ABSTRACT
Mass vaccination is critical to control the pandemic of coronavirus disease 2019 (COVID-19). Fear of adverse events (AEs) after COVID-19 vaccination is a main factor associated with vaccination hesitancy. We aimed to analyze AEs in healthcare workers (HCWs) vaccinated with COVID-19 vaccines (Aikewei or CoronaVac) composed of inactivated virus. We used a structured self-administered questionnaire to conduct two surveys on COVID-19 vaccination among HCWs in perinatal medicine and obstetrics/gynecology from April 5 to April 21, 2021. In total, 1392 HCWs who had received at least one vaccine dose were included. Of them, 1264 (90.8%) were females and 1047 (75.2%) received two doses. The overall incidence of any AEs after the first and second dose was 38.2% (532/1392) and 31.0% (325/1047) respectively (χ2 = 13.506, P = .0002). Female and HCWs aged 18–30 y were more likely to report AEs. The most common AEs were local reaction, accounting for 48.1% and 67.4% of all AEs after the first and second dose respectively. The systemic AEs were mainly neurological (9.8% and 4.8% after the first and second injection respectively) and flu-like symptoms (6.3% and 3.2%). Overall, most of AEs were mild, only 5.1% (after the first dose) and 2.8% (after the second dose) of individuals with AEs received symptomatic treatment or sick leaves, and none of them required hospitalization. Our data added more evidence that inactivated COVID-19 vaccines are highly safe. The data are valuable to overcome vaccine hesitancy associated with concerns about the safety of COVID-19 vaccines.
Introduction
Coronavirus disease 2019 (COVID-19) has lead to global healthcare crisis because its causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been transmitted to all over the world and COVID-19 has a high fatality rate.Citation1,Citation2 In addition, COVID-19 has caused adverse effects in patients with other diseases and in those who may be immunocompromised.Citation3–6 Universal vaccination in all populations should be the most effective way to control the pandemic of COVID-19. Since December 2020, several kinds of COVID-19 vaccines, including mRNA-based vaccine, non-replicating viral vector vaccine, inactivated virus, or protein subunit, have been developed, licensed, and recommended for use in human.Citation7–11 However, the real-world COVID-19 vaccination coverage in general populations as well as in healthcare workers (HCWs) was not as high as expected.Citation12–16 The reasons for the suboptimal coverage of COVID-19 vaccination are complicated, and an important one appears the concern about the safety of COVID-19 vaccines,Citation15,Citation16 although clinical trials demonstrated that the vaccines are highly safe.Citation17–20 Therefore, more safety data from the real-world applications of COVID-19 vaccines are critical to increase the coverage of COVID-19 vaccination. In the current study, we presented the self-reported adverse events (AEs) in Chinese HCWs who were vaccinated with COVID-19 vaccine (Aikewei or CoronaVac) composed of inactivated SARS-CoV-2.
Subjects and methods
Participants
The China Health Authority issued the first licensed COVID-19 vaccine (Aikewei, Beijing Institute of Biological Products/Sinopharm, Beijing, China) composed of inactivated SARS-CoV-2 for emergency use in adult populations (at 18–60 y age) at risk for infection on December 30, 2020, and issued the second licensed inactivated COVID-19 vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China) on February 5, 2021. The recommended full vaccination requires two injections at an interval 2–4 weeks. The COVID-19 vaccination was compulsory for all persons employed in hospitals as well as other populations at high risk for infection of SARS-CoV-2 during the initial period of the vaccination campaign. It was planned to complete the COVID-19 vaccination in all HCWs between January 1 and March 31, 2021. Each hospital made every effort to have all staff to receive COVID-19 vaccination.
