ABSTRACT
Invasive meningococcal disease (IMD) caused by Neisseria meningitidis (Nm) continues to be a global public health concern. Understanding the prevalence of Nm serogroups in IMD is critical for developing strategies for meningococcal vaccination. We used the keywords “cerebrospinal meningitis”, “meningococcal”, “Neisseria meningitidis’’, “meningococcal meningitis”, “serogroup’’ and “China’’ to search five databases, including PubMed, CNKI, CBM (Chinese BioMedical Literature Database), WanFang and VIP from 2010 to 2020. The age distributions, proportions of Nm serogroups and serogroup changes in IMD were analyzed. A total of 14 studies were included according to PRISMA guidelines. In China, from 2010 to 2020, the highest proportion of Nm in IMD was NmC, with 49.7% (95% CI: 35.8%–63.5%), followed by NmB with 30.2% (95%CI:17.3%–43.0%) and NmW with 23.8% (95%CI: 7.0–40.7%). Before 2014, NmC was the major circulating serogroup, with 59.6% (95% CI: 43.8%-75.4%), followed by NmW with 24.4% (95% CI: 5.9%–42.9%). After 2015, IMD cases caused by NmB were increasing, the proportion of NmB reached to 52.4% (95% CI: 31.8%–73.1%). The age groups of children from 0 to 5 years and from 6 to 10 years represented, respectively, 29.6% (95% CI: 16.8%–42.4%) and 28.9% (95% CI: 12.1%–45.8%) of all IMD cases were reported. In China, NmB, NmC and NmW were the major serogroups causing IMD between 2010 and 2020. Since 2015, the proportion of NmB increased rapidly. The current serogroup distribution in China highlights the need of replacing the meningococcal polysaccharide vaccines that are being used in the National Immunization Program with more appropriate vaccines.
Introduction
Neisseria meningitidis (Nm), a human-specific gram-negative pathogen, can cause invasive meningococcal disease (IMD).Citation1,Citation2 The fatality rate of IMD ranges to 4 to 20%, even with appropriate clinical treatment.Citation3 10–20% of IMD survivors reported have long-term serious sequelae, such as amputations, hearing loss, cognitive difficulties, and other neurologic disorders.Citation4,Citation5
Nm is categorized into 12 serogroups on the basis of the capsular polysaccharide. Serogroups A, B, C, W, X and Y are responsible for most IMD cases worldwide.Citation6,Citation7 The incidence and serogroup dirstribution of IMD vary by geographic region and over time.Citation7,Citation8 Although IMD can occur at all ages, infants are at the highest risk, followed by adolescents and young adults.Citation8 In Europe, the overall notification rate was 0.62 cases per 100,000 population in 2017, ranged from .45 (Northern and Southern Europe) to 1.33 (UK and Ireland) cases per 100,000 population.Citation9 In the United States, the incidence has declined to 0.11 cases per 100,000 population in 2019.Citation10 In African meningitis belt, with the introduction of MenA conjugate vaccine (MenAfriVac) in 2010, the IMD incidence has dramatically declined to 0.02 cases per 100,000 during 2010–2103.Citation11 In China, based on data from the National Notifiable Disease Reporting System (NNDRS), the reported IMD average incidence was 0.0078/100,000 during 2015–2019.Citation12
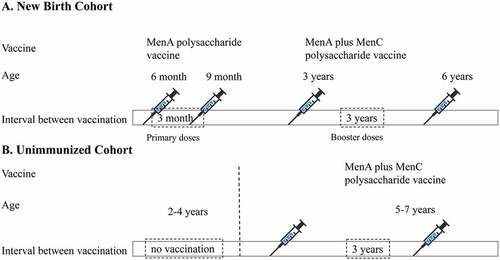
China has experienced several previous NmA meningococcal epidemics. MenA polysaccharide vaccines have been introduced into routine immunization programs since the 1980s.Citation13 In 2003, serogroup C cc4821 emerged and was responsible for the meningococcal disease outbreak in Anhui Province, China, then NmC cc4821 has rapidly spread to most provinces in China.Citation14 Therefore, the group A plus C polysaccharide vaccine was introduced into the Chinese Expanded Program for Immunization (EPI) in 2008. Chinese EPI schedule for meningococcal vaccination as follows (): two primary doses of MenA polysaccharide vaccines were used for infants at 6 months and 9 months ages, two booster doses of group A plus C polysaccharide vaccines were inoculated for children at 3 years and 6 years. If the child aged 2 to 4 years have not received MenA polysaccharide vaccine, they would receive two dose MenA plus MenC polysaccharide vaccine for initial vaccination and the booster with an interval of 3 years.Citation13 The above polysaccharide vaccines were EPI cost-free vaccines, which were paid by government. Ideally, the vaccination coverage rate could reach to 100% in China. The incidence of IMD caused by NmA and NmC decreased dramatically with mass vaccination. Since 2006, IMD caused by NmW cc11 and NmB cc4821 has become new challenges.Citation15 Few IMD cases caused by NmX and NmY have been reported as sporadic cases in China in recent years.Citation16,Citation17
