ABSTRACT
The study assessed long-term immunopersistence and safety of the Escherichia coli (E. coli)-produced HPV-16/18 bivalent vaccine. In total, 979 participants in the initial immunogenicity noninferiority study, including girls aged 9-14 years who were randomized in a 1:1 ratio to receive 2 doses at months 0 and 6 (n = 301) or 3 doses at months 0, 1 and 6 (n = 304); girls aged 15-17 years (n = 149) and women aged 18–26 years (n = 225) who received 3 doses of the vaccine, were invited to participate in follow-up to 30 months post vaccination (NCT03206255). Serum samples were collected at months 18 and 30, and anti-HPV-16/18 IgG antibodies were measured by enzyme-linked immunosorbent assay. Serious adverse events (SAEs) occurred from month 7 through month 30 were recorded. At month 30, in the per-protocol set, all participants remained seropositive, except for one girl in the 9-14 years (2 doses) group who seroconverted to negative for HPV-18. HPV-16 and HPV-18 antibody levels were higher in girls aged 9-17 years who received 3 doses (125.3 and 60.2 IU/ml) than in women aged 18-26 years who received 3 doses (72.6 and 28.3 IU/ml), and those in girls aged 9-14 years who received 2 doses (73.2 and 24.9 IU/ml) were comparable to those in women aged 18-26 years who received 3 doses. No SAEs were reported to be causally related to vaccination. The E. coli-produced bivalent HPV-16/18 vaccine is safe and induces persistent protective antibodies for up to 30 months after vaccination in girls aged 9-17 years receiving 2 or 3 doses.
Introduction
Human papillomavirus (HPV) infection has been identified as a cause of cervical cancer, which is the fourth most common cause of cancer-related morbidity and mortality in women worldwide.Citation1,Citation2 Annually, nearly 600,000 new cases of and 340,000 deaths due to cervical cancer occur worldwide.Citation2 In China, the estimated numbers are 109,741 and 59,060, respectively.Citation3 Among oncogenic HPV types, HPV-16 and HPV-18 are the most common and account for approximately 70% of cervical cancer cases.Citation4,Citation5
As an effective primary cervical cancer prevention tool, HPV prophylactic vaccines are expected to reduce the impact of this public health problem. Four safe and highly effective vaccines against HPV have been available since 2006, including two HPV-16/18 bivalent vaccines (Cervarix®, GSK; and Cecolin®, Xiamen Innovax), the HPV-6/11/16/18 quadrivalent vaccine (Gardasil®, Merck), and the HPV-6/11/16/18/31/33/45/52/58 9-valent vaccine (Gardasil ®9, Merck), and vaccination programs are being implemented. Due to the high incidence of cervical HPV infection in women attributable to their first sexual relationship and considering the nature of HPV prophylactic vaccines, vaccination before the onset of sexual activity is an ideal public health strategy.Citation6 The World Health Organization (WHO) recommends the vaccination of adolescent girls in all countries; a 2-dose schedule with a 6-month interval between doses is recommended for girls aged 9-14 years, given that the current evidence indicates that the immunogenicity induced by 2 doses of the HPV vaccine in girls aged 9-14 years is noninferior to that induced by 3 doses in women aged 15-24 years.Citation7 However, the risk of HPV infection persists throughout a woman’s sexual life. Thus, the duration of protection provided by HPV vaccination is critical to the vaccine’s overall effectiveness.
