor biopsies taken from patients shortly after TIL infusion (before endogenous T-cell recovery after lymphodepletion) showed infiltration of highly PD-1+ and B and T lymphocyte attenuator (BTLA)+ CD8+ T cells.Citation57 A variety of suppressive monocytes, including myeloid-derived suppressor cells (MDSC), tumor-associated neutrophils (TAN), type 2 tumor-associated macrophages (TAM2) and CD4+CD25+FoxP3+ Tregs,Citation58 have been reported in preclinical and clinical studies.
Previous studies have indicated that depleting Tregs can enhance the outcome of immunotherapies with immune-modulating doses of cyclophosphamide.Citation59 In most patients, additional immunomodulation is needed, possibly including targeted MDSC, TAM and TAN, in order to more effectivelyCitation60 transfer the balance from predominantly cumulative T-cell down-regulation signals toward a majority of T-cell–activating signals.Citation61 Other modulators, such as IDO inhibitors and modulators of Tregs and MDSCs, are also being considered as combination therapy with adoptive cell therapies, but the focus in the near future will be on combining TILs with checkpoint blockade.
Checkpoint blockade is a method to stimulate T-cell function by blocking monoclonal antibodies (mAbs) that inhibit T-cell receptors, whereas T-cell co-stimulation is the method aiming at activating T-cell function with mAbs that target their stimulatory receptors. Published data revealed that cervical cancer has a medium burden of neoantigen, indicating its relative low response to check point inhibitors. However, as a solid tumor, cervical cancer is a multi-gene disease with complicated signal pathway disorders.Citation62 A direct interaction between PD-1 on lymphocytes with their ligands on tumor cells resulting in apoptosis of lymphocytes, which plays an important role in the background of cytotoxic effect inhibition. Blockade of PD-1/PD-L1 pathway using a fully humanized antagonistic monoclonal antibodies increased the number and functionality of tumor-specific T cells and has been demonstrated as novel anticancer reagents with broad spectrum. As previous described, Pembrolizumab, the inhibitory antibody against PD-1, has been approved by FDA not only in the first-line treatment but also in the second-line setting.Citation63
HPV infection can improve PD-1 expression, which is involved in the development of cervical cancer. It was observed that PD-L1 was highly expressed on the surface of cervical cancer cells so as to avoid the cytotoxicity of the CD8+ T cells.Citation64 There were up to 59.1% of cervical cancer cells expressing PD-L1, and 60.6% TILs expressing PD-1 at the same time.Citation65 Besides, the proportion of TILs with PD-1 expression was negatively correlated with patient prognosis.Citation66 A great promotion of PD-1 expression has been identified during the adoptive cell therapy.Citation67 Based on the above evidence, inhibition PD-1 pathway may have a great potential to improve the activity of TILs. An optional choice is PD-1 blockade before the tissue sample collection which may also improve the T cells infiltration into the tumor microenvironment. Thus, pretreatment of PD-1 blockade may also improve the ratio of tumor antigen reactive T cells. A recent report demonstrated that functional anti-tumor immune responses were detected in 19/23 check-point resistant melanoma patients (83%) who were treated with TIL therapy. Not only CD8+ (in 18/23 patients, 78%) but also CD4+ (in 16/23 patients, 70%) TILs could recognize autologous tumors and exerted antitumor activity.Citation68 This result highlighted a possible synergistic effect between TIL therapy with checkpoint-blockade. In China, Yujie Tan et al.Citation69 explored the efficacy of adoptive transfer of TILs and anti-PD1 antibody in patients with metastatic cervical cancer showing low microsatellite instability (MSI) expression and negative for PDL1. Interestingly, they found that this combination therapy registered promising antitumor effects and a satisfactory objective response, with clinical tumor regression observed in 20 out of the 80 patients (25.0%). The medium time for PFS and OS was 6.1 and 11.3 months, respectively. These findings suggest that a combination therapy of TILs and anti-PD1 may potentially modulate the growth of metastatic cervical cancers in patients with low MSI expression and negative for PDL1. Currently, a series of clinical trials combining checkpoint inhibitor with adoptive T-cell therapy are going on (NCT03638375, NCT03645928, NCT03073525, etc.). In addition, recent research also indicated other target to inhibit to promote adoptive T-cell treatment efficiency, like TIM-3,Citation70 LAG-3Citation71,Citation72 and CXCR4, etc. The effects of immune checkpoint inhibitors are mediated by TILs in the tumor microenvironment. Therefore, a combination of immune checkpoint inhibitors and TILs may display superior antitumor effects on treating metastatic cervical cancer than the other current therapies.
