 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The human papillomavirus (HPV) vaccine is the simplest, most economical, convenient, and effective method of preventing cervical cancer. However, the current HPV vaccine is supplied as a single-dose vial with a relatively high cost per dose, which hinders its supply to low- and middle-income countries (LMICs), where the demand for HPV vaccine is highest. Therefore, it is necessary to develop a multi-dose HPV vaccine to promote large-scale affordable vaccination in LMICs. Moreover, the addition of preservatives is required to reduce the risk of microbial contamination in multi-dose vaccines within a single vial. In this study, we investigated the effects of six preservatives on HPV 16L1 and 18L1 virus-like particles in solution, as well as the aluminum adsorption status, under normal and high-temperature conditions. Multiple methods were employed, including dynamic light scattering, differential scanning calorimetry, an in vitro relative potency assay, and an in vivo potency assay in mice. Based on the above results, four types of selected preservatives were further studied, and an antimicrobial effectiveness test was performed on the HPV-2 vaccine, which was employed as a model HPV vaccine. Finally, three preservatives were selected based on their performance to evaluate the long-term stability of the HPV-2 vaccine. The results indicated that 0.12% methylparaben is the most suitable preservative for the multi-dose HPV-2 vaccine, guaranteeing the shelf life for at least three years and meeting “B” standards for antimicrobial effectiveness. The formula developed in this study can contribute toward combating cervical cancer in LMICs.
Introduction
Cervical cancer refers to a malignant tumor of the epithelial and glandular epithelium in the cervix.Citation1 According to statistical reports of the International Agency for Research on Cancer (IARC) in 2018,Citation2 nearly 570,000 new cases and 311,000 deaths each year are attributed to cervical cancer worldwide, with almost 90% occurring in low- and middle-income countries (LMICs), making cervical cancer the fourth most common malignant tumor in women worldwide, behind only breast cancer (2.09 million cases), colorectal cancer (790,000), and lung cancer (730,000). Although the IARC assessment reportCitation2 shows that China does not have a high incidence of cervical cancer, the age-adjusted incidence rate is 7.5 per 100,000. In 2012, China had 61,700 estimated new cases of cervical cancer and 29,500 deaths, accounting for 11% of global cases and deaths. Cervical cancer is the eighth most common cancer in Chinese women and the second most common cancer in Chinese women aged 15–44 years, second only to breast cancer.Citation3 According to the World Health Organization (WHO),Citation4 if effective measures are not taken, the number of new cervical cancer cases is expected to increase from 570,000 to 700,000 over the next 10 years, and the number of deaths per year is expected to increase from 311,000 to 400,000. Notably, LMICs will account for more than 60% of these deaths, which is more than twice as many as in high-income countries.Citation5
Persistent human papillomavirus (HPV) infection is the main cause of cervical cancer.Citation6 HPV-related genes are found in the cancer tissues of 99.7% of cervical cancer patients, and 70% of cervical cancer is caused by HPV types 16L1 and 18L1.Citation5 However, three HPV vaccines have been successfully developed (Gardasil, Gardasil 9, and Cervarix) and are currently available worldwide. If global vaccination coverage is rapidly increased to approximately 80–100%, 6.7–7.7 million cases could be avoided between 2020 and 2069, which would significantly reduce the future incidence of cervical cancer.Citation7 However, by 2014, an estimated 33.6% of girls and women aged 10–20 years in high-income countries had received the full course of the HPV vaccine, compared with 2.7% of such females in LMICs.Citation7,Citation8 In November 2020, following years of work by the WHO Director-General, WHO launched a global strategy to accelerate the elimination of cervical cancer, which is expected to reduce new cases of the disease by more than 40% and prevent 5 million related deaths by 2050.
Because of insufficient supply, currently available HPV vaccines cannot fully meet the increasing global market demand (currently 35.8 million doses per year).Citation9 Simultaneously, high transport costs severely limit the ability to introduce HPV vaccines to LMICs, especially in the context of the COVID-19 pandemic. These challenges are partially attributed to cost. Currently available HPV vaccines are all single-dose configurations with relatively high costs and large storage and transportation spaces. Conversely, multi-dose HPV vaccines can significantly reduce costs, facilitate transportation, and promote wider use of the vaccine in LMICs. To avoid the introduction of microorganisms during the repeated use and long-term storage of multi-dose products (i.e., food, cosmetics, and drugs), preservatives are commonly used to kill or inhibit the growth of potential microorganisms.Citation10
Commonly used preservatives include thiomersal, phenol, phenoxyethanol, m-cresol, benzyl alcohol, chlorobutanol, and methylparaben. However, only three types of preservatives have previously been used for vaccines: phenol (for typhoid fever vaccines), thimerosal (for diphtheria, tetanus, pertussis, influenza, Japanese encephalitis, hepatitis B, and pneumococcus vaccines), and phenoxyethanol (for polio, pneumococcal, and typhoid fever vaccines).Citation11 The above applications of preservatives in vaccines are also listed in the 2020 edition of “Chinese pharmacopoeia” vaccine products. Generally, preservatives exhibit more or less destructive/negative effects on protein stability;Citation11–15 however, no preservative has yet been applied to HPV vaccines.Citation16,Citation17
Previously, our group submitted an HPV-2 vaccine with a single-dose vial format to the China Food and Drug Administration (CFDA) for marketing applications, which is expected to be approved in the near future. In this study, with the support of the Bill Gates Foundation and the most up-to-date research,Citation18–20 we analyze a variety of preservatives to identify the most suitable candidate for use in the development of a HPV-2 vaccine with a multi-dose vial configuration.