The new HPV-16/18 vaccine from Xiamen Innovax Biotech, Xiamen, China (Cecolin®) is a mixture of two aluminum hydroxide adjuvant-absorbed recombinant HPV-16 and HPV-18 L1 virus-like particles (VLPs) expressed in Escherichia coli (E. coli), which is stable, highly productive and inexpensive.Citation8 A phase III, double blind, randomized, controlled trial (NCT01735006) showed that the new vaccine was highly effective against HPV-16/18 persistent infection and associated cervical intraepithelial neoplasia grade 2 or higher (CIN2+) in Chinese women aged 18-45 years.Citation9 Clinical endpoints could not be analyzed in girls due to ethical considerations, and immunobridging results were used to indirectly demonstrate the vaccine’s effectiveness in young girls. A single-centered, age-stratified immunobridging study (NCT02562508) demonstrated noninferiority of the immune response 1 month after the full course of vaccination in Chinese girls aged 9-14 years who received 2 doses and girls aged 9-17 years who received 3 doses compared to that in young women aged 18-26 years who received 3 doses.Citation10 Based on these positive results, Cecolin® was licensed in China on 31 December 2019, and is the only HPV vaccine approved for a 2-dose schedule in China to date. Moreover, Cecolin® was accepted by the WHO for prequalification on 14 October 2021. Accordingly, the coverage of HPV vaccines will be greatly increased in China and other developing countries, which is of great significance to achieve the global strategic goal of eliminating cervical cancer by 2030.Citation11
Currently available data have demonstrated that the vaccine induces robust protection for up to 5.5 years following vaccination in women aged 18-45 years, and the antibody levels at month 7 were inversely associated with age;Citation12,Citation13 however, the antibody kinetic profiles in females in different age groups are unknown. Moreover, girls aged 9-14 years may not engage in sexual activity for an additional 3-8 years, as a multicenter survey has suggested that the median age of sexual debut is approximately 17 years in Chinese females;Citation14 therefore, exploring the duration of immune responses in girls after the completion of a full or reduced-dose series to determine whether a booster is necessary and if so, when to administer the booster before the implementation of vaccine programs is important and urgent. Here, we present the results of an immunobridging follow-up study (NCT03206255) that assessed the persistence of the immune response against HPV-16 and HPV-18 for up to 30 months after the first vaccination.
Methods
Study design and population
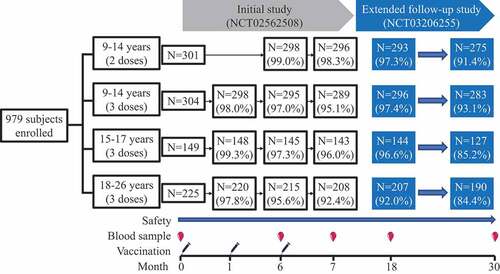
This study is an ongoing extension of an immunobridging study on an E. coli-produced HPV bivalent vaccine (Cecolin®, Xiamen Innovax Biotech, Xiamen, China) (Initial study, NCT02562508). The initial study was completed in July 2016, and the protocol and results of the trial have been reported previously.Citation10 In brief, from 5 December 2015, to 13 December 2015, 754 healthy Chinese girls aged 9-17 years and 225 healthy Chinese women aged 18-26 years from Sheyang County, Jiangsu Province, China, were enrolled to receive an E. coli-produced recombinant HPV-16 and HPV-18 bivalent vaccine; this study aimed to compare the immunogenicity induced by the HPV vaccine in girls receiving 2 or 3 doses with that in young adult women receiving 3 doses. Girls aged 9–14 years were randomized to receive 2 doses (at months 0 and 6) or 3 doses (at months 0, 1 and 6) in an age-stratified manner (9-11 years and 12-14 years, with a ratio of 1:1). Girls aged 15-17 years and young adult women aged 18-26 years were allocated to receive 3 doses (at months 0, 1 and 6). Blood samples were collected on day 0, in month 6, and in month 7 of the study to evaluate immunogenicity, and safety data were collected and assessed. The trial excluded individuals who were pregnant, had immunosuppressive/immunodeficient conditions, had allergic disease, had serious medical conditions or were previously vaccinated against HPV. In this extended follow-up study, all subjects were invited to donate blood samples at month 18 and month 30 for immunogenicity analysis, and serious adverse events (SAEs) that occurred between month 7 and month 30 were carefully investigated and recorded for safety assessment (). Written informed consent was obtained from each participant, or their legal guardian, who was invited to participate in this extension study. The trial was registered with ClinicalTrails.gov (NCT03206255), and the protocols were approved by the Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention (JSJK2017-B005-02).
