ABSTRACT
Objective
To evaluate the immunogenicity, safety and lot-to-lot consistency of an inactivated enterovirus 71 (EV71) vaccine cultured in bioreactors with different specifications after full immunization.
Methods
A randomized, double-blind trial was performed in 3,000 children aged 6 ~ 35 months with six vaccine batches, which were prepared in 40 L and 150 L bioreactors for three consecutive batches respectively. Children were immunized on day 0 and 28, serum samples were collected on day 0 and 56, and neutralizing antibody titers were determined by the microcytopathic method. Immediate reactions were recorded within 30 min, local and systemic symptoms were recorded within 0 ~ 28 days, and serious adverse events were recorded within 6 months.
Results
After immunization with two doses of the inactivated EV71 vaccine, the neutralizing antibody GMT was 825.52 ± 4.09, and the positive conversion rate was 96.18%, with no significant difference. The 95% CI of the serum neutralizing antibody GMT ratio between the two groups after immunization with the three vaccine batches produced in the 150 L and 40 L bioreactors ranged from .67 ~ 1.5. The overall incidence of adverse reactions, mainly grade 1 reactions, for all 6 batches from 0 to 28 days after vaccination was 49.62%, with no significant difference (p = .8736). The incidence of systemic adverse reactions, primarily fever and diarrhea, was 45.14%; the incidence of local adverse reactions, primarily erythema and tenderness, was 9.43%.
Conclusion
The EV71 vaccine was highly immunogenic and safe in children aged 6–35 months, and 6 consecutive batches produced by the two bioreactors with different specifications were consistent.
Introduction
Hand-foot-mouth disease (HFMD) is a common infectious disease in children and infants. It is a category C notifiable infectious disease in China, the main symptoms of which are fever and rashes or herpes on the hands, feet, and mouth. A small number of HFMD patients may develop aseptic meningitis, encephalitis, acute flaccid paralysis, and myocarditis neurogenic pulmonary edema. For a few severely ill children, the disease may progress rapidly and can lead to death.Citation1 The pathogens that cause HFMD are mainly enterovirus 71 (EV71), coxsackie virus A16, coxsackie virus A6, and coxsackie virus A10. Among them, EV71 is one of the main pathogens causing severe HFMD.Citation2,Citation3
According to the requirements of the China Food and Drug Administration, the safety and efficacy of vaccines need to be further evaluated after they are marketed, and different batches of vaccines need to be clinically evaluated to further validate the consistency of the vaccine production process and quality. In accordance with Chinese regulations and the “Clinical Considerations for Evaluation of Vaccines for Prequalification” promulgated by the World Health Organization (WHO),Citation4 clinical studies have been conducted on 3 different batches of an EV71 vaccine in phase III clinical studies. The results of the study showed that lot-to-lot equivalence is achieved and that there is consistency between batches.Citation5 The EV71 vaccine was produced by Wuhan Institute of Biological Products Co. Ltd. (hereafter referred to as WIBP) and was approved and issued a national approval number in December 2016.Citation6–8 Both 40 L and 150 L bioreactors were approved for use in the production of the inactivated EV71 vaccine. After the vaccine is marketed, it needs to be further evaluated for immunogenicity, safety and lot-to-lot consistency of the commercial-scale vaccine in a large-scale population. Except for the different specifications of the upstream culture bioreactors, the hardware structure design, function control, and production process control are basically the same, and the downstream ultrafiltration purification and final bulk preparation process are identical. The vaccines produced by the two reactors with different specifications meet the quality specifications. In this study, two bioreactors with different specifications, 40 L and 150 L, were used to consecutively produce 3 batches of an inactivated EV71 vaccine to immunize infants and to further evaluate the immunogenicity, safety and lot-to-lot consistency of the inactivated EV71 vaccine in Chinese infants aged 6–35 months in order to validate the consistency of the production process and provide data supporting the large-scale use of the vaccine.
Materials and methods
Study design and subjects
This phase IV clinical study was performed with a single-center, randomized, double-blind, equivalence design. Six batches (201803014, 201804021, and 201806028 produced in 40 L reactors and 201804020, 201804022, and 201805025 produced in 150 L reactors) of the inactivated EV71 vaccine were produced in the GMP facility of WIBP with the production process approved by the National Medical Products Administration (NMPA). The vaccine was prepared by virus culture, harvest, concentration, purification, inactivation and aluminum hydroxide adsorption (containing a neutralizing antibody titer not less than 3.0 EU) and used for this phase IV clinical study after passing the National Institutes for Food and Drug Control (NIFDC) tests.