Materials and methods
Reagents
The aluminum phosphate adjuvant was prepared in-house. Phenol (PH, CAS: 108-95-2), phenoxyethanol (PE, CAS: 122-99-6), m-Cresol (CR, CAS: 108-39-4), benzyl alcohol (BA, CAS: 100-51-6), methylparaben (MP, CAS: 99-76-3), and sorbic acid (SA, CAS: 110-44-1) were purchased from Spectrum China Ltd. In addition, 95% ethanol (for dissolving MP) was purchased from Meihekou Fukang alcohol Co., Ltd (Jilin, China).
Preparation of recombinant HPV virus-like particles
The virus-like particles (VLPs) of HPV 16L1 and 18L1 were expressed in Pichia pastoris, harvested, then homogenized using a high-pressure homogenizer. The cell lysates were collected, and VLPs were obtained after multiple steps of column chromatography purification. For HPV 16L1, the VLPs from purification were treated with dithiothreitol followed by ultrafiltration to complete the disassembly and reassembly procedure, ensuring uniformity of the VLP particles. HPV VLPs were formulated with at least two concentrations of the selected preservative.
HPV-2 vaccine formulation
The HPV VLPs were adsorbed on aluminum phosphate adjuvant at target concentrations of 80 μg/mL (HPV 16L1) and 40 μg/mL (HPV 18L1) with 450 μg/mL aluminum, 1.6 mg/mL histidine (Shanghai Xiehe Amino Acid Co., Ltd), 19 mg/mL sodium chloride (Hunan ER-KANG Pharmaceutical Co., Ltd), and 100 μg/mL polysorbate 80 (Nanjing Well Pharmaceutical Co., Ltd). According to typical in-use concentrations,Citation10,Citation11 the selected preservative was added simultaneously in at least two of the following concentrations for each of the formulations: PH: 0.25%, 0.5%; PE: 0.1%, 0.5%; CR: 0.15%, 0.30%; BA: 0.5%, 1.0%; MP: 0.10%, 0.14%, 0.18%; SA: 0.10%, 0.15%, 0.20%.
Dynamic light scattering (DLS)
Nano ZS (Malvern) was used for dynamic light scattering (DLS) analysis. The sample was first equilibrated at 25°C for 120 s before each sample was measured three times automatically.
Differential scanning calorimetry (DSC)
A fully automatic capillary differential scanning calorimeter (VP-CAP-DSC, Malvern) was used to analyze the structural thermal stability of the HPV VLPs. Prior to measurement, liquid samples and reference sample without the HPV VLP antigen were prepared in the same buffer. A suitable heating rate (90°C per hour), start temperature (25 °C), and end temperature (100 °C) were selected, and each sample and corresponding buffer (reference) were paired into the sample cells. The heat exchange was then monitored automatically and the heat exchange profile was generated and recorded. At the end of the test, OriginTM software pre-installed in the instrument was used to process the data, the DSC profile was generated, and the Tm values were recorded.
In vitro relative potency (IVRP)
A ForteBio molecular interaction instrument (catalog No: Octet Red 96, PALL) was used to determine the in vitro relative potency (IVRP) of HPV VLP antigens in HPV 16L1 and HPV 18L1 bulk or HPV-2 vaccine. The antigen bulk sample (without aluminum adjuvant) was used directly for IVRP analysis. Conversely, for the HPV-2 vaccine, the sample must be treated by dissolving the aluminum adjuvant with the desorption buffer (0.12 M trisodium phosphate (Sinopharm Chemical Reagent Co., Ltd), 2 M sodium chloride (Sinopharm Chemical Reagent Co., Ltd), 0.2 M trisodium citrate (Sinopharm Chemical Reagent Co., Ltd), 0.8% PS80, pH 6.7) overnight at room temperature to recover the HPV VLPs antigen prior to IVRP analysis.
For sample load detection, a ForteBio probe and Protein G sensor (PALL) were immersed in the following: a 96-well plate (catalog No. 655209, Greiner Bio-One), sequence buffer, antibody solution (sensor-bound antibody), sample buffer (fluid was changed and excess antibody was washed away), sample to be tested (where the antibody binds to the antigen in the sample to be tested), regeneration buffer (where the antibody, i.e., HPV 16L1: V5; HPV 18L1: J4, and antigen on the sensor were washed off), and neutralization buffer to complete detection. The device then quantifies the antigen content of the test sample according to the binding curve of the reference product to provide an IVRP value.