Antibody detection
All serum samples were evaluated for anti-HPV-16 and anti-HPV-18 IgG using a type-specific enzyme-linked immunosorbent assay (ELISA), as reported previously.Citation10,Citation12,Citation15 In brief, each well of a 96-well microtiter plate was coated with HPV-16 or HPV-18 VLPs expressed by E. coli. After blocking, serially diluted serum samples were added, and then horseradish peroxidase-conjugated goat anti-human IgG was added. After the color reaction, the optical density (OD) was read at 450/620 nm. Antibody titers were calculated using diluted samples with an OD that fell within the working range of the standard curve. References traceable to WHO International Standards for antibodies against HPV-16 (NIBSC code 05/134) or HPV-18 (NIBSC code 10/140) were used for serum quantification. Seropositivity was defined as an antibody level greater than or equal to the assay threshold of 3.0 international units per mL (IU/ml) for HPV-16 and 2.1 IU/ml for HPV-18. Seronegative samples were assigned an arbitrary value of half the threshold value as the antibody level.
Statistical analysis
The primary objective of this study was to evaluate the durability of vaccine-induced serum HPV-16 and HPV-18 antibodies following the administration of a 2-dose regimen of the E. coli-produced HPV bivalent vaccine in girls aged 9-14 years and a 3-dose regimen in girls aged 9-17 years. The secondary objective was to evaluate the long-term safety of the vaccine during follow-up. For comparison of immunogenicity levels among the vaccination groups, the Newcombe-Wilson methodCitation16 was used to calculate differences in seropositivity rates, and the ratio of geometric mean concentrations (GMCs) was computed using the T test for log-transformed concentrations.
The primary outcome of interest was analyzed in the per-protocol set (PPS), which was composed of participants who met the following requirements: 1) seronegativity for the corresponding IgG antibody at Day 0; 2) receipt of all the scheduled vaccine doses, with no important protocol violations; and 3) available results for serological IgG antibody at month 18 or month 30. The results of the intention-to-treat (ITT) population are also presented, which included all participants who received at least one dose, regardless of their antibody status at entry and whether they adhered to the protocol. Safety analyses were based on all the subjects who participated in this extended follow-up study.
Statistical analyses were performed with SAS version 9.4 software (SAS Institute, Cary, North Carolina).
Results
Study participants
All 979 participants in the initial study were invited to participate in this follow-up study, initiated at month 18 post first vaccination. Study compliance was high; 96.0% (940/979) of the participants were followed at month 18, and 89.4% (875/979) of the participants were followed at month 30. Compliance in 9- to 17-year-old girls was higher than that in 18- to 26-year-old young women (). However, age was still balanced between the 2-dose and 3-dose subgroups of the 9- to 14-year-old group who participated at both month 18 and month 30. A total of 83.0% (780/940) and 87.1% (819/940) of the participants were included in the PPS analysis of HPV-16 and HPV-18 at month 18, respectively. The percentage of participants included in the PPS analysis at month 30 (83.1%, 727/875 for HPV-16 and 87.5%, 766/875 for HPV-18) was similar to that at month 18 (). The most common reason for exclusion from the PPS analysis was seropositivity for the corresponding HPV type IgG at Day 0 (before vaccination). The seropositivity rates and the GMC level of natural infection at Day 0 among groups were reported previously in the initial study.Citation10
Table 1. Characteristics of the participants.
Immunogenicity
In the PPS, all participants remained seropositive for IgG antibodies against HPV-16 and HPV-18 (both 100%) at month 18 after the first vaccination. At month 30, the seropositivity rate of HPV-16 IgG antibodies was also 100%, and only one girl in the 9-14 years (2 doses) group seroconverted to negative for HPV-18 IgG antibodies (). In the ITT analysis, the majority of the participants (>99.0%) remained seropositive for HPV-16 and HPV-18 IgG for the duration of follow-up through month 30 (Table S1).
