ABSTRACT
There is a wealth of data suggesting that the effectiveness of existing vaccines against the Omicron variant, the most mutated SARS-CoV-2 variant to date, has been substantially reduced if only primary vaccination is administered. Therefore, the effectiveness of booster vaccination against the Omicron variant has become a topic of current interest. We conducted a comprehensive search in PubMed, Embase, and the Cochrane Library to collect various pseudovirus neutralization tests or live virus neutralization tests for the Omicron variant, with serum specimens from booster vaccinees. We extracted neutralization titers for the Omicron variant, the original strain, and the other variants before and after booster vaccination, and then manually calculated the fold increase or decrease in neutralization titers for the Omicron variant relative to the other variants, and the fold increase in neutralization titers for the Omicron variant after booster vaccination compared with that before booster vaccination. In the two-dose vaccination regimen, the neutralization titers against the Omicron variant decreased substantially compared to the original strain and other variants. However, after booster vaccination, both homologous and heterologous booster vaccination, the neutralization of serum antibodies against the Omicron variant was significantly improved, although still lower than that of the original strain and other variants. The booster vaccination program based on existing vaccines can produce broad but incomplete immunity against the Omicron variant.
Introduction
With the rapid spread of the Omicron variant (B.1.1.529), the effectiveness of existing vaccines has once again become the focus of the world. However, the Omicron variant is far more mutated than previous variants, potentially giving it an unprecedented ability to escape immunity and thus making existing vaccines less effective. As the most mutated syndromic coronavirus 2 (SARS-CoV-2) variant to date, the Omicron variant is known to contain more than 50 variants, 32 of which are located in the spike protein.Citation1,Citation2 As a binding protein in SARS-CoV-2 that recognizes host cells, the spike protein mediates viral invasion of human cells and drives the host immune response by binding to the angiotensin-converting enzyme 2 (ACE2) receptor.Citation3 Therefore, most of the existing coronavirus disease 2019 (COVID-19) vaccines have been developed with the spike protein as the primary target. However, a large number of mutations in the spike protein, especially in the receptor binding domain (RBD), may not only increase the binding affinity of the RBD-hACE2 complex significantly, but also create an unpredictable immune escape capacity, making vaccines, especially mRNA vaccines targeting the spike protein, significantly less effective.
In fact, these are not just speculations obtained through microstructural studies, but ideas that have been validated in further serum neutralization tests.Citation4–6 Although the studies varied in methodology and subject characteristics, they all demonstrated that the various types of COVID-19 vaccines available were not effective against the Omicron variant if only the primary vaccination was administered, a finding that has caused anxiety in the community.
Fortunately, the researchers found that a booster vaccination program may provide satisfactory protection against the Omicron variant.Citation5,Citation7,Citation8 In this systematic review, we incorporated the currently available literature on booster vaccination against the Omicron variant and summarized the effects of various regimens on the seroneutralizing effect of the Omicron variant.
Materials and methods
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Citation9
Search strategy
Using “SARS-CoV-2”, “COVID-19”, “COVID-19 Vaccines”, “Omicron” and “B.1.1.529” as keywords, we firstly conducted a comprehensive search in PubMed, Embase, and Cochrane Library on 6 January 2022, and then an updated search for newly published articles on 27 February 2022. The specific search formula is shown in the Supplementary Material. The reference lists of relevant reviews were also manually searched for potentially eligible investigations.
Study selection
Studies reporting on neutralizing antibodies against the Omicron variant were included, regardless of language or publication status. By using live virus or pseudovirus neutralization assays, the included studies evaluated the neutralization of the Omicron variant by serum antibodies and compared neutralization titers against the Omicron variant with those of the original strain or other variants of concern (VOCs), including the Alpha, Beta, Gamma and Delta variants. Serum samples were obtained from vaccinees who received a booster vaccination program, regardless of the type of vaccine received, and there were no restrictions on their age or sex. If the serum specimens were from people with a history of prior infection, pregnant women, patients with cancer, patients on dialysis therapy, and other immunocompromised population, the trial would be excluded.