Meningococcal vaccination is very beneficial for the control and prevention of IMD. To modify the strategies for meningococcal vaccination and to develop new meningococcal vaccines, such as MenB vaccines, evidence-based data including the prevalence of Nm serogroups and the epidemiological characteristics of the age group distribution, is necessary. In this study, we collected and systematically reviewed the published literatures related to IMD for further meta-analysis to illustrate the trends in the prevalence of serogroups in China.
Methods
Sources of data and search strategies
Following the PRISMA guidelinesCitation18 we identified articles published from 1 January 2010 to 31 December 2020 that reported invasive meningococcal disease serogroup data from 5 databases: PubMed, CNKI, CBM (Chinese BioMedical Literature Database), WanFang, and VIP. The detailed search strategies are presented in Appendix 1 (in the supplementary material). All related studies were reviewed independently by two reviewers (Juan Xu and Mengmeng Yue). To gather the required data, the following medical subject headings (MeSH) terms and text words were chosen and combined: “Cerebrospinal meningitis”, “Meningococcal”, “Neisseria meningitidis’’, “Meningococcal meningitis”, “Serogroup’’, “China’’ and their synonyms from the text, title, or abstract. No restriction was made based upon language. PubMed was searched in English and the other databases were searched in Chinese.
Inclusion and exclusion criteria
Preliminary screening was conducted by reading the title and abstract of the literature and clearly unqualified studies were removed, and then full-text articles were reviewed in detail against the inclusion and exclusion criteria. Questions concerning the appropriateness for inclusion in the analysis were discussed among the group members and agreed upon prior to proceeding to analysis.
Studies were eligible for inclusion with the following items: 1. the studies were peer-reviewed and published from 1 January 2010, to 31 December 2020, 2. reported cases were limited to China, 3. the study included humans, 4. the serogroup prevalence was reported, and at least one or more of the following indicators, such as age, time, and vaccine type, 5. it was published in Chinese or English.
Studies with the following items were excluded: 1. studies that targeted the N. meningitidis carrier population rather than IMD, 2. the articles were systematic reviews, meta-analyses or case reports, 3. reporting data from outbreak investigations, 4. the study data were incomplete and did not include serogroup information or were unable to calculate the proportion of serogroups based on the study data, 5. duplicated publications of the same study, 6. the publication year of the case report was before 2010 or did not explicitly exclude data from prior to 2010.
Quality assessment
Quality assessment of the studies was performed by two reviewers (Juan Xu and Mengmeng Yue) independently using an existing checklist modified by Hoy D.Citation19 The checklist has nine questions. Each question could be answered “yes” or “no”, and the “yes” answer could get one score. Thus, final scores for each study could range from zero to nine. If the score was 0 to 3, it was low quality, 4 to 6 was moderate quality and 7 to 9 was high quality.
Data extraction and data collection
Two reviewers (Juan Xu and Mengmeng Yue) independently conducted data extraction and collection from the literature. For each included article, two researchers extracted the following data into an Excel sheet: title, study region, publication date, study design, research period, age groups of the individuals, sample type, identification method, sample size, number of IMD cases, number of N. meningitidis serogroups (A, B, C, Y, X, W and others), and serogroup distribution in different years and age groups. If any of the desired data were not found in the publications, they were marked as “UN (unknown)”.