Table 2. Seropositive rate of IgG antibodies in the PPS cohort.
The immunological kinetic profiles showed that both vaccine genotype antibodies peaked at month 7 and then subsequently declined through month 30, but the declining trend slowed with time (). At month 30, the GMCs (95% CI) for HPV-16 IgG antibodies were 125.3 (115.3, 136.1) IU/ml in girls aged 9-17 years who received 3 doses, 73.2 (66.0, 81.1) IU/ml in girls aged 9-14 years who received 2 doses, and 72.6 (63.9, 82.6) IU/ml in women aged 18-26 years who received 3 doses. The GMCs of HPV-18 antibodies were 60.2 (54.8, 66.2) IU/ml, 24.9 (22.4, 27.6) IU/ml and 28.3 (24.6, 32.6) IU/ml, respectively. At months 18 and 30, the GMCs for HPV-16 antibodies (between 1.33- and 1.92-fold) and HPV-18 antibodies (between 1.47- and 2.67-fold) were higher in girls aged 9-14 years and girls aged 15-17 years who received 3 doses than in women aged 18-26 years who received 3 doses. The GMCs in girls aged 9-14 years who received 2 doses were comparable to those in women aged 18-26 years who received 3 doses (GMC ratio: 1.01-1.05) for HPV-16, while the GMCs in girls aged 9-14 years who received 2 doses were slightly lower than those in women aged 18-26 years who received 3 doses (GMC ratio: 0.88-0.91) for HPV-18 ( and Table S2). The results between the ITT analysis and PPS analysis were consistent (Table S3). Moreover, similar to the previously reported correlation between GMC levels and age at month 7,Citation12 the GMCs of IgG antibodies against both HPV-16 and −18 decreased with increasing age at month 30. Remarkably, however, in girls who received 2 doses at months 0 and 6, the immune response against HPV-16 and HPV-18 was the weakest in those aged 9 years (58.8 IU/ml and 19.4 IU/ml); the GMC increased to the peak level in those aged 10 years for HPV-16 (84.1 IU/ml) and aged 12 years for HPV-18 (31.0 IU/ml) ().
Figure 2. Immunological kinetic profiles in the per-protocol set. Error bars represent 95% confidence intervals; IU/ml, international units per ml; 9-14 years (2 doses): girls aged 9-14 years who were vaccinated at 0 and 6 months; 9-14 years (3 doses): girls aged 9-14 years who were vaccinated at 0, 1, and 6 months; 9-17 years (3 doses): girls aged 9-17 years who were vaccinated at 0, 1, and 6 months; 18-26 years (3 doses): girls aged 18-26 years who were vaccinated at 0, 1, and 6 months.
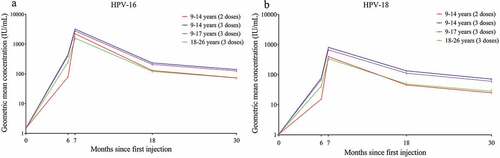
Figure 3. Geometric mean concentrations (GMCs) of IgG antibodies at month 30 after the first dose (PPS). (a): GMCs of IgG antibodies against HPV-16, (b): GMCs of IgG antibodies against HPV-18; 3 doses, received 3 doses at 0, 1 and 6 months; 2 doses, received 2 doses at 0 and 6 months; IU/ml, international units per ml; GMCs associated with natural infection were obtained from women aged 18-26 years in the initial studyCitation10.
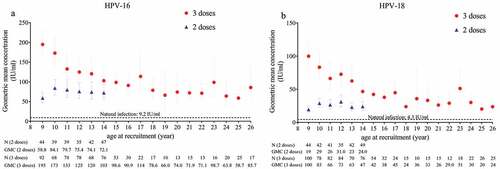
Table 3. Geometric mean concentrations (GMCs) of IgG antibodies in the PPS cohort.
Safety
Twenty SAEs in nineteen participants were reported: five in the 9-14 years age group (2 doses), seven in the 9-17 years age group (3 doses), and eight in the 18-26 years age group (3 doses). However, none were fatal, and none were considered by the investigator to be related to the study vaccine (Table S4).