Vaccine in combination with TIL therapy
Vaccines constitute an active form of immunotherapy since they rely on the recipient to generate T cell response and are not dependent on the injection of large numbers of in vitro derived effector cells. The premise, an adaptive anti-tumor immune response, can be elicited by presenting exogenous tumor antigens to the immune system, is the basic element of therapeutic anti-tumor vaccination. In prior decades, tumor vaccine has been at the forefront of cancer immunotherapy research. Cancer vaccines can be divided into three categories generally, including the use of lymphocyte-defined tumor antigens (LDTAs), whole autologous or allogeneic tumor cell vaccines, and LDTA-pulsed dendritic cells.Citation73 Each has its advantages and disadvantages. Although there were no relevant clinical trials about vaccines combining with TIL therapy in cervical cancer, a few successes had been achieved in the practice of other cancers.
In a clinical trial of 59 resectable pancreatic cancer patients who received preoperative allogeneic tumor cell vaccine treatment, T-cell infiltration and development of tertiary lymphoid structures were observed in the surgery tumor tissue, which suggested that the combination of vaccine and TIL therapy can convert a “non-immunogenic” neoplasm in to an “immunogenic” neoplasm.Citation74,Citation75 Microdissection and gene array analyses demonstrated that the decrease of Treg and the increase of Th17 immune effector signatures within the vaccine-induced intra-tumoral lymphoid aggregates were related to the enhanced response of mesoderm-specific T cells in the system after vaccination with higher intra-tumoral Teffector/Treg ratios and longer patient survival.Citation75 Similar results were also obtained in other types of cancers, including melanomaCitation76 and NSCLCCitation77 It reminded that antitumor vaccine applied before tissue derivation may help harvesting vigorous T cells with greater potential. Utilizing antigen-loaded dendritic cells (DCs) as immunogens is popular in vaccine trials since DCs are professional antigen-presenting cells that can be used as potent inducers of tumor-specific immune responses in a vaccine setting. The successful case of TIL therapy combining with DC vaccination was reported in a phase I trialCitation78(NCT01946373) in metastatic melanoma patients progressing on immune checkpoint inhibitors. An initial cohort (5 patients) received TIL therapy alone and a second cohort (five patients) received TIL combined with autologous tumor lysate-loaded DC vaccination. All patients received cyclophosphamide/fludarabine preconditioning prior to, and intravenous IL-2 after, TIL transfer. The DC vaccine was given as five intradermal injections after TIL and IL-2 administration. In the first cohort, all patients had a mixed response or stable disease, but none was durable. In the combination cohort, two patients experienced complete responses (CR) that are still ongoing (>36 and >18 months, respectively). In addition, two patients had partial responses (PR), one still ongoing (>42 months) with only a small bone-lesion remaining. The results may be attributed to an increase in CD127- expressing and interferon-g-producing CD8+ T cells.Citation79 Although the trial had several limitations, it provided the information that vaccination can act as a key prerequisite for central effector memory CD8+ T cells to exhibit their superior antitumor properties.
Prior study revealed that vaccination against human papilloma viruses leads to a favorable cytokine profile of specific T cells. Cervical cancers harbor a variable number of somatic mutations. Neoantigens from tumor mutation and cancer testis antigens could stimulate T cell recognition as well as HPV protein.Citation24 It can be expected that tumor vaccine treatment may help boost the tumor immune reaction that may bring efficient TILs during the future treatment.