This study followed the principle of informed consent and voluntary participation to recruit 3,000 infants aged 6–35 months in Pei County, Jiangsu Province, as subjects. We obtained written informed consent from the guardians of all the participants and approval from the institutional review board of the Jiangsu Provincial Center of Disease Control and Prevention (JPCDC) before initiation of the study. The trial was undertaken by the JPCDC in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The subjects were randomly assigned to be administered the vaccines at a ratio of 1:1:1:1:1:1 First, 1,500 were evaluated for the lot-to-lot consistency of the EV71 vaccine produced in the 150 L reactor (500 subjects for each batch), blood was collected on day 0 and on the 28th day after two injections (day 56), and the lot-to-lot consistency of antibody levels after the immunization was compared. Active safety observation after each dose was conducted to evaluate the safety of the vaccine. The other 1,500 infants aged 6–35 months were used to evaluate the lot-to-lot consistency of the EV71 vaccine produced in the 40 L reactor (500 subjects for each batch), and the study method was consistent with that for the 150 L reactors.
Immunogenicity assessment
Intravenous blood was collected before vaccination (day 0) and at day 56 after the first dose. The serum was separated and stored below −20°C and the NIFDC was responsible for immunogenicity testing. This study mainly evaluates the level of neutralizing antibody at 56 days after immunization. The neutralizing antibody titer of clinical serum samples was determined by the microcytopathic method,Citation9 and the neutralizing antibody titer (geometric mean titer; GMT) was calculated; that is, the sample was diluted 1:8 and then serially diluted 2-fold, mixed with 100 TCID50 of EV71 and neutralized for 2 hours. The cell suspension was added, cultured at 37°C and incubated with 5% CO2 for 7 days to determine the result. The highest dilution that could inhibit 50% of the cytopathic changes was judged as the EV71-neutralizing antibody titer.
A neutralizing antibody titer ≥1:8 was considered antibody positive. The susceptible population was composed of those who were negative for antibodies (neutralizing antibody titer <1:8) before immunization, and the nonsusceptible population was composed of those who were positive for antibodies before immunization. It is recommended by WHO guidelines and Food and Drug Administration/Center for Biologics Evaluation and Research(FDA/CBER) that in the vaccine clinical trials, the GMT non-inferiority boundary value should be .67–1.5.Citation10,Citation11 If all the 95% CIs of the GMT ratios fell within .67 ~ 1.5, there was consistency. Seroconversion was defined as (1) a reciprocal neutralizing antibody titer <1:8 before vaccination and ≥1:8 after vaccination or (2) a reciprocal neutralizing antibody titer ≥1:8 before vaccination and at least a 4-fold increase after vaccination.
Safety assessment
Active safety observations were conducted after each dose of the vaccine, and adverse reactions within 0–28 days and serious adverse reactions within 6 months were recorded to evaluate the safety of the vaccine. Local adverse reactions included tenderness, hardness, erythema, swelling, and itching. Systemic adverse reactions mainly included fever, allergies, fatigue, irritability, loss of appetite, nausea and vomiting. Adverse event classification as per all adverse reactions was recorded according to the prescribed procedures. All adverse events were judged according to the “Guiding Principles for Classification Standards of Adverse Reactions in Clinical Trials of Preventive Vaccines” issued by the China Food and Drug Administration.Citation12 The grading standards are as follows:
Level 1 (Mild): short-term discomfort (<48 hours), no medical treatment required;
Level 2 (Moderate): mild to moderate restriction of daily activities, no or only minimal medical intervention required;
Level 3 (Severe): Significant limitation of activities of daily living, requiring daily care, medical treatment, and possibly hospitalization;
Level 4 (Life-threatening): Extreme restriction of activities of daily living, significant need for daily caring, medical treatment and hospitalization.
Statistical analysis
SAS 9.4 (SAS Institute Inc., Cary, NC, USA.) was used for statistical analysis. The chi-square test or Fisher’s analysis was used to compare the adverse reactions within 0 ~ 28 days and the serious adverse events within 28 days to 6 months of administration of each of the six vaccine batches. The chi-square test was used to compare the positive conversion rate. All hypothesis tests for safety, positive conversion rate and antibody level analyses were two-sided. For comparisons among groups, when the test level (α = .05), had a P value ≤.05, the difference was considered statistically significant.