Discussion
The duration of protection induced by vaccines is a critical factor in their utility as public health interventions. This follow-up analysis of the immunobridging study on the E. coli-produced HPV-16/18 bivalent vaccine demonstrates that the vaccine administered to adolescent girls with a 2- or 3-dose regimen and young women with a 3-dose regimen induces a long-term immune response. Although this study was not designed to assess efficacy, the levels of antibodies against HPV-16 and −18 in adolescent girls who received 3 doses at month 30 were higher than those in women aged 18-45 year. In girls aged 9-14 years who received 2 doses, the levels were comparable to the plateau levels observed in women aged 18-45 years in a previous study, in which sustained efficacy of the HPV-16/18 bivalent vaccine against infections and cervical lesions was demonstrated up to 66 months.Citation13 In addition, there were no SAEs reported to be causally related to the investigational vaccine during the follow-up period, indicating the safety of the vaccine. To our knowledge, this is the first report on the duration of the immune response in young adolescent girls receiving a reduced-dose schedule of the E. coli-produced HPV-16/18 bivalent vaccine for up to 30 months, which will help inform public health program planning.
In this study, serum antibodies against HPV-16 and -18 were measured using a type-specific ELISA, which detects IgG antibodies that bind to the HPV L1 VLP coating. Two other serological assays are also widely used for measuring HPV antibodies: the pseudovirion-based neutralization assay (PBNA), which measures total neutralizing antibodies that can block the entry of pseudovirions into cultured cells, and the competitive Luminex immunoassay (cLIA), which measures antibodies competing with a dominant monoclonal antibody against a neutralizing epitope.Citation17 Among these assays, PBNA is considered the gold standard; however, it is difficult to use in large sample studies due to its complexity and labor intensity. Fortunately, earlier studies have shown that neutralizing antibodies measured by PBNA and IgG antibodies measured by ELISA correlate well in analyses of post-HPV vaccination antibodies.Citation15,Citation18 Therefore, IgG antibodies measured by ELISA were adopted to assess the immunogenicity persistence induced by the vaccine in this study.
The kinetics of anti-HPV-16 and −18 antibody responses in all groups were consistent and were similar to those observed in previous clinical trials: the antibody levels declined after month 7; however, the declining trend slowed with time.Citation19–25 In addition, similar to the immune response at month 7, the GMC level was inversely associated with age, and in the participants who received 2 doses, the GMC was lowest in 9-year-old girls at month 30, with GMCs of 58.8 IU/ml for HPV-16 and 19.4 IU/ml for HPV-18, which were approximately 6.5- and 4.5-fold higher than those acquired from natural infection, respectively. Although there are no data after 30 months in this study, it is expected that the antibody levels would be maintained at levels several-fold higher than natural infection levels for a long time, as reported previously, in which vaccine-induced IgG antibodies decreased during the first year after the final vaccination and then remained relatively stable. The critical threshold of anti-HPV antibodies representing an immunological correlate for vaccine effectiveness has not been determined. Nevertheless, studies reported that low vaccine-induced antibody concentrations after one dose induced levels of protection against vaccine-targeted HPV infections similar to those induced by high antibody concentrations from two or three doses until 7 years post vaccination with Gardasil®Citation26 and 11 years postvaccination with Cervarix®.Citation27 Moreover, some studies indicated that naturally acquired antibodies were associated with a reduced risk of subsequent homotypic infection.Citation28–32 These data suggest that the protective antibody level for HPV might be even lower. Given the above results, the vaccine could induce long-term protection in adolescent girls aged 9-17 years who receive 3 doses and in girls aged 9-14 years who receive 2 doses. Nevertheless, more follow-up data are necessary. Considering the vaccination schedule and characteristics of sexual debut and sexual behavior, it may be optimal to initiate immunization programs in girls of 10 to 14 years of age.