The primary outcome indicator was the neutralization of serum antibodies against the Omicron variant as well as the original strain or other VOCs, 7 or 14 days after receiving the booster vaccination regimen. Secondary outcome indicators included (1) the neutralization of serum antibodies to the Omicron variant as well as to the ancestral strain or other VOCs, 7 or 14 days after receiving the primary vaccination regimen; (2) The folds decrease in neutralization of the Omicron variant, compared to the ancestral strain or the Delta variant, after receiving the primary vaccination regimen or booster vaccination program; (3) The folds increase in the neutralizing effect of serum antibodies against each variant after booster, compared to pre-booster vaccination. Information on more than three variants, or data on the original strain and more than two variant should be provided. Studies not related to the neutralization of Omicron by the serum would be excluded. Preference was given to serum neutralization titers after 7 or 14 days post-vaccination. If data for the two time points of 7 or 14 days after vaccination were not available, 28 days post-inoculation would be chosen as the endpoint. If the original study did not provide data for these three time points, other time points would be arbitrarily selected as endpoints. Two reviewers independently assessed the eligibility of studies for inclusion in the systematic review, and all disagreements would be resolved by discussion or consultation with a third reviewer.
Data extraction and quality assessment
Based on a pre-developed extraction form in Microsoft Excel software, two reviewers independently extracted data in duplicate, including title, first author, year of publication, vaccination protocol, applied neutralization assay, characteristics of participants, and outcome indicators with the time point at which they were collected. If the original article did not provide the required data in the form we expected, we would calculate them manually based on the available information. When data were provided in the form of images only, the two reviewers would perform a critical evaluation and then discard any unclear information. To avoid missing information, we attempted to refine the data collection by reading supplementary materials of the included literature. Due to time constraints, we did not obtain additional trial details or raw trial data by contacting the corresponding authors of these articles. Given that this review was based on laboratory studies and not population studies, the risk of bias evaluation was not applicable. If any disagreement was encountered, consensus would be reached through negotiation or consultation with a third reviewer.
Data analysis
Considering the heterogeneity among studies, it may be not feasible to directly compare the serum neutralization titers among studies. Therefore, we mainly determined the effect of booster vaccination on the Omicron variant indirectly, by generalizing the fold changed in the neutralization of the Omicron variant compared to other variants, and increased Omicron neutralization folds after booster vaccination compared to pre-booster vaccination. If necessary, we would perform subgroup analyses based on the type of vaccine, the vaccination regimen (Homologous and heterologous booster vaccination programs), and the neutralization assay (Live virus and pseudovirus neutralization assays).
Result
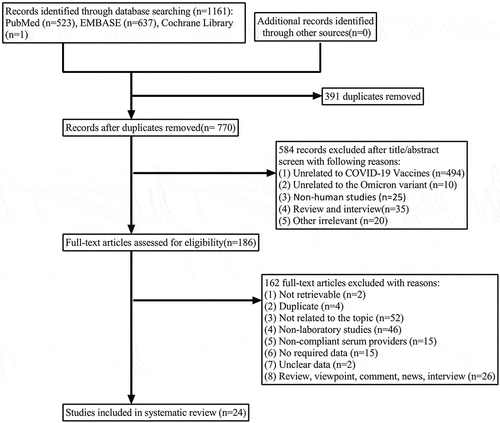
A total of 1161 documents were retrieved, and 770 documents remained after removing duplicates. By reading the titles and abstracts, two reviewers filtered out 584 literatures and included the remaining 186 in the full-text review. Ultimately, a total of 24 literatures were included in this review.Citation7,Citation10–32 During the full-text review process, 162 articles were excluded for various reasons, mainly including unavailability of full text (n = 2), duplicates (n = 4), irrelevant topics (n = 52), non-laboratory studies (n = 46), non-compliant serum providers (n = 15), lack of required data (n = 15), and unclear data provided (n = 2). Non-compliant serum providers were defined as animals (n = 2), pregnant women (n = 1), cancer patients (n = 2), patients on hemodialysis therapy (n = 2), people receiving allogeneic organ transplants (n = 1), previously infected individuals (n = 2), and vaccine recipients with an unclear history of previous infection (n = 5). There were 2 publications excluded because of unclear data, as the neutralization titers for the Omicron variant were only shown as pictures without specific values. In addition, a total of 13 studies were excluded due to unavailability of comparable data for other variants and the original strain, although the researchers discussed the neutralization of the Omicron variant by the serum after booster vaccination.