The present study was the detailed analysis of self-reported AEs in HCWs who were included in two cross-sectional surveys to investigate the actual acceptance of COVID-19 vaccination during the first three months period of the vaccination campaign among HCWs in the field of perinatal medicine and obstetrics/gynecology in China.Citation15,Citation16 One survey was conducted among HCWs who participated in a nation-wide symposium on the perinatal medicine held Taiyuan city, April 16–18, 2021, and the survey was conducted by an online platform from April 9–21, 2021. The questionnaires contained the detailed questions in Chinese about AEs after the first and second dose of COVID-19 vaccination (Supplementary Material). In total, 1087 HCWs participated in the survey. Of them, 36 (3.3%) who provided incomplete responses were excluded, and 1051 (96.7%) who completed the survey were included in the analysis. Of these 1051 eligible participants, 86.2% (906) received at least one dose of COVID-19 vaccine.Citation15
Another survey was conducted in Jiangsu province, involving HCWs who participated in a Jiangsu provincial symposium in perinatal medicine held in Nanjing city, April 10–11, 2021, and HCWs in the Department of Obstetrics and Gynecology, Nanjing Drum Tower Hospital, April 5–11. The questionnaire form used in this survey was same as that used in the above nation-wide symposium in Taiyuan city except that ethnic minority and religion were deleted, because 99.5% of the population in Jiangsu province is Han nationality. This survey was performed by distributing questionnaire form on-site so that we were able to directly communicate with the participants to exclude those who were planning to participate in the nation-wide symposium in Taiyuan city and those who were employed in Nanjing Drum Tower Hospital. Among the 269 participants in the symposium, 250 questionnaire forms were distributed, because 8 HCWs declined and 11 HCWs were not eligible. Of them, 22 did not submit the forms and 2 submitted the incomplete forms, and finally 226 (90.4%) forms were included in the analysis. Among all 422 HCWs in Obstetrics and Gynecology, Nanjing Drum Tower Hospital, 412 questionnaire forms were distributed, because 6 HCWs were not accessible and 4 HCWs were not eligible. Of them, 19 did not submit the forms and 1 submitted an incomplete form, and finally 392 (95.1%) forms were included in the analysis. Therefore, a total of 618 HCWs were included, and 79.0% (488) of them were vaccinated with at least one dose of COVID-19 vaccine.Citation16 These two surveys were approved by the Ethics Committee of the Nanjing Drum Tower Hospital (2021-138-01).
COVID-19 vaccines
The COVID-19 vaccines initially used in China were mainly composed of inactivated SARS-CoV-2 adsorbed on aluminum hydroxide adjuvant (Aikewei, Beijing Institute of Biological Products/Sinopharm, or CoronaVac, Sinovac Life Sciences, Beijing, China).Citation21,Citation22 This study only included HCWs who received these two types of inactivated COVID-19 vaccines. The vaccine was intramuscularly injected in the deltoid muscle (usually left arm).
Survey contents about AEs after vaccination
In addition to questions on the demographic characteristics in the questionnaire form, we prepared a question of whether any AEs occurred after the first or second vaccine dose. If any AEs occurred, nine categories of questions about the detailed AEs were provided and the severity of AEs was included. The questionnaire form was in Chinese, which is presented in the Supplementary Material with English translation.
Statistical analyses
Categorical variables were reported as number and percentage. We used chi-squared test or the chi-squared test with Yates’ correction for continuity to compare the rates between participants with reported AE and those without reported AE. P < .05 was considered statistically significant. Multivariable logistic regression analysis was performed to explore the risk factors associated with AEs after COVID-19 vaccination. All statistical analysis was performed using the R software (R version 4.04).
Results
Participant characteristics and the incidence of AEs in HCWs vaccinated with the first and second COVID-19 vaccine
In total, 1394 HCWs received COVID-19 vaccination. All, except for two who received the recombinant adenovirus vaccine, were vaccinated with inactivated SARS-CoV-2 vaccines (Aikewei or CoronaVac). Thus, 1392 HCWs were included in this study. The demographic characteristics, educational levels, and professional roles of HCWs who accepted the first COVID-19 vaccination are summarized in . Of them, 860 (61.8%) reported no any AEs, and 532 (38.2%) reported one or more AEs, including local pain at the injection site. The comparison of demographic characteristics between those with and without AEs showed that females, younger individuals, and those with junior profession titles were more likely to have AEs after the first vaccination, while other parameters, such as roles in hospital, hospital levels and university hospitals, were not associated with the occurrence of AEs ().
Table 1. Comparison of demographic characteristics between vaccinees with and without adverse events (AEs) after the 1st dose of inactivated COVID-19 vaccine.