One of the limitations of this study is the limited follow-up time. Although the risk of cervical HPV infection is highest soon after the first sexual contact, women remain susceptible throughout their sexual lives.Citation6 Whether the duration of protection covers the entire sexual life expectancy and whether a booster dose is required, especially in girls who receive 2 doses, remain to be studied; however, such studies are in progress. Serum samples at month 60 post the first dose have been collected, and longer time of follow-up is planned. Another limitation is the lack of data on clinical efficacy. The initial study was not designed as an efficacy study, and the evaluation of the vaccine’s efficacy was difficult without a control group. A further limitation is the fact that due to the very conserved attitude toward sexual activity, we did not collect information about sexual behavior of these girls to identify whether subjects had been potentially exposed to HPV in the follow-up period; therefore, we could not assess whether the antibody levels were affected by HPV exposure.
In conclusion, this follow-up study showed that the E. coli-produced bivalent HPV-16/18 vaccine is generally safe and well tolerated and is immunogenically noninferior at 30 months after vaccination in adolescent girls receiving 2 doses or 3 doses to that in adult women receiving 3 doses, among whom the extremely high efficacy of the vaccine has been demonstrated. Additional data are needed to determine longer-term immunopersistence and the clinical efficacy of the vaccine in adolescent girls after they become sexually active. However, the presently available evidence supports the introduction of the vaccine into immunization programs targeting young adolescent girls.
Author contributions
T Wu, Y Hu, N Xia, J Zhang, X Yao and W He contributed to the study design. X Yao, W He, X Wu, J Gu, J Zhang, B Lin, Z Bi, S Huang, and Y Hu contributed to sample collection and experiment or data interpretation. X Yao, T Wu, Y Su, and J Zhang contributed to the data analysis and writing of the report. All authors reviewed or revised the manuscript and approved the final draft for submission.
Supplemental Material
Download MS Word (37.6 KB)Acknowledgments
We thank all the study participants.
Disclosure statement
Bizhen Lin reports being a current employee of Xiamen Innovax Biotech Company, which provided the vaccines in the immunobridging study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2061248.
Additional information
Funding
References
- de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–8. doi:10.1016/j.bpobgyn.2017.08.015.
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J; Bosch FX, dS S ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 22 October 2021. [accessed 2022 Jan 15].
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, Bosch FX, and dS S ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in China. Summary Report 22 Oct 2021. [accessed 22 Oct 2021].
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi:10.1016/S1470-2045(10)70230-8.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi:10.1002/ijc.30716.
- Collins S, Mazloomzadeh S, Winter H, Blomfield P, Bailey A, Young LS, Woodman CB. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. Bjog. 2002;109(1):96–98. doi:10.1111/j.1471-0528.2002.01053.x.
- Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec. 2017;92(19):241–68. http://www.ncbi.nlm.nih.gov/pubmed/28530369 .
- Gu Y, Wei M, Wang D, Li Z, Xie M, Pan H, Wu T, Zhang J, Li S, Xia N. Characterization of an Escherichia coli-derived human papillomavirus type 16 and 18 bivalent vaccine. Vaccine. 2017;35(35 Pt B):4637–45. doi:10.1016/j.vaccine.2017.06.084.
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human Papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020;112(2):145–53. doi:10.1093/jnci/djz074.
- Hu YM, Guo M, Li CG, Chu K, He WG, Zhang J, Gu JX, Li J, Zhao H, Wu XH, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. 2020;63(4):582–91. doi:10.1007/s11427-019-9547-7.
- WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. https://www.who.int/publications/i/item/9789240014107.
- Chen Q, Zhao H, Yao X, Lin Z, Li J, Lin B, Wang R, Huang Y, Su Y, Wu T, et al. Comparing immunogenicity of the Escherichia coli-produced bivalent human papillomavirus vaccine in females of different ages. Vaccine. 2020;38(39):6096–102. doi:10.1016/j.vaccine.2020.07.030.
- Specification of Cecolin®. http://www.innovax.cn/research.aspx?BaseInfoCateID=121&CateID=121 .