In 12 of these 24 literatures, researchers used live virus neutralization assays,Citation11,Citation12,Citation16–19,Citation22,Citation23,Citation25,Citation27,Citation30,Citation32 while in the remaining 12 literatures, pseudotyped virus neutralization assays were selected.Citation7,Citation10,Citation13–15,Citation20,Citation21,Citation24,Citation26,Citation28,Citation29,Citation31 A total of four types of COVID-19 vaccines were included in this review, namely mRNA vaccine (BNT162b2 vaccine and mRNA-1273 vaccine), inactivated vaccine (BBIBP-CorV and CoronaVac), vector vaccine (ADZ1222 vaccine, Ad26.COV2.S vaccine and BriLife vaccine), and recombinant protein subunit vaccine (ZF2001 vaccine). The different types of COVID-19 vaccines were combined into eight primary vaccination regimens as well as thirteen booster regimens, seven of which were homologous (3×BBIBP-CorV, 3×CoronaVac, 3×BNT162b2 vaccine, 3×mRNA-1273 vaccine, 3×ADZ1222 vaccine, 3×BriLife vaccine, 3×ZF2001 vaccine), four of which were heterologous (BNT162b2 vaccine + CoronaVac + BNT162b2 vaccine, 2×CoronaVac +1×BNT162b2 vaccine, 2×BNT162b2 vaccine +1×Ad26.COV2.S1 vaccine, 2×BBIBP-CorV +1×ZF2001 vaccine), and two were unknown. All primary vaccination programs required a double dose, except for the Ad26.COV2.S vaccination programCitation14 which required only one dose. The specific screening process and characteristics of the included studies are shown in and . Given that all included studies were in vitro neutralization trials, quality assessment was not applicable.
Table 1. The characteristics of the included studies.
All studies demonstrated neutralization of the Omicron variant, the original strain, or other VOCs before and after booster vaccination. As shown in , two reviewers performed independent secondary processing of the data provided in all articles to obtain the desired multiplicity.
Table 2. Fold decrease in neutralization compared to Prototype/D614 G or other variant.
Table 3. Increased fold in neutralization after booster compared to pre-booster.
After primary inoculation, the neutralization titer against the Omicron variant decreased sharply and was lower than that of the original strain and any of the VOCs (). Using the original strain as a control, the neutralization of the Omicron variant decreased by 1.15 to 109.87-fold, while compared to Delta, the neutralization titers decreased by 1 to 21.5 fold. The neutralization of the Omicron variant by serum antibodies was significantly enhanced in all studies after booster vaccination (). The neutralization titers of the Omicron variant increased 1.17 to 96.94-fold after booster vaccination compared to pre-booster vaccination. In comparison, the original strain increased by 1 to 53.83-fold, the Alpha variant by 3.15-fold, the Beta variant by 2.94 to 119.84-fold, and the Delta variant by 1.80 to 53.81-fold. In addition, the multiple between the neutralization titers of the Omicron variant and that of other strains was significantly reduced, compared to that before the booster inoculation. After booster vaccination, the neutralization of the Omicron variant decreased by 2.2 to 26.87 times compared to the original strain, and 0.77 to 15.66 times compared to the Delta variant. In one study, serum antibodies neutralized the Omicron variant even more than the Delta variant.
Live virus and pseudovirus neutralization assays
Based on the specific neutralization assays applied by the researchers, the subgroup analysis was conducted (). The data showed that the booster vaccination not only led to a significant increase in neutralization titers against the Omicron variant, but also resulted in a reduction in the multiple between the neutralization titers of the Omicron variant and other strains, in both the pseudovirus and live virus assay groups.
Table 4. Increased fold in neutralization after booster compared to pre-booster, with live virus or pseudovirus neutralization assay.
Table 5. Fold decrease in neutralization compared to Prototype/D614 G or other variant, with live virus or pseudovirus neutralization assay.
In the pseudovirus neutralization assay group, booster vaccination resulted in 1 to 34-fold, 3.15-fold, 4.72 to 89.22-fold, 1.80 to 42.80-fold, and 4 to 44.99-fold increases in the neutralization titers of the original strain, Alpha variant, Beta variant, Delta variant, and Omicron variant, respectively. Before booster vaccination, the neutralizing titer of the Omicron variant decreased 11.16 to 109.87-fold and 5.81 to 21.5-fold compared to the original and Delta strains, while after booster vaccination, the fold difference between the Omicron variant and the original and Delta strains was reduced to 2.2 to 15.97-fold and 0.77 to 15.66-fold, respectively.
Using live virus neutralization assay, sera from booster vaccine recipients showed 1.85- to 53.83-fold, 2.94- to 119.84-fold, 2.52- to 53.81-fold, and 1.17- to 96.94-fold greater neutralization of the original strain, Beta variant, Delta variant, and Omicron variant, respectively, compared with sera from primary vaccine recipients. Before receiving the booster vaccination, the neutralizing effect of the serum against the Omicron strain decreased by 1.59 to 104.89 and 1.59 to 14.84 times compared to the original strain and the Delta variant, respectively; after the booster, the fold difference with the original strain and the Delta variant changed to 2.9 to 26.87 and 2.47 to 13.9 times, respectively.