In vivo potency assay
BALB/c mouse immunization and pseudovirus neutralization methods were used to evaluate the in vivo potency of HPV 16L1 and HPV 18L1 antigens of the HPV-2 vaccine. After diluting the test sample (HPV-2 vaccine) for eight gradients, each of the diluted samples were injected subcutaneously into the abdomen of 10 female BALB/c mice at the age of 6–8 weeks to prompt the production of a specific antibody against the HPV-2 vaccine in the mice. Mouse serum was then separated from the collected blood of the mice after 28 days of raising.
Serum samples were diluted to a fixed multiple and neutralized with a pseudovirus. Then, 96 well plates with 293 FT cells were added, and the same amount of pseudovirus was added as a positive control. The plates were incubated in a 5% CO2 incubator at 37°C for three days. The cell plate was scanned using a fluorescent spot analyzer, and the number of fluorescent spots was calculated. The average number of fluorescent spots in multiple holes of each serum sample was calculated; if less than or equal to the cutoff value, the sample was judged as positive, and if greater than the cutoff value, the sample was judged as negative (EquationEquation (1)(1)
(1) ). The percentage of positive serum samples in each dose group was calculated, and the ED50 value was calculated using a probit model (EquationEquation (2)
(2)
(2) ).
Antimicrobial effectiveness test (AET)
According to the Chinese Pharmacopoeia antimicrobial effectiveness test (AET) method, the samples were divided into five parts and inoculated with the test microorganisms (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Candida albicans, and Aspergillus niger). The amount of microorganism used for each inoculation of 1 mL sample was guaranteed to be 105–106 cfu, and the volume of the inoculation liquid did not exceed 1% of the volume of the test sample. The samples were stored in the dark at 20–25°C According to the interval specified in , subsamples were taken from five samples, and the number of microorganisms in each test sample was determined by the plate counting method. Bacteria were determined using trypsin soybean peptone agar medium, and fungi were determined using Sabouraud glucose agar medium.
Table 1. Chinese Pharmacopoeia antimicrobial effectiveness test standards.
High-Performance liquid chromatography (HPLC) assay
A high-performance liquid chromatography (HPLC) assay was used to determine the content of preservatives in the HPV-2 vaccines according to the standard curve. The chromatographic conditions were as follows: a Waters Acquity Peptide BEH C18 300 A (150 mm × 2.1 mm, 1.7 μm) column was selected; mobile phases were A = 0.1% trifluoroacetic acid (Merck UVASOL) aqueous solution, B = 0.1% trifluoroacetic acid acetonitrile (J.T. Baker) solution, and the elution gradient is shown in ; the flow rate was 0.2 ml/min; the column temperature is 40°C the sample tray temperature was 12°C and the detection wavelength was 254 nm.
Table 2. Elution gradient of preservative content detected by HPLC.
Results
Effect of preservatives on HPV VLPs
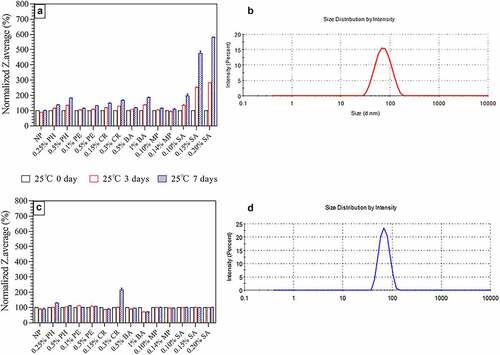
According to the DLS method, all VLP groups exhibited similar particle sizes (~65 nm for HPV 16L1 and ~70 nm for HPV 18L1) on day zero, which met our own quality standards (With the approval of CFDA, based on batches statistics analysis our company has established our own quality standards for DLS, i.e., 45 nm ~85 nm.). After storing at 25°C for seven days, the SA group exhibited the most considerable impact on the morphology of HPV 16L1 VLP (the particle size increased by 2–6 times compared with day zero). As the concentration of the agent increased, the particle size also slightly increased (up to two times) (). However, only high-dose (0.3%) CR caused the greatest damage to the particle morphology of HPV 18L1 VLPs () (doubled from day zero), and none of the other preservatives had a remarkable impact (<30%). The particle size and distribution of VLPs are correlated with their immunogenicity. The closer to the real virus particle (<100 nm), the more symmetrical the VLP icosahedron and the stronger its immunogenicity,Citation21 as confirmed by our clinical phase III research results (results are not shown). Therefore, according to the change of particle size, at least 0.25% PH, 0.5% PH, 0.1% PE, 0.5% PE, 0.15% CR, 0.5% BA, 1.0% BA, 0.10% MP, and 0.14% MP can be used as preservatives for the HPV-2 vaccine.
Figure 1. Particle size (Z-average) of HPV 16L1 and HPV 18L1 antigens in solution in the presence of six preservatives under high-temperature conditions (25 ± 2°C protected from light). A) HPV 16L1 Z-average results. B) DLS spectrum of HPV 16L1 without preservatives. C) HPV 18L1 Z-average results. D) DLS spectrum of HPV 18L1 without preservatives. Antigen concentrations in samples were 0.2 mg/ml. All Z-average results were normalized to the respective T0 results. Z-average values are the average of three independent measurements from DLS analysis; error bars indicate standard deviation (SD) values.