Immunogenicity analysis was performed at 28 days after the second injection. The EV71-neutralizing antibody levels of the groups after immunization with the six batches of vaccine were expressed as geometric mean, standard difference and 95% confidence interval, and single factor square difference analysis was used for comparison. The interval method was used to conduct lot-to-lot consistency inspection on the GMT of the groups immunized with the three batches of vaccine produced in each reactor. The interval method was used to test the equivalence of the GMT of the two groups with vaccines from the different reactors. The equivalence interval of the lot-to-lot consistency study was preset at .67 to 1.5. The t-test was used to calculate the difference in the GMT value between the two groups after logarithmic transformation. When the 95%CI of the difference between the two groups fell within the range of .67 to 1.5 after anti-logarithmic transformation (the 95%CI of the difference between the two groups after logarithmic transformation fell within −.176 and .176), it was considered that the different batches of vaccine were consistent.
Results
Demographic characteristics
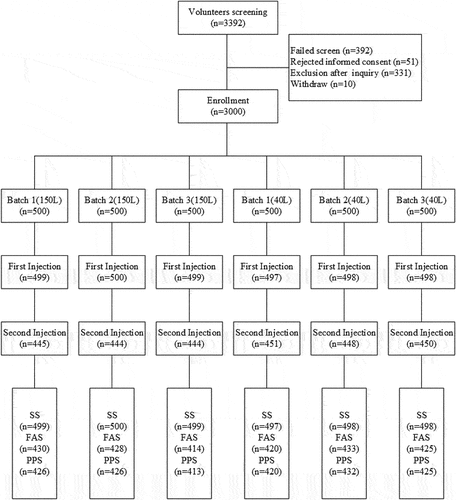
From May 2019 to August 2020, a total of 3,392 volunteers were recruited at the test site in Pei County, Jiangsu Province, and randomly assigned to be immunized with the vaccine from 1 of the 3 batches of inactivated EV71 vaccine produced in a 150 L reactor or 1 of the 3 batches of inactivated EV71 vaccine produced in a 40 L reactor at a ratio of 1:1:1:1:1:1; 500 recipients were enrolled in each batch group. Ultimately, 2,991 recipients were vaccinated with the first dose of vaccine, and all completed blood collection before immunization; 2,682 recipients received 2 doses to complete full immunization, and 2,551 recipients completed blood collection on the 28th day after full immunization (), 2,550 of whom were included in the full analysis set (FAS), and 1 (No. A0063) was not included in the FAS due to incorrect vaccination. A total of 2,542 subjects were included in the PPS (7 subjects were excluded because of protocol violations). The demographic characteristics of participants that received the six different vaccine batches were similar ().
Table 1. Demographic characteristics of the participants in the three groups.
Immunogenicity and lot-to-lot consistency analysis
In the immunogenicity per-protocol set (PPS), the serum anti-EV71 neutralizing antibody GMT level of the subjects on the 56th day after immunization was 825.52 ± 4.09, and the serum neutralizing antibody positive conversion rate on the 56th day after immunization was 96.18% (2,445/2,542). The serum anti-EV71 neutralizing antibody GMT level and positive conversion rate of the susceptible population on day 56 after immunization were 712.76 ± 3.88 and 99.15%, respectively. There was no statistically significant difference in serum anti-EV71 neutralizing antibody GMT or positive conversion rate of subjects in the whole population and those in the susceptible population for each vaccine batch group 56 days after immunization (see ).
Table 2. Serum neutralizing antibody level GMT (PPS) of subjects on the 56th say after immunization.
Table 3. Serum neutralizing antibody positive conversion rate (PPS) of subjects on the 56th day after immunization.
The 95% CI of the ratio of serum neutralizing antibody GMT between the two groups after immunization with the three vaccine batches produced in the 150 L and 40 L reactors were all between .67 and 1.5; the 95% CI of the postvaccination serum neutralizing antibody GMT ratios for the 150 L and 40 L reactors were all between .67 and 1.5 ().
Table 4. Lot-To-Lot consistency of each vaccine batch and of the vaccines from the two reactors (PPS).
Safety analysis
According to the criteria for judging the relevance of adverse events, after judging the relevance of adverse events within 0–28 days after vaccination, all events judged to be related to vaccination were regarded as adverse reactions. The overall adverse reaction rate for the 6 vaccine batches 0–28 days after vaccination was 49.62% (1,484/2,991), and there was no statistically significant difference in the overall adverse reaction rate among batches (see ). Within 0–28 days after immunization, the main adverse reactions were grade 1 and grade 2 reactions. There was no statistically significant difference in the incidence of overall adverse reactions at any level between batches (see ). The overall incidence rate of systemic adverse reactions for the 6 batches was 45.14% (1,350/2,991), and the main symptoms were fever(38.85%,1,162/2991) and diarrhea(9.16%,274/2,991). The overall local adverse reaction rate for the 6 batches was 9.43% (282/2,991), and the main local symptoms were erythema (8.39%, 251/2,991) and tenderness (.7%, 21/2,991). During the 6-month safety observation period, 71 severe adverse events (SAEs) were reported, none of which were related to the vaccine.