Homologous and heterologous booster vaccination programs
Subgroup analysis was performed according to homo- and heterozygosity of the booster vaccine (). Both in the heterologous and homologous booster groups, the third dose of the vaccine resulted in a substantial increase in the neutralization of the serum against the Omicron variant. Among those who received the homologous booster vaccine, the neutralization titers against the Omicron variant were increased by 1.17 to 96.94-fold, while the original strain, the Alpha variant, the Beta variant and the Delta variant were increased by 1.85 to 53.83-fold, 3.15-fold, 2.94 to 119.84-fold and 1.80 to 53.81-fold, respectively. Similarly, neutralization titers against the Omicron variant, the original strain, the Beta variant and the Delta variant were found to be increased 2 to 15.87-fold, 18.4 to 36.1-fold, 17.4 to 89.22-fold and 27.9 to 42.80-fold, respectively, in the heterologous enhanced inoculation group.
Table 6. Increased fold in neutralization after homologous booster vaccination compared to pre-booster vaccination.
Table 7. Fold decrease in neutralization after homologous vaccination compared to Prototype/D614 G or other variant.
Different types of COVID-19 vaccine
Inactivated vaccine booster group
In the inactivated vaccine group (), serum neutralization of the Omicron variant decreased 1.59 to 68.25-fold and 5.1 to 16.5-fold, respectively, before and after the booster vaccination, using the original strain as a control. After receiving the third dose of vaccine, serum neutralization titers against the Omicron variant increased 1.17 to 8.07-fold in vaccinees, compared to 4.24 to 7.58-fold for the original strain, 2.94 to 24.37-fold for the Beta variant, and 2.52 to 11.79-fold for the Delta variant. Subgroup analyses were performed according to specific vaccine types and neutralization assays. The data showed an 8.07-fold and 1.17 to 7.33-fold increase in neutralization titers for the Omicron variant in the BBIBP-CorV homologous booster and CoronaVac homologous booster groups, as well as a 1.17 to 7.33-fold and an 8.07-fold increase in neutralization titers against the Omicron variant in the live virus and pseudovirus neutralization groups, respectively.
Table 8. Increased fold in neutralization after booster compared to pre-booster in different vaccine groups.
Table 9. Fold decrease in neutralization compared to Prototype/D614 G or other variant in different vaccine groups.
mRNA vaccine booster group
In the mRNA vaccine group (), neutralization titers against the Omicron variant increased 5.47 to 96.94-fold after booster, compared with a 1 to 53.83-fold increase for the original strain, a 3.15-fold increase for the Alpha variant, a 4.72 to 119.84-fold increase for the Beta variant, and a 1.80 to 53.81-fold increase for the Delta variant. For vaccinees who received a double dose of mRNA vaccine, the neutralization of serum antibodies against the Omicron variant decreased by 4 to 109.87-fold if the original strain was used as a control, and by 2.64 to 17.44-fold with the Delta variant as the control. After receiving the booster vaccine, the fold difference between Omicron variant and other strains was significantly reduced. The neutralization of Omicron variant by serum antibodies was reduced by 2.2 to 26.87-fold and 0.77 to 11.79-fold when the original and Delta strains were used as controls, respectively.
Subgroup analysis was performed according to different vaccine types. The data showed that after the third dose of BNT162b2 homologous vaccine, neutralization titers increased 5.53 to 96.94-fold for the Omicron variant, while 2.3 to 53.83-fold, 4.72 to 119.84-fold, and 4.63 to 53.81-fold for the original strain, Beta variant, and Delta variant, respectively. Before and after booster inoculation, the neutralization titers against Omicron decreased 4 to 104.89-fold and 2.2 to 26.87-fold, respectively, compared to the original strain. For mRNA-1273 homologous booster vaccine recipients, the booster sera showed 24.07- to 32.29-fold, 1.86- to 2.80-fold, and 7.48- to 9.85-fold increases in neutralization titers against the Omicron variant, the original strain, and the Beta variant, respectively. Before and after booster vaccination, the neutralization of the Omicron variant by serum antibodies decreased 42.6 to 84-fold and 4.2 to 16.7-fold, respectively, when the original strain was used as a control. In the mRNA-1273/BNT162b2 homologous booster group, the third dose of vaccine resulted in a 26.27- to 44.99-fold, 3.76- to 6.54-fold, and 1.80- to 4.94-fold increase in neutralization titers against the Omicron variant, the original strain, and the Delta variant, respectively. Before and after the booster, serum neutralization against the Omicron variant was respectively reduced by 22.88 to 109.87-fold and 3.28 to 15.97-fold compared to the original strain.