Epigenetic modulation of tumor cells
The epigenome controls genome structure and regulation. DNA methylation abnormality is a characteristic change of HPV infection, and HPV E7 protein can enhance the activity of DNA methylase.Citation80 Tumor cells with HPV infection were found to have an obviously reduced level of tumor antigen expression together with the expression level of molecules that help present tumor antigen. On the other hand, abnormal methylation can reduce T cell vitality, immune cells secrete cytokines disorder, and Treg cell activation.Citation81 DNA methyltransferase inhibitors (DNMTi) can enhance the cytotoxicity of CD8 + T cells, as well as assist CD4 + T cells by inducing the expression of key immunostimulatory cytokines.Citation82–85 Besides, DNMTi could downregulate the suppressive factor,Citation86,Citation87 including the expression of Treg cells, MDSC and M1 macrophages. It could also promote KIR expression on NK cell surface, bind to MHC class I molecules to recognize abnormal cells, and increase NKG2D-dependent NK cell-mediated killing of these cells in vitro.Citation82 DNMTi is expected to play an important role in cancer immunotherapy. However, DNMTi modulation of immune cells is closely related to the state of cell activity, and the drug dose and regulatory mechanism need to be further elaborated in basic experiments.
Histone deacetylase inhibitors (HDACi) as potential immunomodulating agents were also well studied in the treatment of cancers. The activity of HDACs can affect the expression of MHC and co-stimulatory molecules. Based on the preclinical data, HDACi could induce cell cycle arrest, differentiation and cell death in cancer cells, reduce angiogenesis, and modulate the immune response.Citation88 It converted cancer cells into sensitive cells susceptive to immunotherapy by raising expression levels of tumor antigen.Citation89 HDACi are capable of inhibiting apoptosis of CD4+ T cells within the tumor, thereby enhancing responses of anti-tumor immune and suppressing the growth of melanoma cells.Citation90 Besides, some HDACi can also enhance the activity of CD8+ T cells and NK cells in anticancer immune responses.Citation91–93 Based on the above reasons, there is a prospect to combine TIL therapy with methylation inhibitors or deacetylase inhibitors to improve the treatment outcome.Citation94
Summary and prospects
With the rapid progression of genome-editing technique, adoptive immunologic cells transfusion therapy is being developed quickly. Adoptive TIL therapy is still the most established form of adoptive T-cell therapy. Due to the natural heterogeneity of TILs, tumor-obtained adoptive T cells therapy shows great prospect. A TIL-based treatment model that combining TILs culture, and check-point blockade, tumor vaccination as well as epigenetic modulation will be promising anti-tumor therapy that may defeat malignancy completely (). Pilot research has revealed that such a combination therapy with TILs may show great potential in treating advanced cervical cancer. A great problem is that many of the immune therapies are only effective in a minority of patients, and so is TIL therapy in cervical cancer. Utilizing tumor and mesenchyme targeted therapies to reverse tumor immune resistance and sensitize tumors to TIL-based adoptive cell therapy may prove to be a safe and effective way to treat advanced cervical cancer.
Authors’ contributions
Yahui Zhu, Jing Zhou, Lijing Zhu, Wenjing Hu, Baorui Liu and Li Xie conceived, wrote and edited the manuscript. All authors read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–11. doi:10.3322/caac.21492.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. doi:10.3322/caac.21338.
- Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654–63. doi:10.1016/s0140-6736(17)31607-0. PMID:Pmc5714293.
- Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–88. doi:10.1016/j.ccell.2020.07.005.
- Wu Y, Chen W, Xu ZP, Gu W. PD-L1 distribution and perspective for cancer immunotherapy-blockade, knockdown, or inhibition. Front Immunol. 2019;10:2022. doi:10.3389/fimmu.2019.02022. PMID:Pmc6718566.
- Gorabi AM, Hajighasemi S, Sathyapalan T, Sahebkar A. Cell transfer-based immunotherapies in cancer: a review. IUBMB Life. 2020;72(4):790–800. doi:10.1002/iub.2180.
- Strizova Z, Bartunkova J, Smrz D. The challenges of adoptive cell transfer in the treatment of human renal cell carcinoma. Cancer Immunol Immunother. 2019;68(11):1831–38. doi:10.1007/s00262-019-02359-z.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi:10.1002/(sici)1096-9896(199909)189:1<12:aid-path431>3.0.co;2-f.
- Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–99. doi:10.1016/s0140-6736(13)60022-7.
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–36. doi:10.1016/0092-8674(90)90409-8.
- Ganti K, Broniarczyk J, Manoubi W, Massimi P, Mittal S, Pim D, Szalmas A, Thatte J, Thomas M, Tomaić V, et al. The human papillomavirus E6 PDZ binding motif: from life cycle to malignancy. Viruses. 2015;7(7):3530–51. doi:10.3390/v7072785. PMID:Pmc4517114.
- Dyson N, Howley PM, Münger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243(4893):934–37. doi:10.1126/science.2537532.
- Zhou F, Chen J, Zhao KN. Human papillomavirus 16-encoded E7 protein inhibits IFN-γ-mediated MHC class I antigen presentation and CTL-induced lysis by blocking IRF-1 expression in mouse keratinocytes. J Gen Virol. 2013;94(Pt 11):2504–14. doi:10.1099/vir.0.054486-0.
- Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, Lee CW, Kim S, Woo JW, Park KS, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. PMID:PMC4220493 which is developing GX-188E vaccine. The remaining authors declare no competing financial interests. doi:10.1038/ncomms6317.
- Liao SJ, Deng DR, Zeng D, Zhang L, Hu XJ, Zhang WN, Li L, Jiang XF, Wang CY, Zhou JF, et al. HPV16 E5 peptide vaccine in treatment of cervical cancer in vitro and in vivo. J Huazhong Univ Sci Technolog Med Sci. 2013;33(5):735–42. doi:10.1007/s11596-013-1189-5.
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. a preliminary report. N Engl J Med. 1988;319(25):1676–80. doi:10.1056/nejm198812223192527.
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–21. doi:10.1126/science.3489291.
- Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–79. doi:10.1038/s41586-018-0130-2.
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–39. doi:10.1200/jco.2008.16.5449. PMID:Pmc2652090.
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–57. doi:10.1158/1078-0432.ccr-11-0116. PMID:Pmc3131487.
- Merhavi-Shoham E, Itzhaki O, Markel G, Schachter J, Besser MJ. Adoptive cell therapy for metastatic melanoma. Cancer J. 2017;23(1):48–53. doi:10.1097/ppo.0000000000000240.
- Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3). doi:10.1093/jnci/dju435. PMID:Pmc4565530.
- Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry RM, Kammula US, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543–50. doi:10.1200/jco.2014.58.9093. PMID:PMC4417725 online at Author contributions are found at the end of this article.
- Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356(6334):200–05. doi:10.1126/science.aak9510. PMID:Pmc6295311.
- Stevanović S, Helman SR, Wunderlich JR, Langhan MM, Doran SL, Kwong MLM, Somerville RPT, Klebanoff CA, Kammula US, Sherry RM, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–93. doi:10.1158/1078-0432.ccr-18-2722. PMID:Pmc6397671.
- Rohaan MW, Wilgenhof S, Haanen J. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474(4):449–61. doi:10.1007/s00428-018-2484-0. PMID:Pmc6447513.
- Faltas BM, Prandi D, Tagawa ST, Molina AM, Nanus DM, Sternberg C, Rosenberg J, Mosquera JM, Robinson B, Elemento O, et al. Clonal evolution of chemotherapy-resistant urothelial carcinoma. Nat Genet. 2016;48(12):1490–99. doi:10.1038/ng.3692. PMID:Pmc5549141.
- Blank CU, Haanen JB, Ribas A, Schumacher TN. Cancer Immunology. the “cancer immunogram”. Science. 2016;352(6286):658–60. doi:10.1126/science.aaf2834.
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–21. doi:10.1038/nature22991. PMID:Pmc5577644.
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi:10.1126/science.aaa4971.
- Chacon JA, Sarnaik AA, Chen JQ, Creasy C, Kale C, Robinson J, Weber J, Hwu P, Pilon-Thomas S, Radvanyi L. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21(3):611–21. doi:10.1158/1078-0432.ccr-14-1934. PMID:Pmc4315752.
- Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ Jr. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014;20(1):44–55. doi:10.1158/1078-0432.ccr-13-0945. PMID:Pmc3947326.
- Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18(24):6758–70. doi:10.1158/1078-0432.ccr-12-1177. PMID:Pmc3525747.
- Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, Alvarez-Downing MM, Lee CC, Singh S, Li T, et al. The stoichiometric production of IL-2 and IFN-γ mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med. 2012;4(149):149ra120. doi:10.1126/scitranslmed.3004306. PMID:Pmc6453124.
- Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother (1991). 2008;31(8):742–51. doi:10.1097/CJI.0b013e31818403d5. PMID:Pmc2614999.
- Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, White DE, Nathan D, Restifo NP, Steinberg SM, et al. Randomized selection design trial evaluating CD8±enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31(17):2152–59. doi:10.1200/jco.2012.46.6441. PMID:PMC3731980 found at the end of this article.
- Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, Kudchadkar R, Zager J, Gibney G, Sondak VK, et al. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother (1991). 2012;35(8):615–20. doi:10.1097/CJI.0b013e31826e8f5f. PMID:4467830.
- O’-Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–102. doi:10.1126/science.1178334. PMID:2997673.
- Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Ménoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187(7):3555–64. doi:10.4049/jimmunol.1101244. PMID:3178659.
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–13. doi:10.1038/nature08511. PMID:2789415.
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–45. doi:10.1126/science.1251102.
- Veatch JR, Lee SM, Fitzgibbon M, Chow IT, Jesernig B, Schmitt T, Kong YY, Kargl J, Houghton AM, Thompson JA, et al. Tumor-Infiltrating BRAFV600E-specific CD4+ T cells correlated with complete clinical response in melanoma. J Clin Invest. 2018;128(4):1563–68. doi:10.1172/jci98689. PMID:5873881.
- Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24(6):724–30. doi:10.1038/s41591-018-0040-8. PMID:6348479.
- Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255–62. doi:10.1056/NEJMoa1609279. PMID:5178827.
- Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A, Funnell T, Little N, de Souza CPE, Laan S, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173(7):1755–69.e1722. doi:10.1016/j.cell.2018.03.073.
- Donia M, Kjeldsen JW, Andersen R, Westergaard MCW, Bianchi V, Legut M, Attaf M, Szomolay B, Ott S, Dolton G, et al. PD-1(+) polyfunctional T cells dominate the periphery after tumor-infiltrating lymphocyte therapy for cancer. Clin Cancer Res. 2017;23(19):5779–88. doi:10.1158/1078-0432.ccr-16-1692. PMID:7115919.
- de Vos van Steenwijk PJ, Heusinkveld M, Ramwadhdoebe TH, Löwik MJ, van der Hulst JM, Goedemans R, Piersma SJ, Kenter GG, van der Burg SH. An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res. 2010;70(7):2707–17. doi:10.1158/0008-5472.can-09-4299.
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–30.e814. doi:10.1016/j.immuni.2018.03.023. PMID:5982584.
- Adurthi S, Mukherjee G, Krishnamurthy H, Sudhir K, Bafna UD, Umadevi K, Jayshree RS. Functional tumor infiltrating TH1 and TH2 effectors in large early-stage cervical cancer are suppressed by regulatory T cells. Int J Gynecol Cancer. 2012;22(7):1130–37. doi:10.1097/IGC.0b013e318262aa53.
- Radvanyi LG. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. 2015;21(6):450–64. doi:10.1097/ppo.0000000000000162.
- Schumacher TN, Scheper W, Kvistborg P. Cancer neoantigens. Annu Rev Immunol. 2019;37:173–200. doi:10.1146/annurev-immunol-042617-053402.
- Tang Y, Zhang AXJ, Chen G, Wu Y, Gu W. Prognostic and therapeutic TILs of cervical cancer-current advances and future perspectives. Mol Ther Oncolytics. 2021;22:410–30. PMID:Pmc8430272. doi:10.1016/j.omto.2021.07.006.
- Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, Powell DJ Jr., Riley JL, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17(14):4719–30. doi:10.1158/1078-0432.ccr-11-0351. PMID:3612507.
- Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365–86. doi:10.7150/thno.58390. PMID:Pmc8039952.