Table 5. Occurrence of adverse reactions 0–28 dDays after fFull immunization.
Table 6. Classification of overall adverse reactions of each group 0–28 days after immunization.
Discussion
This is a postmarketing phase IV clinical study of a vaccine produced on a large scale. It evaluates the immunogenicity, safety and lot-to-lot consistency of the vaccine produced on a commercial scale. The vaccines used in the study all obtained a Certificate for the Release of Biological Products from the NIFDC. The results of phase I, II, and III clinical studies of this product have shown thatCitation9,Citation13,Citation14the vaccine has good immunogenicity and safety. Additionally, consistency studies on 3 batches have been carried out, and the results showed that the differences between batches were not statistically significant and that the 3 batches could reach equivalence.Citation5 Batch consistency is very important for vaccine development and application. After the inactivated EV71 vaccine (Vero Cell), was approved for marketing, the consistency of the immunogenicity and safety of the vaccine produced in 40 L and 150 L reactors was evaluated to provide a basis for the large-scale application of the vaccine.
With reference to the method recommended by the FDA/CBER,Citation10 this study conducted a lot-to-lot consistency analysis of 3 batches of the vaccine produced in a 40 L reactor and 3 batches of the vaccine produced in a 150 L reactor, that is, pairwise comparison of the postimmunization serum neutralizing antibody GMT for 6 vaccine batches. If the GMT ratio and its 95% CI fell within the preset equivalence range of .67 ~ 1.5, it can be considered to meet the lot-to-lot consistency requirements. The results for the 6 batches of finished product showed that irrespective of whether the same size reactor was equivalent between batches or between 40 L and 150 L reactors, good uniformity was observed. At 28 days after immunization, the neutralizing antibody GMT was 825.52 ± 4.09, which was much higher than the EV71 immunological surrogate endpoints of 1:16-1:32.Citation15 This result further shows that the EV71 vaccine has good immunogenicity.
In addition, this study used active safety monitoring methods to evaluate safety. There were no significant differences in overall adverse reactions between 0 and 28 days after immunization among the finished products for the 6 batches produced by the two bioreactors with different sizes. Most adverse events were mainly mild, and there were no grade 4 adverse reactions. The results are similar to the phase III clinical data of this product and the results of the Sinovac and Kunming InstituteCitation9,Citation16,Citation17and no rare or serious adverse reactions have been found. After the EV71 vaccine was launched in the 2017–2019 safety observation study in Jiangsu Province,Citation18 it was shown that the general reaction was mainly fever and local redness and swelling, which was consistent with these observations. The above results show that the EV71 vaccine has good safety.
Vaccine accessibility is critical to disease prevention, requiring vaccination in a large population to produce an immune barrier. At present, three EV71 vaccines have been approved for marketing in China. Currently, only WIBP uses bioreactors to produce EV71 vaccines. After the 40 L reactor is scaled up to the 150 L reactor, the output will be greatly improved. The bioreactor process parameters are controlled within control ranges for both sizes, and the EV71 vaccine produced meets the specifications. The results of clinical studies show that the production processes with the 40 L and 150 L reactors have good consistency in vaccine quality.
To date, three EV71 vaccines have been marketed in China. In this clinical trial, both the active and passive methods were used for the safety observation. The overall adverse reaction results were similar to those obtained by post-marketing active monitoring of adverse reaction rates (42.33%) by the Institute of Medical Biology.Citation19 Sinovac conducted a lot-to-lot consistency study on the EV7 l vaccine in July 2012, and the main adverse reaction was fever (39.8%), which was similar to the results of this observation. The 95% CIs of the pairwise differences in GMTs between batches were between −.176 and .176, with good consistency.Citation20 The consistency analysis of EV71 vaccine conducted in Guangxi by the Institute of Medical Biology also proved consistency between vaccine batches.Citation21 The neutralizing antibody GMT levels after vaccination with two doses of the EV71 vaccine manufactured by the Institute of Medical Biology and Sinovac were 255.7 and 220.6, respectively, which were lower than the results of this clinical study.Citation22
HFMD is one of the main infectious diseases in infants. It mainly occurs in children under 5 years of age. Among the etiological agents, EV71 is the main pathogen causing hospitalization and fatality cases.Citation2,Citation23,Citation24 EV71 vaccination is still the primary method for prevention of HFMD caused by EV71 infection, which is the main cause of disease.Citation6,Citation25 A large-scale production and control quality system has been established for this product, which can provide technical support for the subsequent development of multivalent HFMD vaccines. The large-scale application of this product on the market is of great significance for the prevention and control of HFMD. This study evaluated the quality, immunogenicity and safety of the postmarketing vaccine. In the future, we will pay more attention to the trend of antibody titer changes and long-term safety under natural laws after vaccination and provide a basis for subsequent booster immunization and application.