As of the completion of the survey, of the 1392 HCWs who accepted the first vaccine dose, 1047 (75.2%) received the second dose and 345 (24.8%) did not yet receive the second dose due to the shorter interval (less than 2 weeks), temporary lack of vaccine, catching cold, acute toothache, or other reasons. Of those vaccinated with the second dose, 722 (69.0%) reported no any AEs and 325 (31.0%) reported one or more AEs, including local pain at the injection site (). The overall incidence of any AEs after the second dose was significantly lower than the incidence of 38.2% after the first vaccine dose (χ2 = 13.506, P = .0002). The comparison of demographic characteristics between those with and without AEs after the second vaccination showed that female HCWs were more likely to have AEs, whereas other parameters, such as ages, educational levels, professional titles, roles in hospital, hospital levels and university hospitals, were not associated with the occurrence of AEs ().
Table 2. Comparison of demographic characteristics between vaccinees with and without adverse events (AEs) after the 2nd dose of inactivated COVID-19 vaccine.
Detailed AEs in vaccinees
The detailed AEs in the HCWs after the first and second vaccine dose are listed in . The most common self-reported AEs were local pain, redness, swelling, or itch at the injection site in the absence of any other AEs, which accounted for 48.1% and 67.4% of all AEs after the first and second vaccine dose, respectively (). The second and third most common AEs were neurological (9.8% and 4.8% after the first and second dose respectively) and flu-like (6.3% and 3.2%) symptoms respectively. The other AEs included fever (2.15% and 0.9%), gastroenterological (3.1% and 1.1%) and respiratory (1.1% and 0.7%) symptoms and allergic reactions (1.4% and 0.8%). Notably, 22 and 10 had menstrual disorders after the first and second vaccine dose respectively.
Table 3. The incidence and proportion of adverse events (AEs) in vaccinees after the 1st and 2nd dose of inactivated vaccine.
also shows that, of the 532 HCWs with AEs after the first vaccine dose, 505 (94.9%) just had mild symptoms and required no treatment, 27 (5.1%) received symptomatic treatment or took sick leaves, and none (0%) required hospitalization. Of the 325 HCWs with AEs after the second vaccine dose, 316 (97.2%) required no treatment, 9 (2.8%) received the treatment, and none (0%) needed hospitalization ().
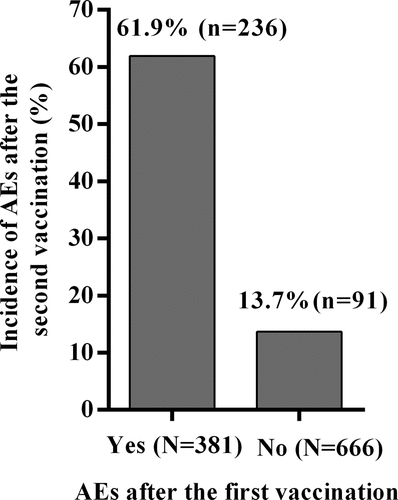
Incidence of AEs after the second vaccine dose in HCWs with or without AEs after the first dose
Of the 532 HCWs who had AEs and the 860 HCWs who had no AEs after the first vaccination, 381 (71.6%) and 666 (77.4%) received the second vaccination respectively. The incidence of AEs in these HCWs after the second vaccination is presented in . Following the second vaccination, those who had AEs after the first vaccination reported higher frequencies of AEs than those who did not have AEs after the first vaccination (61.9% vs 13.7%, χ2 = 263.012, P < .0001).
Comparison of incidence of AEs after the first and second vaccine doses in HCWs
In total, 1047 HCWs were fully vaccinated with two doses of inactivated COVID-19 vaccine. We compared the incidences of AEs after the first and second vaccine doses (). The results showed that the incidence of AEs after the second vaccine dose was significantly lower than that after the first vaccine dose (31.0% vs. 36.7%, χ2 = 14.565, P = .0001).
Table 4. The incidence of adverse events (AEs) after the 1st and 2nd vaccine dose in 1047 subjects who received the full vaccination*.
Risk factors associated with AEs after COVID-19 vaccination
presents the risk factors associated with AEs after the first and second vaccine dose determined with the binary logistic regression analysis. Compared with male subjects, female subjects appeared to have reported more AEs. In addition, compared to those aged 18–30 y, subjects aged 51–60 y were less likely to have AEs after the first vaccination.
Table 5. Risk factors of adverse events after the 1st and 2nd vaccine dose analyzed with binary logistic regression.