Effect of preservatives on the in vitro potency and stability of HPV VLPs
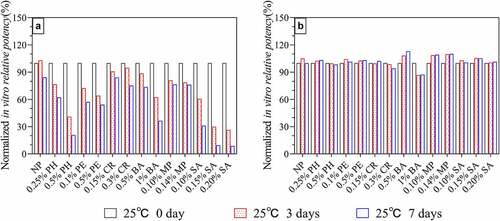
The ForteBio molecular interaction method was used to detect the in vitro potency after incubation at 25°C for seven days. The SA treatment group exhibited the most severe damage to the antigenic potency of HPV 16L1 (according to the preservative concentration, the antigenic potency was reduced by 70–90% compared to day zero), followed by PH (60–80%). The slowest decline in antigen potency was observed in MP, CR, and low-dose BA groups, which maintained 70% of the antigen potency (). The HPV 18L1 antigen potency results () showed that all preservative groups maintained antigen potency of more than 80% after being kept at 25°C for seven days. According to the results shown in , the ability of HPV 18L1 to resist preservatives was considerably higher than that of HPV 16L1, which may be due to the better stability of HPV 18L1. According to the in vitro potency stability, if the shelf life standard of the HPV-2 vaccine (i.e., less than 40% potency loss) is taken as the acceptable standard, at least 0.25% PH, 0.1% PE, 0.5% PE, 0.15% CR, 0.3% CR, 0.5% BA, 0.10% MP, and 0.14% MP can be used as preservatives for the HPV-2 vaccine.
Figure 2. In vitro potency of HPV 16L1(A) and HPV 18L1(B) antigens in solution in the presence of six preservatives under high-temperature conditions (25 ± 2°C protected from light). Antigen concentrations in samples were 0.1 mg/ml. All in vitro potency results were normalized to the respective T0 results and are the average of two independent measurements from ForteBio analysis; error bars indicate SD values.
Effect of preservatives on the thermal stability of HPV VLPs
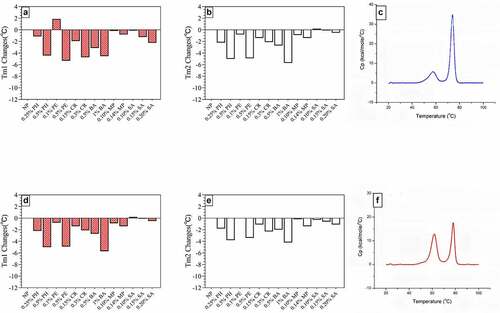
DSC experiments were performed by rapidly heating the sample and measuring the difference in heat capacity between the sample and the control solution being monitored. The temperature at the highest point of each peak was defined as the thermal transition midpoint temperature (Tm). All samples showed two distinct thermal transitions during unfolding; therefore, all samples had two Tm values (Tm1 and Tm2).Citation22 According to previous studies,Citation23 Tm1 is related to VLP structural changes, whereas Tm2 may be due to the unfolding of individual L1 monomers.
The DSC results () showed that SA and MP had the least impact on the thermal stability of HPV VLPs, followed by low-concentration PE, CR, and PH. The preservative-free (NP) group had the following Tm values for HPV16L1 and HPV18L1: Tm1 58.0°C Tm2 73.5°C and Tm1 61.0°C Tm2 78.5°C respectively. According to the thermal stability changes of each group, taking the NP group ±2°C as the standard, at least 0.25% PH, 0.1% PE, 0.15% CR, 0.10% MP, 0.14% MP, and 0.10–0.20% SA can be used as the preservatives for HPV-2 vaccines.
Figure 3. Transition temperature (Tm) for antigens in solution in the presence of six preservatives. A) HPV 16L1 Tm1 changes. B) HPV 16L1 Tm2 changes. C) DSC spectrum of HPV 16L1 without preservative. D) HPV 18L1 Tm1 changes. D) HPV 18L1 Tm2 changes. E) DSC spectrum of HPV 18L1 without preservatives. Antigen concentration in samples was 0.2 mg/ml. Tm changes indicate the difference in Tm values for preservative-treated and NP samples from DSC analysis.
Effect of preservatives on the in vitro potency and stability of the HPV-2 vaccine
The ForteBio molecular interaction method was also used to detect the in vitro potency and all the group retained at least 80% potency except SA groups(i.e., more than 60% potency loss. Data not shown)at day zero. showed that the five concentrations of PH, PE, CR, BA, and MP were negatively correlated with HPV antigen stability under accelerated condition (25 ± 2°C protected from light). SA had a greater impact on the initial antigen at zero points, so the stability of the antigen was not dependent on the concentration of SA. According to the stability of the HPV-2 vaccine, and using the shelf life standard (i.e., less than 40% potency loss) as the acceptable standard, at least 0.25% PH, 0.1% PE, 0.15% CR, 0.5% BA, and 0.10% MP can be used as preservatives for the HPV-2 vaccine.
Figure 4. In vitro potency of HPV 16L1(A) and HPV 18L1(B) antigens in HPV-2 vaccine in the presence of six preservatives under accelerated conditions (25 ± 2°C protected from light). Each sample contained 80 μg/mL HPV 16L1 and 40 μg/mL HPV 18L1 antigens. All in vitro potency results were normalized to the respective T0 results and are the average of two independent measurements; error bars indicate SD values. Results are presented as the relative binding rate of the neutralizing antibody.