Subgroup analysis based on different neutralization assays showed that the neutralization titers of the Omicron variant increased by 5.90 to 44.99-fold and 5.47 to 96.94-fold, respectively, in the pseudovirus and live virus neutralization assay groups. Boosted vaccination resulted in a reduction in the fold difference between the Omicron variant and the other strains, in both the live virus and pseudovirus neutralization assay groups.
Viral vector vaccine booster group
In the vector vaccine group (), the neutralization titers increased 2.71-fold against the Omicron variant, 1.85-fold against the original strain, and 2.75-fold against the Delta variant after booster vaccination compared to before booster vaccination. Before and after booster vaccination, the neutralization of serum antibodies against Omicron decreased 18.57-fold and 2.9 to 12.7-fold, respectively, with the original strain as a control. Similarly, further specific analyses were performed for different types of vector vaccines and neutralization assays. In the ADZ1222 homologous booster group and the BriLife homologous booster group, serum neutralization to the Omicron variant was reduced by a factor of 12.7 and 2.9 in booster vaccinees, respectively, relative to the original strain. There was no available pseudovirus neutralization test in the vector vaccine group.
Recombinant protein subunit vaccine booster group
For vaccinees who received the homologous recombinant protein subunit booster vaccination, the serum antibodies were 3.1 to 10.6-fold and 4.13 to 10.16-fold less neutralizing to the Omicron variant compared to the original strain and the Delta variant, respectively ().
Mixed vaccine booster group
In addition, in the combination vaccination group (), serum neutralization titers against the Omicron variant increased by 2 to 15.87-fold after booster vaccination compared with those before booster vaccination, while those against the original strain, Beta variant and Delta variant increased by 18.4 to 36.1-fold, 17.4 to 89.22-fold and 27.9 to 42.80-fold, respectively. The neutralization titers of the Omicron variant increased by 2 to 4.76 and 15.87-fold using live virus and pseudovirus neutralization assays, respectively.
Discussion
The neutralizing effect of serum antibodies against the Omicron variant after booster vaccination was indirectly assessed, by pooling the fold decrease in neutralizing titers against the Omicron variant compared to other strains after primary and booster vaccination, as well as the fold increase in neutralizing titers against various strains after booster compared to before booster vaccination. Pooling the available evidence, we found that primary vaccination regimens showed a precipitous decrease in neutralizing titers against the Omicron variant, regardless of the type of COVID-19 vaccine administrated, while booster vaccination did provide good protection against the Omicron variant. After booster vaccination, the Omicron variant neutralization titer increased significantly, and the fold difference between it and other variants was significantly reduced, regardless of the type of vaccine administered, the homology of the booster regimen performed, and the method of neutralization assay used, suggesting that booster vaccination improves not only the level of serum neutralizing antibodies, but also the breadth of the neutralizing response.
Notably, in one study,Citation15 the neutralizing effect of serum antibodies against the Omicron variant even exceeded that against the Delta variant. In this trial,Citation15 the researchers found that after receiving the primary vaccination, the serum titer against Omicron (NT50 121) was much lower than that of the Delta strain (NT50 1354). However, after receiving the booster vaccination, the rise in neutralizing titers against the Omicron variant was much higher (26.27-fold increase) than the Delta strain (1.80-fold increase). After primary vaccination, the neutralization of serum antibodies against the Omicron and Delta variants respectively decreased by 22.88-fold and 2.05-fold, compared to the original strain. After the third dose of vaccine, the fold difference between the neutralizing titers against Omicron and the original strain was reduced to 3.28 fold, while those against the Delta variant and the original strain was increased to 4.28 fold. These data again suggest that booster vaccination can significantly increase the titer and breadth of neutralizing antibodies, especially against the Omicron variant. However, the result that serum neutralization against the Omicron variant exceeded that of the Delta variant after booster vaccination should be viewed with caution. After all, the immune escape capacity of the Omicron variant is far superior to all previous variants, including the Delta variant, both theoretically and from the extensive information available. Furthermore, of the 24 included neutralization trials, only in this one did the researchers report this surprising result, and the statistical power of this trial was limited by the small sample size. Therefore, the contingency of this result should be carefully considered.