A limitation of this clinical trial is the immunogenicity evaluation of 6 batches of vaccine on day 28 after immunization, therefore, the long-term persistence of antibodies in the real world after the vaccine is released on the market needs further study. The subjects in this trial will continue to be followed up to detect long-term immunogenicity and safety to evaluate the trend of neutralizing antibody levels after vaccination with the EV71 vaccine.
Abbreviations
| HFMD | = | Hand-foot-mouth disease |
| WHO | = | World Health Organization |
| EV71 | = | Enterovirus 71 |
| WIBP | = | Wuhan Institute of Biological Products Co., Ltd |
| NMPA | = | National Medical Products Administration |
| NIFDC | = | National Institutes for Food and Drug Control |
| GMT | = | Geometric mean titer |
| AEs | = | Adverse events |
| SAEs | = | Severe adverse events |
| PPS | = | Per-protocol set |
Author contributions
Fengcai Zhu, Kai Duan, Xiaoqi Chen, Xiuling Li, Pengfei Jin, Jinhua Chen, Xinguo Li, Wei Chen, Fanyue Meng were responsible for development of the study concept, and design and performance of the research. Jinhua Chen and Pengfei Jin wrote the manuscript. Meizhi Du, Xiujuan Li, Weiwei Lu, Xing Yan, Xiang Guo, Qingliang Li, Changfu Guo, Tingbo Xie, Li Li, Hongqiao Du contributed to implementation of the study. Pei Liu performed the data analysis. Qunying Mao, Fan Gao performed serum samples testing and provided technical support.
Acknowledgments
We would like to thank all the investigators from Jiangsu Provincial Center for Disease Control and Prevention, the National Institute for Food and Drug Control, and the Pei County Center for Disease Control and Prevention.
Disclosure statement
Clinical Trials identifier: CTR20191004, May 29, 2019.
Additional information
Funding
References
- Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, et al. Neurologic complications in children with enterovirus 71 infection[J]. N Engl J Med. 1999;341(13):936–7. PMID: 10 498488. doi:10.1056/NEJM199909233411302.
- Chang PC, Chen SC, Chen KT. The current status of the disease caused by enterovirus 71 infections: epidemiology, pathogenesis, molecular epidemiology, and vaccine development. Int J Environ Res Public Health. 2016;13(9):890. PMID: 27618078.
- Liu SL, Pan H, Liu P, Amer S, Chan T-C, Zhan J, Huo X, Liu Y, Teng Z, Wang L, et al. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China fro-m 2008 to 2014. Rev Med Virol. 2015;25(2):115–28. PMID: 25704797.
- WHO. Clinical considerations for evaluation of vaccines for prequalification. Points to consider for manufacturers of human vaccines; 2010 [accessed 2013 Set 12]. http://www.who.int/immunization/standards/vaccine/quality/clinicalconsiderations/oct10.pdf
- Chen YJ, Meng FY, Mao Q, Li J-X, Wang H, Liang Z-L, Zhang Y-T, Gao F, Chen Q-H, Hu Y, et al. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large-scale phase 3 clinical trial. Hum Vaccin Immunot Her 2014;10(5):1366–72. PMID:24633366. doi:10.4161/hv.28397.
- Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines. 2016;15(5):599–606. PMID: 26732723. doi:10.1586/14760584.2016.1138862.
- Li Z-Q, Qin ZQ, Tan HF, Zhang C-H, Xu J-X, Chen J, Ni L-H, Yun X-X, Cui M, Huang Y, et al. Analysis of the coverage of inactivated enterovirus 71 (EV71) vaccine and adverse events following immunization with the EV71 vaccine among children from 2016 to 2019 in Guangzhou. Expert Rev Vaccines. 2021;20(7):907–18. PMID: 34036862. doi:10.1080/14760584.2021.1933451.