Discussion
In the present study, we revealed that AEs occurred in 38.2% and 31.0% of HCWs after the first and second dose of inactivated COVID-19 vaccines respectively, and the local reactions accounted for 48.1% and 67.4% of all AEs respectively. The most systemic AEs were headache (9.8% and 4.8%) and flu-like symptoms (6.3% and 3.2%). Only 5.1% and 2.8% of HCWs who had AEs after the first and second vaccination respectively received symptomatic treatment or sick leave, and none of them required hospitalization. These data indicate that the COVID-19 vaccines composed on inactivated SARS-CoV-2 are highly safe in the real-world application.
In the phase 1/2 clinical trials, any AEs occurred in 15.0% (36/240) to 29.0% (42/144) of the Chinese subjects injected with the inactivated COVID-19 vaccines (CoronaVac and Aikewei).Citation21–23 In the phase 3 clinical trials of these inactivated COVID-19 vaccines, the United Arab Emirates reported any AEs in 41.7% (5623/13471) to 44.2% (5957/13464) of the participants,Citation17 and Turkey reported any AEs in 18.9% (1259/6646) of the CoronaVac recipients.Citation24 The considerably varied incidence of AEs after the vaccination was also observed in clinical trials of other types of COVID-19 vaccines.Citation25 In our present survey, the incidence (38.2% after the 1st dose and 31.0% after the 2nd dose) of self-reported AEs appeared to be within the range reported in clinical trials. Other investigators reported a wide range of the incidence (from fewer than 3% to 82.6%) of any AEs following inactivated COVID-19 vaccination in the actual application.Citation26–31 The substantial difference in the reported incidence of AEs after the vaccination might be related with different populations and different definitions of AEs.
In the present study, the vaccinees who had AEs after the first vaccine dose were much more likely to have AEs after the second dose (), which is in agreement with the findings reported by Zhang et al.Citation27 This suggests that some individuals are prone to have AEs. The binary logistic regression analysis showed that females and young subjects were more likely to experience AEs (), which have been observed in individuals injected with mRNA or adenovirus-vector COVID-19 vaccines and other vaccines.Citation28,Citation29,Citation31–34
Notably, the data in clinical trials and real-world applications showed that, among those who were injected with mRNA or adenovirus-vectored vaccines against COVID-19, any AEs occurred in as high as more than 70% to 95% of the recipients and the systemic SEs were observed in more than 47% to 85.9%.Citation13–37–Citation39 Studies showed that inactivated COVID-19 vaccines have the lowest reported AEs.Citation31,Citation40 On the other hand, mRNA vaccines appear to have higher antibody response and better protection against COVID-19 than inactivated vaccines.Citation41,Citation42 These results suggest that the occurrence of AEs may be related to the strength of the immune response; the higher the immune responses, the more frequency of AEs.
In addition to the local and systemic AEs, we found that 2.1% and 1.0% of women aged 18–50 y reported menstrual changes after the first and second COVID-19 vaccination respectively (). Irregular menstrual changes have also been observed in women vaccinated with other types of COVID-19 vaccines.Citation38,Citation43 Whether the irregular menstrual change was associated with COVID-19 vaccination or was a co-incident event requires further investigation.
Of interesting, we revealed that AEs occurred less frequently after injection of the second dose of inactivated COVID-19 vaccine (), which was also observed in Jordanian population,Citation31 or in Indian population vaccinated with different inactivated COVID-19 vaccine.Citation44 These findings are practically useful. In the real-world practice, some individuals were reluctant to receive second vaccine dose because of the AEs occurred after the first vaccination. The finding of the lower incidence of AEs after the second dose can be used by health providers to encourage those who had AEs after the first dose to receive the second dose.
This study has several limitations. First, because HCWs in the present study were from perinatal medicine and obstetrics and gynecology, the female subjects accounted for more than 90% of total participants, which may over-estimated the incidence of AEs, since females are more likely to have AEs after vaccination.Citation28,Citation29,Citation31–34 Second, since the participants were vaccinated in the first period of the vaccination campaign, during which those who had chronic diseases were mostly not vaccinated, whether those with chronic diseases may have more AEs is unknown. Third, since the vaccinated subjects were at the age of 18–60 y, the incidence of AEs in those who are older than 60 y or younger than 18 y is still unknown. Fourth, we did not compare the frequency of AEs between the two types of inactivated COVID-19 vaccines. Fifth, we did not follow up the long-term AEs after COVID-19 vaccination.