However, overall, even with booster injections, the neutralization of the Omicron variant in vaccinees remained generally lower than that of the original strain and other variants, suggesting that the immunity produced by the existing booster regimen against the Omicron variant is incomplete. Relevant data showed that neutralization against the Omicron variant by three doses of vaccine was comparable to that against the original strain after a double-dose regimen.Citation5,Citation7 There were also disseminated case reports from around the world that complete protection against the Omicron variant is not guaranteed even after receiving booster shots.Citation33 Fortunately, 80% of the epitopes that mediate T-cell immunity in spiking proteins are affected to a limited extent, and vaccine recipients retained the vast majority of their CD4+ and CD8+ T-cell responses to the Omicron variant.Citation34–36 Recently, the UK Health Security Agency (UKHSA) provided data on the protective efficacy of booster vaccination in the real world, where protection against hospitalization and death was maintained at 97–99% with a third dose of vaccine, in the presence of a co-prevalence of Delta and Omicron dual variants, suggesting that booster vaccination remains important for the prevention of serious illness.Citation37
Four different types of COVID-19 vaccines were included in our review, and overall, the mRNA vaccines, especially the mRNA-1273 vaccine, were highly effective against the Omicron variant, which is largely consistent with previous findings.Citation38,Citation39 Furthermore, heterologous booster vaccination regimens did not appear to be inferior to the homologous booster vaccination regimens in our review. In fact, in resource-limited areas, heterologous vaccination is often an unavoidable option.Citation40 Currently, there are significant gaps in research on the efficacy and safety of heterologous vaccination programs, but this may be a very promising vaccination program. If the best combination of vaccines can be found,Citation41 perhaps a heterologous vaccination regimen will bring unexpectedly good results, even surpassing those of homologous vaccination. In addition, mixing different types of vaccines may significantly increase the level of multifunctional T cells, which is also of special interest for the prevention of severe diseases.Citation42
There are some limitations in this review. First of all, as all included were laboratory studies, there was no suitable scale to assess the quality of the literature. However, a large number of non-peer-reviewed preprints was included, and most of the included trials were small sample size studies, which introduced large fluctuations to the data and affected the reliability of the results. Nevertheless, the literature we included showed a relatively high consistency in confirming the neutralizing effect of the serum against the Omicron variant and other variants after booster vaccination, which to some extent suggests that the results we obtained are not coincidental. Furthermore, there were significant differences among studies in terms of study population, study methods, timing of serum collection, and outcome indicators. To minimize heterogeneity among studies, we strictly regulated inclusion criteria and excluded trials with immunocompromised and previously infected populations as serum suppliers. To improve the comparability of data among groups, we excluded studies without three or more virulent strains and avoided direct comparison of virus neutralization titers across studies by converting the values of neutralization titers to multiples. Besides, detailed subgroup analyses were taken according to the type of vaccine, vaccination regimen, and neutralization assay. Nevertheless, the impact of these factors on our results was unavoidable. Moreover, the included studies were in vitro neutralization trials, and data from real-world populations are still vacant. In addition, this review focuses on neutralizing antibody titers, while T-cell immune potency was not assessed.
Conclusion
Overall, this review confirms the protective effect of booster vaccination against the Omicron variant, although the protection is not complete. However, given the limitations, the results of this review should be viewed with caution. In the face of constantly mutating SARS-CoV-2, stacking neutralizing antibody levels may be a quick and effective but clumsy way. Anyway, the development of a new specific vaccine against a variant or with broad-spectrum properties must be on the agenda as soon as possible, but until then, booster vaccination may become a powerful tool to protect ourselves.
Authorsʼ contributions
YD and YS conceived and designed the study. YD and LC conducted the database search and extracted the data. YD and YS performed the data analysis and prepared the figures and the tables. YD wrote the manuscript. LC and YS revised it critically for important intellectual content. YS is the guarantor.
Supplemental Material
Download Zip (778.2 KB)Data availability statement
The information in this review is derived from pre-existing literature, all of which is listed in the references and available online.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2062983
Additional information
Funding
References
- Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2021. doi:10.1002/jmv.27526.
- Cameroni E, Saliba C, Bowen JE, Rosen LE, Culap K, Pinto D, De Marco A, Zepeda SK, di Iulio J, Zatta F, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shift. BioRxiv. 2021. doi:10.1101/2021.12.12.472269.
- Genovese L, Zaccaria M, Farzan M, Johnson W, Momeni B. Investigating the mutational landscape of the SARS-CoV-2 Omicron variant via ab initio quantum mechanical modeling. BioRxiv. 2021. doi:10.1101/2021.12.01.470748.
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako D, et al. SARS-CoV-2 omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. MedRxiv. 2021. doi:10.1101/2021.12.08.21267417.