- Li ML, Shih SR, Tolbert BS, Brewer G. Enterovirus A71 vaccines. Vaccines (Basel). 2021;9(3):199. PMID: 33673595. doi:10.3390/vaccines9030199.
- Zhu FC, Meng FY, Li JX, Li X-L, Mao Q-Y, Tao H, Zhang Y-T, Yao X, Chu K, Chen Q-H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381(9882):2024–32. PMID: 23726161. doi:10.1016/S0140-6736(13)61049-1.
- Nauta J. Vaccine equivalence and noninferiority immunogenicity trials. Statistics in clinical vaccine trials. Springer; 2011. doi:10.1007/978-3-642-14691-6_6.
- Guidelines on clinical evaluation of vaccines: regulatory expectations[J]. WHO Technical Report Series, 2004. Accept from: https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9
- National Medical Products Administration. Guidelines for adverse event classification standards for clinical trials of preventive vaccines. [DC]. National Medical Products Administration, December 31; 2019. Available from: http://english.nmpa.gov.cn/2019-12/31/c_448385.htm
- Zhu F-C, Liang Z-L, Li XL, Ge H-M, Meng F-Y, Mao Q-Y, Zhang Y-T, Hu Y-M, Zhang Z-Y, Li J-X, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2013;381(9871):1037–45. PMID: 23352749. doi:10.1016/S0140-6736(12)61764-4.
- Meng FY, Li JX, Li XL, Chu K, Zhang Y-T, Ji H, Li L, Liang Z-L, Zhu F-C. Tolerability and immunogenicity of an inactivated enterovirus 71 vaccine in Chinese healthy adults and children: an open label, phase 1 clinical trial. Hum Vaccin Immunother. 2012;8(5):668–74. PMID: 22634437. doi:10.4161/hv.19521.
- Zhu W, Jin P, Li JX, Zhu FC, Liu P. Correlates of protection for inactivated enterovirus 71 vaccine: the analysis of immunological surrogate endpoints. Expert Rev Vaccines. 2017;16(9):945–49. PMID: 28548626. doi:10.1080/14760584.2017.1335603.
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370(9):818–28. PMID: 24571754. doi:10.1056/NEJMoa1304923.
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370(9):829–37. PMID: 24571755. doi:10.1056/NEJMoa1303224.
- Gao J, Tang F, Wang Z, et al. Post-Marketing safety surveillance for inactivated enterovirus 71 vaccines in Jiangsu, China from 2017 to 2019. Vaccine. 2021;39(9):1415–19. PMID: 3354 1 795. doi:10.1016/j.vaccine.2021.01.048.
- Bai YH, Li LI, Zhang JN, et al. Post-Marketing assessment on safety of inactived enterovirus type 71 vaccine by proactive monitoring[J]. Chin J Public Health. 2017;33(7):1045–1047. doi:10.11847/zgggws2017-33-07-03.
- Hu Y-M, et al. Immunogenicity, safety, and lot consistency of a novel inactivated enterovirus 71 vaccine in Chinese children aged 6 to 59 months. Clin Vaccine Immunol. 20(12):1805–11. PMID: 24108780. doi:10.1128/CVI.00491-13.
- Zhang Y, Liu L, Dong C, et al. Consistency of various batches of inactivated enterovirus 71 vaccine [J]. Chin J Biol. 2015;28(3):6.
- Xu Q, et al. Interchangeability of two enterovirus 71 inactivated vaccines in Chinese children: a phase IV, open-label, and randomized controlled trial. Vaccine. 2020;38(12):2671–77. PMID: 32067817. doi:10.1016/j.vaccine.2020.02.013.
- Komatsu H, Shimizu Y, Takeuchi Y, Ishiko H, Takada H. Outbreak of severe neurologic involvement associated with enterovirus 71 infection. Pediatr Neurol. 1999;20(1):17–23. PMID: 10029254. doi:10.1016/s0887-8994(98)00087-3.
- Lum LC, Wong KT, Lam SK, Chua KB, Goh AY. Neurogenic pulmonary oedema and enterovirus 71 encephalomyelitis. Lancet. 1998;352(9137):1391. PMID: 9802304. doi:10.1016/s0140-6736(05)60789-1.
- Klein MH. EV71 vaccines: a first step towards multivalent hand, foot and mouth disease vaccines. Expert Rev Vaccines. 2015;14(3):337–40. PMID: 25536888.