Formula design and antimicrobial effectiveness of different preservatives
The “A” standard of the Chinese Pharmacopoeias AET is extremely stringent. Because the concentration of most preservatives added to meet the “A” standard has already adversely affected vaccine products and even reached toxic levels. Therefore, the Chinese Pharmacopoeia and WHO have lowered the standards, requiring that the antibacterial effect of preservatives in vaccines meet the “B” standard, which is also acceptable.Citation24
In this study, four types of preservatives were selected (PE, BA, MP, and CR) at three concentrations (high, medium, and low, see for specific concentrations) for the AET and in vitro potency tests. The results in confirm that the three concentrations of PE and the low concentration of BA and MP did not meet the “B” standards for the AET. Pharmacopoeia B standards indicate that HPV-2 vaccine preparations affect the antimicrobial effectiveness of these three concentrations of preservatives.
Table 3. AET results for HPV-2 vaccine containing different concentrations of different preservatives.
Table 4. The percentage results of HPV 16 L1 mice responded under eight gradient in each group.
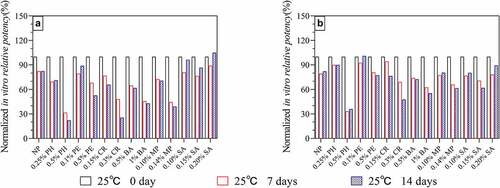
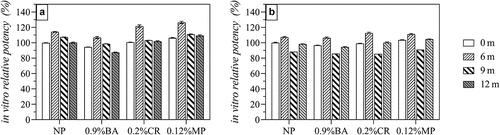
According to the AET results and the observed effects of preservatives on HPV antigens (), the remaining three acceptable preservatives were selected, and lower concentrations (0.9% BA, 0.12% MP, and 0.20% CR) were prepared to produce a HPV-2 vaccine containing preservatives. The accelerated (25 ± 2°C protected from light) and long-term (5 ± 3°C protected from light) stability of the resulting vaccine was then studied. The accelerated stability results () showed that there was no remarkable difference (<10%) in in vitro antigenic potency on day 0 in all groups. When the HPV-2 vaccine containing these three preservatives were stored 25°C for 28 days, the 0.9% BA group decrease ~50% in vitro antigenic potency while the decline degree of IVRP of 0.12% MP group and 0.2% CR group was the same as that of NP group(~20%). The results of long-term stability also support this conclusion. The results in showed that the IVRP of 0.12% MP and 0.2% CR groups was basically the same as that of NP group(>90%) after being placed at 2–8°C for one year, while 0.9% BA group was slightly worse(~85%).
Figure 5. In vitro potency of HPV 16L1(A) and HPV 18L1(B) antigens in HPV-2 vaccine in the presence of three preservatives under accelerated conditions (25 ± 2°C protected from light). Each sample contained 80 μg/mL HPV 16L1 and 40 μg/mL HPV 18L1 antigens. All in vitro potency results were normalized to the T0 results for the NP group and are the average of two independent measurements; error bars indicate SD values.
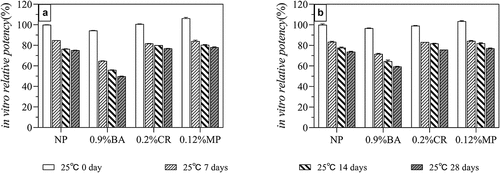
Figure 6. In vitro potency of HPV 16L1(A) and HPV 18L1(B) antigens in HPV-2 vaccine in the presence of three preservatives under long-term conditions (5 ± 3°C protected from light). Each sample contained 80 μg/mL HPV 16L1 and 40 μg/mL HPV 18L1 antigens. All in vitro potency results were normalized to the T0 results for the NP group and are the average of two independent measurements; error bars indicate SD values.
The in vivo antigenicity difference between these four group was also evaluated by pseudovirus neutralization analysis (the data of mice responded in each group have been shown in and ). reveals that neither relative potency measure was considerably reduced in the HPV-2 vaccine containing 0.9% BA and 0.12% MP after storage at 2–8°C for one year (compared with the control, the decrease is less than 30%). More importantly, the impact of only 0.12% MP is even smaller.
Table 5. The percentage results of HPV 16 L1 mice responded under eight gradient in each group.
Figure 7. In vivo potency of HPV 16L1(A) and HPV 18L1(B) antigens in HPV-2 vaccine in the presence of three preservatives under long-term conditions (5 ± 3°C protected from light). Each sample contained 80 μg/mL HPV 16L1 antigen and 40 μg/mL HPV 18L1 antigen. Female BALB/c mice (n = 10) were immunized through abdominal injection and mice serum was collected after 28 days of raising. the percentage of positive serum samples in each dose group was calculated, and the ED50 value was calculated using a probit model. All in vivo relative potency ED50 values were normalized to the T0 ED50 value for the NP group.