- Pfizer. Pfizer and biontech provide update on omicron variant. The United States; 2021 Dec 8 [accessed 2021 Dec 20]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant .
- Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, Metzler M, Kohmer N, Hoehl S, Helfritz FA, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. MedRxiv. 2021. doi:10.1101/2021.12.07.21267432.
- Doria-Rose NA, Shen X, Schmidt SD, O’-Dell S, McDanal C, Feng W, Tong J, Eaton A, Maglinao M, Tang H, et al. Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization. MedRxiv. 2021. doi:10.1101/2021.12.15.21267805.
- Moderna. Moderna announces preliminary booster data and updates strategy to address omicron variant. 2021 Dec 20 [accessed 2021 Dec 26]. https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Preliminary-Booster-Data-and-Updates-Strategy-to-Address-Omicron-Variant/default.aspx .
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1.
- Ai J, Zhang H, Zhang Y, Lin K, Zhang Y, Wu J, Wan Y, Huang Y, Song J, Fu Z, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2021;1–19. doi:10.1080/22221751.2021.2022440.
- Dejnirattisai W, Huo J, Zhou D, Zahradník J, Supasa P, Liu C, Duyvesteyn HME, Ginn HM, Mentzer AJ, Tuekprakhon A, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–84. doi:10.1016/j.cell.2021.12.046.
- Edara VV, Manning KE, Ellis M, Lai L, Moore KM, Foster SL, Floyd K, Davis-Gardner ME, Mantus G, Nyhoff LE, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. Preprint. bioRxiv. 2021. doi:10.1101/2021.12.20.473557.
- Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, et al. mRNA-Based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–66.e4. doi:10.1016/j.cell.2021.12.033.
- Lusvarghi S, Pollett SD, Neerukonda SN, Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, Epsi NJ, et al. SARS-CoV-2 Omicron neutralization by therapeutic antibodies, convalescent sera, and post-mRNA vaccine booster. bioRxiv. 2021. doi:10.1101/2021.12.22.473880.
- Zeng C, Evans JP, Qu P, Faraone J, Zheng YM, Carlin C, Bednash JS, Zhou T, Lozanski G, Mallampalli R, et al. Neutralization and Stability of SARS-CoV-2 Omicron Variant. bioRxiv. 2021. doi:10.1101/2021.12.16.472934.
- Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, Kreiss Y, Alroy-Preis S, Regev-Yochay G, Mendelson E, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N Engl J Med. 2021. doi:10.1056/NEJMc2119358.
- Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, Sominsky LA, Clark JJ, Adelsberg DC, Bielak DA, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602(7898):682–88. doi:10.1038/s41586-022-04399-5.
- Khong KW, Liu D, Leung KY, Lu L, Lam HY, Chen L, Chan PC, Lam HM, Xie X, Zhang R, et al. Antibody response of combination of BNT162b2 and CoronaVac platforms of COVID-19 vaccines against Omicron variant. Vaccines (Basel). 2022;10(2):160. doi:10.3390/vaccines10020160.
- Wang K, Jia Z, Bao L, Wang L, Cao L, Chi H, Hu Y, Li Q, Jiang Y, Zhu Q, et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022;603:919–25. doi:10.1038/s41586-022-04466-x.
- Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C, Kurth F, Sander LE, Klein F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022;1–4. doi:10.1038/s41591-021-01676-0.
- Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, Ozhelvaci O, Beckmann N, Güimil Garcia RC, Poran A, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375(6581):678–80. doi:10.1126/science.abn7591.
- Yahalom-Ronen Y, Erez N, Fisher M, Tamir H, Politi B, Achdout H, Melamed S, Glinert I, Weiss S, Cohen-Gihon I, et al. Neutralization of SARS-CoV-2 variants by rVSV-ΔG-spike-elicited human sera. Vaccines (Basel). 2022;10(2):291. doi:10.3390/vaccines10020291.
- Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CBF, Malik AA, De la Cruz E, Jorge A, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–85. doi:10.1038/s41591-022-01705-6.
- Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–56. doi:10.1016/j.cell.2021.12.032.
- Debes AK, Xiao S, Egbert ER, Caturegli P, Sitaras I, Pekosz A, Milstone AM. Comparison of total and neutralizing SARS-CoV-2 spike antibodies against omicron and other variants in paired samples after two or three doses of mRNA vaccine. MedRxiv. 2022. doi:10.1101/2022.01.26.22269819.