- Ding N, Zou Z, Sha H, Su S, Qian H, Meng F, Chen F, Du S, Zhou S, Chen H, et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat Commun. 2019;10(1):1336. doi:10.1038/s41467-019-09296-6. PMID:6430780.
- Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–92. doi:10.1093/annonc/mdw168.
- Ritthipichai K, Haymaker CL, Martinez M, Aschenbrenner A, Yi X, Zhang M, Kale C, Vence LM, Roszik J, Hailemichael Y, et al. Multifaceted role of BTLA in the control of CD8(+) T-cell fate after antigen encounter. Clin Cancer Res. 2017;23(20):6151–64. doi:10.1158/1078-0432.ccr-16-1217. PMID:5748156.
- Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. PMID:4604472. doi:10.1038/srep15179.
- Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, Tewari P, Duncan C, Traube C, McCall D, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16(1):45–63. doi:10.1038/s41571-018-0075-2. PMID:7096894.
- Met Ö, Jensen KM, Chamberlain CA, Donia M, Svane IM. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019;41(1):49–58. doi:10.1007/s00281-018-0703-z.
- Gowrishankar K, Birtwistle L, Micklethwaite K. Manipulating the tumor microenvironment by adoptive cell transfer of CAR T-cells. Mamm Genome. 2018;29(11–12):739–56. doi:10.1007/s00335-018-9756-5.
- Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13(5):273–90. doi:10.1038/nrclinonc.2016.25. PMID:5551685.
- Frenel JS, Le Tourneau C, O’-Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, van Brummelen EMJ, Rugo HS, Thomas S, Saraf S, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the Phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35(36):4035–41. doi:10.1200/jco.2017.74.5471.
- Yang W, Song Y, Lu YL, Sun JZ, Wang HW. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139(4):513–22. doi:10.1111/imm.12101. PMID:3719068.
- Feng YC, Ji WL, Yue N, Huang YC, Ma XM. The relationship between the PD-1/PD-L1 pathway and DNA mismatch repair in cervical cancer and its clinical significance. Cancer Manag Res. 2018;10:105–13. PMID:5783151. doi:10.2147/cmar.s152232.
- Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi:10.1016/j.molmed.2014.10.009. PMID:4282825.
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-Regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi:10.1126/scitranslmed.3006504. PMID:4136707.
- Andersen R, Borch TH, Draghi A, Gokuldass A, Rana MAH, Pedersen M, Nielsen M, Kongsted P, Kjeldsen JW, Westergaard MCW, et al. T cells isolated from patients with checkpoint inhibitor-resistant melanoma are functional and can mediate tumor regression. Ann Oncol. 2018;29(7):1575–81. doi:10.1093/annonc/mdy139.
- Yin H, Guo W, Sun X, Li R, Feng C, Tan Y. Tils and anti-PD1 therapy: an alternative combination therapy for PDL1 negative metastatic cervical cancer. J Immunol Res. 2020;2020:8345235. PMID:Pmc7492938. doi:10.1155/2020/8345235.
- Fucikova J, Rakova J, Hensler M, Kasikova L, Belicova L, Hladikova K, Truxova I, Skapa P, Laco J, Pecen L, et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin Cancer Res. 2019;25(15):4820–31. doi:10.1158/1078-0432.ccr-18-4175.
- He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12(5):814–23. doi:10.1016/j.jtho.2017.01.019.
- Pedersen M, Westergaard MCW, Milne K, Nielsen M, Borch TH, Poulsen LG, Hendel HW, Kennedy M, Briggs G, Ledoux S, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Oncoimmunology. 2018;7(12):e1502905. doi:10.1080/2162402x.2018.1502905. PMID:6279323.
- Yannelli JR, Wroblewski JM. On the road to a tumor cell vaccine: 20 years of cellular immunotherapy. Vaccine. 2004;23(1):97–113. doi:10.1016/j.vaccine.2003.12.036.
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14(5):1455–63. doi:10.1158/1078-0432.ccr-07-0371. PMID:2879140.
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616–31. doi:10.1158/2326-6066.cir-14-0027. PMID:4082460.
- Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72(16):3997–4007. doi:10.1158/0008-5472.can-12-1377.
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-Term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–17. doi:10.1200/jco.2007.15.0284.
- Lövgren T, Wolodarski M, Wickström S, Edbäck U, Wallin M, Martell E, Markland K, Blomberg P, Nyström M, Lundqvist A, et al. Complete and long-lasting clinical responses in immune checkpoint inhibitor-resistant, metastasized melanoma treated with adoptive T cell transfer combined with DC vaccination. Oncoimmunology. 2020;9(1):1792058. doi:10.1080/2162402x.2020.1792058. PMID:Pmc7458624.
- Song S, Zhang K, You H, Wang J, Wang Z, Yan C, Liu F. Significant anti-tumour activity of adoptively transferred T cells elicited by intratumoral dendritic cell vaccine injection through enhancing the ratio of CD8(+) T cell/regulatory T cells in tumour. Clin Exp Immunol. 2010;162(1):75–83. doi:10.1111/j.1365-2249.2010.04226.x. PMID:Pmc2990932.
- Burgers WA, Blanchon L, Pradhan S, de Launoit Y, Kouzarides T, Fuks F. Viral oncoproteins target the DNA methyltransferases. Oncogene. 2007;26(11):1650–55. doi:10.1038/sj.onc.1209950. PMID:3350866.
- Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–53. doi:10.1038/nature15520. PMID:4779053.
- Dan H, Zhang S, Zhou Y, Guan Q. DNA methyltransferase inhibitors: catalysts for antitumour immune responses. Onco Targets Ther. 2019;12:10903–16. PMID:Pmc6913319. doi:10.2147/ott.s217767.
- Covre A, Coral S, Nicolay H, Parisi G, Fazio C, Colizzi F, Fratta E, Di Giacomo AM, Sigalotti L, Natali PG, et al. Antitumor activity of epigenetic immunomodulation combined with CTLA-4 blockade in syngeneic mouse models. Oncoimmunology. 2015;4(8):e1019978. doi:10.1080/2162402x.2015.1019978. PMID:4570131.
- Saleh MH, Wang L, Goldberg MS. Improving cancer immunotherapy with DNA methyltransferase inhibitors. Cancer Immunol Immunother. 2016;65(7):787–96. doi:10.1007/s00262-015-1776-3.
- Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol. 2013;19(29):4808–17. doi:10.3748/wjg.v19.i29.4808. PMID:3732856.
- Sahin M, Sahin E, Koksoy S. Regulatory T cells in cancer: an overview and perspectives on cyclooxygenase-2 and Foxp3 DNA methylation. Hum Immunol. 2013;74(9):1061–68. doi:10.1016/j.humimm.2013.05.009.
- Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, Oshlack A, Harrison LC. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 2013;122(16):2823–36. doi:10.1182/blood-2013-02-481788. PMID:3798997.
- Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7). doi:10.3390/ijms18071414. PMID:Pmc5535906.
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–51. doi:10.1038/nrc3237. PMID:3967236.
- Cao K, Wang G, Li W, Zhang L, Wang R, Huang Y, Du L, Jiang J, Wu C, He X, et al. Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene. 2015;34(49):5960–70. doi:10.1038/onc.2015.46. PMID:4672172.
- Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183(3):1695–704. doi:10.4049/jimmunol.0900592. PMID:2893405.
- Zhang F, Zhou X, DiSpirito JR, Wang C, Wang Y, Shen H. Epigenetic manipulation restores functions of defective CD8+ T cells from chronic viral infection. Mol Ther. 2014;22(9):1698–706. doi:10.1038/mt.2014.91. PMID:4435497.
- Kroesen M, Gielen P, Brok IC, Armandari I, Hoogerbrugge PM, Adema GJ. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget. 2014;5(16):6558–72. doi:10.18632/oncotarget.2289. PMID:Pmc4196144.
- Gomez S, Tabernacki T, Kobyra J, Roberts P, Chiappinelli KB. Combining epigenetic and immune therapy to overcome cancer resistance. Semin Cancer Biol. 2020;65:99–113. PMID:7308208. doi:10.1016/j.semcancer.2019.12.019.