In conclusion, the present study showed that AEs after the first and second dose of COVID-19 vaccines composed of inactivated SARS-CoV-2 are mostly local reactions, and the systemic AEs are generally mild and well tolerated. Female and young individuals are more likely to have AEs after COVID-19 vaccination. The real-world evidence of COVID-19 vaccine safety should be valuable to overcome the vaccine hesitancy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Weekly epidemiological update on COVID-19 Edition 83 - 15 March 2022. [accessed 2022 Mar 16]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—15-march-2022.
- Peng Y, Xu B, Sun B, Han G, Zhou YH. Importance of timely management of patients in reducing fatality rate of coronavirus disease 2019. J Infect Public Health. 2020;13(6):890–7. doi:10.1016/j.jiph.2020.04.015.
- Hess PE, Wylie BJ, Golen T, Shainker SA, Zera C, Li Y. Keeping pregnant patients safe during COVID-19 pandemic. Matern Fetal Med. 2021;3(1):2–6. doi:10.1097/FM9.0000000000000072.
- Nieto-Calvache AJ, Padilla I, Tabares-Blanco MF, López-Girón MC, Vergara Galliadi LM. Fear is the path to the dark side: Unsafe delivery, one of the consequences of fear of the SARS-CoV-2 pandemic, a case report. Matern Fetal Med. 2021;3(4):292–94. doi:10.1097/FM9.0000000000000112.
- Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, Redondo-Pachón MD, Murphy B, Florman S, Cyrino LG, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20(11):3140–48. doi:10.1111/ajt.16185.
- Belsky JA, Tullius BP, Lamb MG, Sayegh R, Stanek JR, Auletta JJ. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021;82(3):329–38. doi:10.1016/j.jinf.2021.01.022.
- Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, Lee GM, Talbot HK, Romero JR, Oliver SE. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1857–59. doi:10.15585/mmwr.mm6949e1.
- Topol EJ. Messenger RNA vaccines against SARS-CoV-2. Cell. 2021;184(6):1401. doi:10.1016/j.cell.2020.12.039.
- Dal-Ré R, Caplan AL, Gluud C, Porcher R. Ethical and scientific considerations regarding the early approval and deployment of a COVID-19 vaccine. Ann Intern Med. 2021;174(2):258–60. doi:10.7326/M20-7357.
- Mallapaty S. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature. 2021;594(7862):161–62. doi:10.1038/d41586-021-01497-8.
- Mehraeen E, Dadras O, Afsahi AM, Karimi A, MohsseniPour M, Mirzapour P, Barzegary A, Behnezhad F, Habibi P, Salehi MA, et al. Vaccines for COVID-19: a review of feasibility and effectiveness. Infect Disord Drug Targets. 2021. doi:10.2174/1871526521666210923144837.
- Gharpure R, Patel A, Link-Gelles R. First-Dose COVID-19 vaccination coverage among skilled nursing facility residents and staff. Jama. 2021;325(16):1670–71. doi:10.1001/jama.2021.2352.
- Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. Spanish Society of Nephrology Council. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021;36(6):363–69. doi:10.1016/j.jhqr.2021.05.002.
- Whiteman A, Wang A, McCain K, Gunnels B, Toblin R, Lee JT, Bridges C, Reynolds L, Murthy BP, Qualters J, et al. Demographic and social factors associated with COVID-19 vaccination initiation among adults aged ≥65 years - United States, December 14, 2020-April 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(19):725–30. doi:10.15585/mmwr.mm7019e4.
- Xu B, Gao X, Zhang X, Hu Y, Yang H, Zhou YH. Real-World acceptance of COVID-19 vaccines among healthcare workers in perinatal medicine in China. Vaccines (Basel). 2021;9(7):704. doi:10.3390/vaccines9070704.
- Zheng Y, Shen P, Xu B, Chen Y, Luo Y, Dai Y, Hu Y, Zhou YH. COVID-19 vaccination coverage among healthcare workers in obstetrics and gynecology during the first three months of vaccination campaign: a cross-sectional study in Jiangsu province, China. Hum Vaccin Immunother. 2021;17(12):4946–53. doi:10.1080/21645515.2021.1997297.