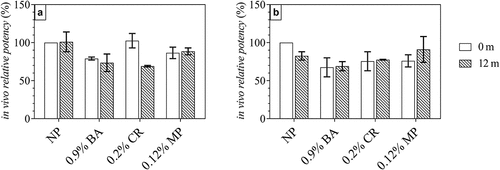
Based on the stability of our upcoming HPV-2 vaccine, the in vivo and in vitro potency of the HPV-2 vaccines without preservative (NP group) remain unchanged under long-term condition (5 ± 3°C protect from light) for more than 3 years. According to the above results (), there is no remarkable difference between the group containing preservative and NP group for 12 months, so we speculate that the HPV-2 vaccines with 0.12%MP may remain unchanged under long-term condition (5 ± 3°C protect from light) for 3 years.
Moreover, shows that the three groups of HPV 2-valent vaccines containing preservatives can still pass the AET of the Chinese Pharmacopoeia “B” standard after storage at 2–8°C for one year. In addition, we quantified the preservatives in the HPV-2 vaccine by HPLC () and found that the three preservatives were not adsorbed by the aluminum phosphate adjuvant on day zero but remained free in the solution. However, after six months of storage at 2–8°C the concentration of CR in the solution dropped by nearly 30% and remained constant thereafter. Considering the AET results, we speculate that either the CR in solution was adsorbed by the aluminum phosphate adjuvant (whereby the aluminum phosphate adjuvant became saturated with CR after 30% adsorption, resulting in constant CR thereafter) or the CR was partially degraded (whereby the product may still have antimicrobial effectiveness after degradation). Conversely, BA and MP were stable (the deviation from the theoretical value is less than 20%) in the solution for one year.
Table 6. AET results for HPV-2 vaccine containing three preservatives under long-term conditions (5 ± 3°C protected from light).
Table 7. Preservative content in HPV-2 vaccine in the presence of three preservatives under long-term conditions (5 ± 3°C protected from light).
Discussion
The various effects of phenol, thimerosal, and phenoxyethanol on proteins have already been proven; therefore, this study analyzes other preservatives available on the market.Citation14,Citation25,Citation26 Although there is no direct evidence of the health risks (diseases related to mercury poisoning) associated with thimerosal in vaccines, the number of vaccines currently in use that contain thimerosal has dropped significantly since 1999.Citation27 In addition, the presence of thimerosal can significantly reduce the conformational stability of HPV VLP.Citation28 Therefore, thimerosal was excluded from this study, and six candidate preservatives (phenol, phenoxyethanol, m-cresol, benzyl alcohol, methylparaben, and sorbic acid) were investigated instead.Citation11–15
The ability to rapidly screen an appropriate preservative and its concentration is very important in vaccine development. A key consideration is that the presence of certain preservatives can cause protein instability such as aggregation, which leads to a loss of protein potency.Citation14,Citation24 Preservatives such as benzyl alcohol cause the aggregation of cytochrome c+, rhIL-1ra, and interferon α2a, as well as changes in the thermal structural stability of proteins;Citation15,Citation25,Citation26 however, the detailed physical mechanism by which these preservatives induce protein aggregation is not fully understood. According to experiments under accelerated conditions, the six preservatives exhibited varying degrees of negative effects on HPV VLPs, manifested by a slight decrease in the aggregation, thermal stability, and in vitro potency of VLP proteins, indicating that preservatives can change the protein structure. For example, high-concentration PH substantially reduced the HPV 16L1 antigen potency in bulk and aluminum adsorption products, and different concentrations of SA led to a substantial decrease in the HPV 16L1 antigen potency and destroyed the structure of HPV VLPs. Thus, a major challenge of developing multi-dose HPV vaccines containing preservatives is the compatibility of preservatives with HPV VLP antigens. Because all preservatives directly or indirectly destroy the proteins in microorganisms to achieve antimicrobial effectiveness (for example, by directly reacting with proteins to inactivate proteins or affecting antigens by changing the antigen microenvironment), this negative impact is inevitable. However, the degree of impact depends on the pH, buffer system, nonionic surfactants, antigenic properties, concentration, and other factors. Therefore, identifying a preservative that has a certain negative impact within an acceptable range is essential for the successful development of a multi-dose HPV-2 vaccine.
To confirm whether the magnitude of the negative effects of preservatives is acceptable and ensure that optimal HPV-2 vaccine preservatives are selected, continuous AET and long-term stability tests of the vaccine are crucial. Therefore, we selected three preservatives (BA, CR, and MP), not only to evaluate their respective antimicrobial effectiveness on the HPV-2 vaccine, but also to evaluate their effect on long-term stability. Compared with the control group, the three groups containing preservatives showed no remarkable decrease in in vitro potency after 12 months of storage at 2–8°C The addition of 0.12% MP had the least impact on the HPV-2 vaccine, and the preservative content did not change substantially (a reduction of less than 10%), which ensured that the antimicrobial effectiveness met “B” standards. We suggest two possible mechanisms for this outcome: 1) aluminum phosphate adjuvant can effectively isolate the contact between preservatives and HPV VLPs and protect the antigen; 2) in storage processing, there will still be partial dynamic contact between the preservative and the antigen, resulting in a slight loss of in vivo potency; however, the structural changes or interaction sites for the two antigens of HPV 16L1 and HPV 18L1 may not completely surround the spatial epitope.