- Tan CS, Collier ARY, Liu J, Yu J, Chandrashekar A, McMahan K, Wan H, He X, Jacob-Dolan C, Sellers D, et al. Homologous and Heterologous vaccine boost strategies for humoral and cellular immunologic coverage of the SARS-CoV-2 Omicron variant. MedRxiv. 2021. doi:10.1101/2021.12.02.21267198.
- Basile K, Rockett RJ, McPhie K, Fennell M, Johnson-Mackinnon J, Agius JE, Fong W, Rahman H, Ko D, Donavan L, et al. Improved neutralization of the SARS-CoV-2 Omicron variant after Pfizer-BioNtech BNT162b2 COVID-19 vaccine boosting. bioRxiv. 2021. doi:10.1101/2021.12.12.472252.
- Muecksch F, Wang Z, Cho A, Gaebler C, Tanfous TB, DaSilva J, Bednarski E, Ramos V, Zong S, Johnson B, et al. Increased potency and breadth of SARS-CoV-2 Neutralizing antibodies after a third mRNA vaccine dose. bioRxiv. 2022. doi:10.1101/2022.02.14.480394.
- Tada T, Zhou H, Dcosta BM, Samanovic MI, Chivukula V, Herati RS, Hubbard SR, Mulligan MJ, Landau NR, et al. Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies. bioRxiv. 2021. doi:10.1101/2021.12.28.474369.
- Haveri A, Solastie A, Ekström N, Österlund P, Nohynek H, Nieminen T, Palmu AA, Melin M. Neutralizing antibodies to SARS-CoV-2 Omicron variant after 3rd mRNA vaccination in health care workers and elderly subjects and response to a single dose in previously infected adults. MedRxiv. 2021. doi:10.1101/2021.12.22.21268273.
- Zhao X, Li D, Ruan W, Zhang R, Zheng A, Qiao S, Zheng X, Zhao Y, Chen Z, Dai L, et al. Reduced sera neutralization to Omicron SARS-CoV-2 by both inactivated and protein subunit vaccines and the convalescents. bioRxiv. 2021. doi:10.1101/2021.12.16.472391.
- Ariën KK, Heyndrickx L, Michiels J, Vereecken K, van Lent K, Coppens S, Pannus P, Martens GA, van Esbroeck M, Goossens ME, et al. Three doses of the BNT162b2 vaccine confer neutralising antibody capacity against the SARS-CoV-2 B.1.1.529 (Omicron) variant of concern. MedRxiv. 2021. doi:10.1101/2021.12.23.21268316.
- Helmsdal G, Hansen OK, Møller LF, Christiansen DH, Petersen MS, Kristiansen MF. Omicron outbreak at a private gathering in the Faroe Islands, infecting 21 of 33 triple-vaccinated healthcare workers. Clin Infect Dis. 2022. doi:10.1093/cid/ciac089.
- Gao Y, Cai C, Grifoni A, Müller TR, Niessl J, Olofsson A, Humbert M, Hansson L, Österborg A, Bergman P, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med. 2022;28:472–76. doi:10.1038/s41591-022-01700-x.
- Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, Khan K, Cele S, Bernstein M, Karim F, et al. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. medRxiv. 2021. doi:10.1101/2021.12.26.21268380.
- Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, Bloom NI, Goodwin B, Phillips E, Mallal S, et al. SARS-CoV-2 vaccination induces immunological memory able to cross-recognize variants from Alpha to Omicron. BioRxiv. 2021. doi:10.1101/2021.12.28.474333.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Bernal JL. Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat Med. 2022. doi:10.1038/s41591-022-01699-1.
- Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021;93(12):6486–95. doi:10.1002/jmv.27203.
- Cheng H, Peng Z, Luo W, Si S, Mo M, Zhou H, Xin X, Liu H, Yu Y. Efficacy and safety of COVID-19 vaccines in phase III Trials: a meta-analysis. Vaccines (Basel). 2021;9(6):582. doi:10.3390/vaccines9060582.
- Zinatizadeh MR, Zarandi PK, Zinatizadeh M, Yousefi MH, Amani J, Rezaei N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed Pharmacother. 2021;146:112527. doi:10.1016/j.biopha.2021.112527.
- UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 33. The United Kingdom; 2021 Dec 16 [accessed 2021 Dec 26]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043807/technical-briefing-33.pdf .
- Zuo F, Abolhassani H, Du L, Piralla A, Bertoglio F, de Campos-Mata L, Wan H, Schubert M, Wang Y, Sun R, et al. Heterologous immunization with inactivated vaccine followed by mRNA booster elicits strong humoral and cellular immune responses against the SARS-CoV-2 Omicron variant. medRxiv. 2022. doi:10.1101/2022.01.04.22268755.