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. Jama. 2021;326(1):35–45. doi:10.1001/jama.2021.8565.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–29. doi:10.1056/NEJMoa2107058.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdox1 nCov-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi:10.1016/S1473-3099(20)30831-8.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–92. doi:10.1016/S1473-3099(20)30843-4.
- Pan HX, Liu JK, Huang BY, Li GF, Chang XY, Liu YF, Wang WL, Chu K, Hu JL, Li JX, et al. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chin Med J (Engl). 2021;134(11):1289–98. doi:10.1097/CM9.0000000000001573.
- Tanriover MD, Doganay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. doi:10.1016/S0140-6736(21)01429-X.
- Blais JE, Wei Y, Chui CSL, Chan EW, Wong ICK. Inconsistent safety outcome reporting in randomized clinical trials of COVID-19 vaccines complicates informed medical decisions. Drug Saf. 2021;44(11):1121–23. doi:10.1007/s40264-021-01108-5.
- Wang G, Zhu L, Zhu Y, Ye Q, Yu X, Fu M, Lu J, Li X, Huang Y, Zhang J, et al. Safety survey by clinical pharmacists on COVID-19 vaccination from a single center in China. Hum Vaccin Immunother. 2021;17(9):2863–67. doi:10.1080/21645515.2021.1913964.
- Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;20(7):891–98. doi:10.1080/14760584.2021.1925112.
- Zhu JS, Zhang MX, Chien CW, Yang WY, Shi GF, Qiu S, Tung TH, Chen HX. Sex differences in adverse reactions to an inactivated SARS-CoV-2 vaccine among medical staff in China. Front Med (Lausanne). 2021;8:731593. doi:10.3389/fmed.2021.731593.
- Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219–26. doi:10.1016/j.ijid.2021.08.013.
- Ali Sahraian M, Ghadiri F, Azimi A, Naser Moghadasi A. Adverse events reported by Iranian patients with multiple sclerosis after the first dose of Sinopharm BBIBP-CorV. Vaccine. 2021;39(43):6347–50. doi:10.1016/j.vaccine.2021.09.030.
- Omeish H, Najadat A, Al-Azzam S, Tarabin N, Abu Hameed A, Al-Gallab N, Abbas H, Rababah L, Rabadi M, Karasneh R, et al. Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Hum Vaccin Immunother. 2022;18(1):1981086. doi:10.1080/21645515.2021.1981086.
- Somiya M, Mine S, Yasukawa K, Ikeda S. Sex differences in the incidence of anaphylaxis to LNP-mRNA COVID-19 vaccines. Vaccine. 2021;39(25):3313–14. doi:10.1016/j.vaccine.2021.04.066.
- Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–49. doi:10.1007/s00281-018-0726-5.
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–99. doi:10.1146/annurev-cellbio-100616-060718.
- Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. doi:10.1056/NEJMoa2027906.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdox1 nCov-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78. doi:10.1016/S0140-6736(20)31604-4.
- Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdox1 nCov-19 vaccine: Independent evidence from two EU States. Vaccines (Basel). 2021 ;9(6):673. doi:10.3390/vaccines9060673.
- Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. Bnt162b2 and ChAdox1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med (Lausanne). 2021;8:760047. doi:10.3389/fmed.2021.760047.
- Mehraeen E, SeyedAlinaghi S, Karimi A. Can children of the Sputnik V vaccine recipients become symptomatic? Hum Vaccin Immunother. 2021;17(10):3500–01. doi:10.1080/21645515.2021.1933689.
- Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. doi:10.1186/s40249-021-00878-5.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Zee JST, Lai KTW, Mks H, Leung ACP, Chan QWL, Esk M, Lee KH, Lau CC, Yung RWH. Serological response to mRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: Preliminary results. Hong Kong Med J. 2021;27(4):312–13. doi:10.12809/hkmj219605.
- Male V. Menstrual changes after COVID-19 vaccination. Bmj. 2021;374:n2211. doi:10.1136/bmj.n2211.
- Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, Aileni VK, Kanungo S, Rai S, Reddy P, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173–84. doi:10.1016/S0140-6736(21)02000-6.