Our results indicate that 0.12% methylparaben (MP) is currently the most suitable choice of preservative for the HPV-2 vaccine. MP is widely used as an antimicrobial preservative in cosmetics, food, and pharmaceutical preparations, and has antimicrobial effectiveness in the pH range of 4–8.Citation10 As the pH increases, the formation of phenolate ions reduces the antiseptic effect.Citation10 At room temperature, an aqueous solution of methyl parahydroxybenzoate with a pH of 3–6 is stable for up to four years.Citation10
Parabens have been used as preservatives for injections and ophthalmic preparations, and are non-mutagenic, non-teratogenic, and non-carcinogenic.Citation10 Moreover, sensitization is rare, with these compounds exhibiting no obvious light contact sensitization or phototoxicity. Hypersensitivity to parabens is generally delayed and manifests as contact dermatitis;Citation10 however, immediate hypersensitivity after injection of preparations containing parabens has also been reported.Citation29 Moreover, the incidence of delayed contact dermatitis is higher when parabens are used topically, but may also occur after oral administration.Citation30–32 Furthermore, some people have expressed concern about the use of MP in infant parenteral products because bilirubin binding may be affected, which is potentially dangerous for neonates with hyperbilirubinemia.Citation33,Citation34
As mentioned earlier, only phenol, thimerosal, and phenoxyethanol are currently used as preservatives in vaccines, whereas MP is widely used as an antimicrobial preservative in cosmetics, food, and other applications. Therefore, our results indicate that MP is a novel potential preservative for the multi-dose HPV-2 vaccine that can guarantee the shelf life of the vaccine for at least three years; thus, we present an effective formula for the future industrialization of this product. The next step in this research will be to study and confirm the mechanism of its impact on VLP and perform animal experiments to evaluate its safety, thereby ensuring that our company is approved for a single-dose HPV-2 vaccine. Subsequently we intend to develop a multi-dose HPV vaccine as soon as possible to help prevent cervical cancer in LMICs.
In addition, future studies should consider compound preservatives that may produce synergistic effects. This method may not only reduce the number of preservatives that have a negative impact on the vaccine but also expand the antibacterial spectrum and improve the antibacterial effects. For example, future studies may attempt the synergistic use of benzyl alcohol and m-cresol or the synergistic use of benzyl alcohol and methyl paraben.
Because of the limited conditions, the following aspects require further research. 1) The preservative concentration of the CR group decreased by approximately 30% after six months, with no further decline in the following 12 months. Nevertheless, the antimicrobial effectiveness in the CR group did not decrease; the reasons for this should be explored. 2) It is important to explore the mechanism by which preservatives affect the structure and conformation of antigens.
Acknowledgements
We thank Kehua Yang and Jiangming Huang for their help with the animal experiments, antimicrobial effectiveness experiments, and preservative content experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Medeiros R, Vaz S, Rebelo T, Figueiredo-Dias M. Prevention of human papillomavirus infection. Beyond cervical cancer: a brief review. Acta Med Port. 2020;33(3):198–10. doi:10.20344/amp.12259.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
- ICO. Chinese HPV and related diseases report. www.hpvcentre.net. p. 87.
- World Health Organization. Cervical cancer. WHO; 2022. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26. doi:10.1016/j.bpobgyn.2017.08.006.
- Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Franco EL, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. J Am Med Assoc. 2002;286(24): 3106–3114. doi:10.1001/jama.286.24.3106.
- Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, Castle PE, Bray F, Canfell K. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol. 2019;20(3):394–407. doi:10.1016/s1470-2045(18)30836-2.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–63. doi:10.1016/S2214-109X(16)30099-7.
- Division US. Human papillomavirus vaccine: supply and demand update. https://www.unicef.org/supply/media/501/file/human%20papillomavirus%20HPV%20vaccine%20supply%20and%20demand%20update.pdf2019.
- Raymond C Rowe PJSaMEQ. Handbook of Pharmaceutical Excipients. Pharmaceutical Press. 2010;6:917.
- Meyer BK, Ni A, Hu B, Shi L. Antimicrobial preservative use in parenteral products: past and present. J Pharm Sci. 2007;96(12):3155–67. doi:10.1002/jps.20976.
- Supriya Gupta EK. Development of a multidose formulation for a humanized monoclonal antibody using experimental design techniques. AAPS PharmScitech. 2003;5.
- Wöhlbrand L, Wilkes H, Halder T, Rabus R. Anaerobic degradation of p-ethylphenol by “Aromatoleum aromaticum” strain EbN1: pathway, regulation, and involved proteins. J Bacteriol. 2008;190(16):5699–709. doi:10.1128/JB.00409-08.
- Hutchings RL, Singh SM, Cabello-Villegas J, Mallela KM. Effect of antimicrobial preservatives on partial protein unfolding and aggregation. J Pharm Sci. 2013;102(2):365–76. doi:10.1002/jps.23362.