- Zhao FH, Tiggelaar SM, Hu SY, Xu LN, Hong Y, Niyazi M, Gao XH, Ju LR, Zhang LQ, Feng XX, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol. 2012;36(4):384–90. doi:10.1016/j.canep.2012.01.009.
- Zhao H, Lin ZJ, Huang SJ, Li J, Liu XH, Guo M, Zhang J, Xia NS, Pan HR, Wu T, et al. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Human Vaccines Immunother. 2014;10(3):740–46. doi:10.4161/hv.27619.
- Newcombe RG. Interval estimation for the difference between independent proportions: Comparison of eleven methods. Stat Med. 1998;17(8):873–90. doi:10.1002/(Sici)1097-0258(19980430)17:8<873:Aid-Sim779>3.0.Co;2-I.
- Lin SW, Ghosh A, Porras C, Markt SC, Rodriguez AC, Schiffman M, Wacholder S, Kemp TJ, Pinto LA, Gonzalez P, et al. HPV16 seropositivity and subsequent HPV16 infection risk in a naturally infected population: comparison of serological assays. PLoS One. 2013;8(1):e53067. doi:10.1371/journal.pone.0053067.
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MPM, Poncelet SM, Pinto LA, Wettendroff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccines. 2008;4(6):425–34. doi:10.4161/Hv.4.6.6912.
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–55. doi:10.1016/S0140-6736(06)68439-0.
- GlaxoSmithKline Vaccine, HPVSG; Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374(9706):1975–85. doi:10.1016/S0140-6736(09)61567-1.
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;27(4):581–87. doi:10.1016/j.vaccine.2008.10.088.
- De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, Sanchez N, Schuind A. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28(38):6247–55. doi:10.1016/j.vaccine.2010.07.007.
- Petaja T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, Lehtinen M, Descamps D. Long-Term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129(9):2147–57. doi:10.1002/ijc.25887.
- Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8(3):390–97. doi:10.4161/hv.18865.
- Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, Zahaf T, Catteau G, Geeraerts B, Descamps D. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10(8):2147–62. doi:10.4161/hv.29532.
- Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, Bhatla N, Nene BM, Shaw J, Poli URR, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36(32):4783–91. doi:10.1016/j.vaccine.2018.02.087.
- Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, Wagner S, Boland J, Schussler J, Lowy DR, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020;112(10):1038–46. doi:10.1093/jnci/djaa011.
- Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, Quint W, van Doorn LJ, Sherman ME, Xhenseval V, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. JNCI J Nat Cancer Inst. 2010;102(21):1653–62. doi:10.1093/jnci/djq384.
- Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human Papillomavirus infection: a systematic review and meta-analysis. J Infect Dis. 2016;213(9):1444–54. doi:10.1093/infdis/jiv753.
- Safaeian M, Castellsague X, Hildesheim A, Wacholder S, Schiffman MH, Bozonnat MC, Baril L, Rosillon D; HPVVT Costa Rica, and Psg. The risk of HPV-16/18 infections and associated cervical abnormalities in women seropositive for naturally acquired antibodies: pooled analysis based on control arms of two large clinical trials. J Infect Dis. 2018;218(1):84–94. doi:10.1093/infdis/jiy112.
- Rosillon D, Baril L, Del Rosario-Raymundo MR, Wheeler CM, Skinner SR, Garland SM, Salmeron J, Lazcano-Ponce E, Vallejos CS, Stoney T, et al. Risk of newly detected infections and cervical abnormalities in adult women seropositive or seronegative for naturally acquired HPV-16/18 antibodies. Cancer Med. 2019;8(10):4938–53. doi:10.1002/cam4.1879.
- Yao X, Chen W, Zhao C, Wei L, Hu Y, Li M, Lin Z, Lin B, Liu X, Hong Y, et al. Naturally acquired HPV antibodies against subsequent homotypic infection: a large-scale prospective cohort study. 2021;13:100196. doi:10.1016/j.lanwpc.2021.100196.