- Bis RL, Mallela KM. Antimicrobial preservatives induce aggregation of interferon alpha-2a: the order in which preservatives induce protein aggregation is independent of the protein. Int J Pharm. 2014;472(1–2):356–61. doi:10.1016/j.ijpharm.2014.06.044.
- Mach H, Volkin DB, Troutman RD, Wang B, Luo Z, Jansen KU, Shi LI. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J Pharm Sci. 2006;95(10):2195–206. doi:10.1002/jps.20696.
- Zhao Q, Potter CS, Carragher B, Lander G, Sworen J, Towne V, Abraham D, Duncan P, Washabaugh MW, Sitrin RD, et al. Characterization of virus-like particles in GARDASIL® by cryo transmission electron microscopy. Hum Vaccines Immunother. 2013;10(3):734–39. doi:10.4161/hv.27316.
- Jain NK, Sahni N, Kumru OS, Joshi SB, Volkin DB, Russell Middaugh C. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv Drug Deliv Rev. 2015;93:42–55. doi:10.1016/j.addr.2014.10.023.
- Shi L, Sanyal G, Ni A, Luo Z, Doshna S, Wang B, Graham TL, Wang N, Volkin DB. Stabilization of human papillomavirus virus-like particles by non-ionic surfactants. J Pharm Sci. 2005;94(7):1538–51. doi:10.1002/jps.20377.
- Gu Y, Wei M, Wang D, Li Z, Xie M, Pan H, Wu T, Zhang J, Li S, Xia N, et al. Characterization of an Escherichia coli-derived human papillomavirus type 16 and 18 bivalent vaccine. Vaccine. 2017;35(35):4637–45. doi:10.1016/j.vaccine.2017.06.084.
- Kim HJ, Cho SY, Park MH, Kim HJ. Comparison of the size distributions and immunogenicity of human papillomavirus type 16 L1 virus-like particles produced in insect and yeast cells. Arch Pharm Res. 2018;41(5):544–53. doi:10.1007/s12272-018-1024-4.
- Shank-Retzlaff ML, Zhao Q, Anderson C, Hamm M, High K, Nguyen M, Wang F, Wang N, Wang B, Wang Y, et al. Evaluation of the thermal stability of Gardasil®. Hum Vaccin. 2006;2(4):147–54. doi:10.4161/hv.2.4.2989.
- Zhao Q, Allen MJ, Wang Y, Wang B, Wang N, Shi L, Sitrin RD. Disassembly and reassembly improves morphology and thermal stability of human papillomavirus type 16 virus-like particles. Nanomed Nanotechnol Biol Med. 2012;8(7):1182–89. doi:10.1016/j.nano.2012.01.007.
- Moser CL, Meyer BK. Comparison of compendial antimicrobial effectiveness tests: a review. AAPS PharmScitech. 2011;12(1):222–26. doi:10.1208/s12249-010-9575-9.
- Singh SM, Hutchings RL, Mallela KM. Mechanisms of m-cresol-induced protein aggregation studied using a model protein cytochrome c. J Pharm Sci. 2011;100(5):1679–89. doi:10.1002/jps.22426.
- Roy S, Jung R, Kerwin BA, Randolph TW, Carpenter JF. Effects of benzyl alcohol on aggregation of recombinant human interleukin-1-receptor antagonist in reconstituted lyophilized formulations. J Pharm Sci. 2005;94(2):382–96. doi:10.1002/jps.20258.
- Gołoś A, Lutyńska A. Thiomersal-Containing vaccines - a review of the current state of knowledge. Przegl Epidemiol. 2015;69(1): 59-64, 157-61.
- Chen S, Huang X, Li Y, Wang X, Pan H, Lin Z, Zheng Q, Li S, Zhang J, Xia N, et al. Altered antigenicity and immunogenicity of human papillomavirus virus-like particles in the presence of thimerosal. Eur J Pharm Biopharm. 2019;141:221–31. doi:10.1016/j.ejpb.2019.05.027.
- Latronica RJ, Goldberg AF, Wightman JR. Local anesthetic sensitivity. Report of a case. Oral Surg Oral Med Oral Pathol. 1969;28(3):439–41. doi:10.1016/0030-4220(69)90240-0.
- Michaëlsson G, Juhlin L. Urticaria induced by preservatives and dye additives in food and drugs. Br J Dermatol. 1973;88(6):525–32. doi:10.1111/j.1365-2133.1973.tb08014.x.
- Warin RP, Smith RJ. Challenge test battery in chronic urticaria. Br J Dermatol. 1976;94(4):401–06. doi:10.1111/j.1365-2133.1976.tb06117.x.
- Kaminer Y, Apter A, Tyano S, Livni E, Wijsenbeek H. Delayed hypersensitivity reaction to orally administered methylparaben. Clin Pharm. 1982;1:469–70.
- Fisher AA. Cortaid cream dermatitis and the “paraben paradox”. J Am Acad Dermatol. 1982;6(1):116–17. doi:10.1016/s0190-9622(82)80208-9.
- Loria CJ, Echeverria P, Smith AL. Effect of antibiotic formulations in serum protein:bilirubin interaction of newborn infants. J Pediatr. 1976;89(3):479–82. doi:10.1016/s0022-3476(76)80558-6.