Data analysis
The age distribution of IMD, proportions of different serogroups (A, B, C, Y, X, W, and others) and changing trends of serogroups of the IMD cases were analyzed. R software (version 4.0.3, Auckland University, USA) was used for the meta-analysis. First, the Shapiro-Wilk statistic was used to test the normality of the original data. According to the results of the normality test, a data form close to the normal distribution was selected for the meta-analysis. When the original data were normally distributed according to the Shapiro-Wilk test, we used the original rate directly in the meta-analysis. Otherwise, the nonnormally distributed data were transformed using the following methods (log, logit, arcsine, or Freeman-Tukey double arcsine transformation). The chi-square-based heterogeneity test (Q test) was used to analyze the heterogeneity (α = 0.10). We used a random-effects model for pooled prevalence estimation. Heterogeneity was measured using I2 values. Publication bias was analyzed by funnel plots and Egger’$3 test with linear regression or Peters test (for a sample size less than 10). All P values were bilateral, and P < .05 indicated statistical significance.
Results
Study selection
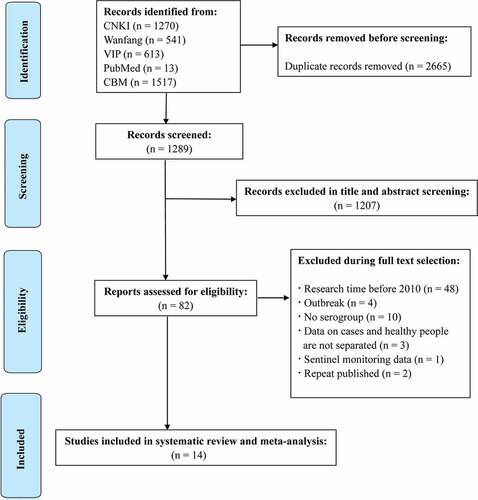
A total of 3954 publications were reviewed in the systematic search, and 2665 duplicates were excluded after the first screening. According to the title, abstract and full text, the literature was screened. Finally, 14 studies were included in the meta-analysis and reported relevant data ().
Among the 14 studies that received a “high” quality assessment score, the results are shown in and Table S1 (in the supplementary material). The 14 studies included 12 articles with data from 8 provinces and 2 studies were national data. The characteristics of the selected articles are summarized in . All studies reported serogroup prevalence by year after 2010 (Table S2 in the supplementary material), and 4 studies clearly reported the number of IMD cases in the different age groups. The average number of N. meningitidis isolates from each study included in the meta-analysis were 42 (specimens or subjects), with a range of 4–296 isolates. Three studies reported that the sample type was CSF (cerebrospinal fluid) and blood, and 11 did not report the sample type.
Figure 3. Quality assessment of the included studies. (a) Summary plot of risk bias; (b) Traffic light plot of risk bias.
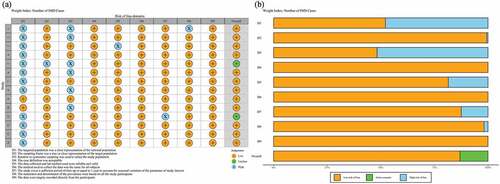
Table 1. Characteristics of the included studies.
The proportion of IMD cases in the different age groups
A total of 4 studies reported IMD cases by age. As shown in , the random-effects model was used to combine the proportion of cases at different ages. As shown in the table, the proportion of IMD cases in children from 0 to 5 years was 29.6% (95% CI: 16.8%–42.4%), followed by children in 6–10 years age group with 28.9% (95% CI: 12.1%-45.8%).
Table 2. The proportion of cases of invasive meningococcal disease of different ages.
The proportion of N. meningitidis serogroups in invasive meningococcal disease
As shown in , a random-effects model was used to evaluate the proportion of Nm serogroups in IMD. In China, during 2010–2020, the highest proportion of Nm serogroups in IMD was NmC with 49.7% (95% CI: 35.8%–63.5%), followed by NmB with 30.2% (95% CI: 17.3%–43.0%) and NmW with 23.8% (95% CI: 7.0%–40.7%). Heterogeneity was high (I2 ≥75%) in NmB, NmW, NmC, NmA and the others.
Table 3. The proportion of N. meningitidis serogroups in IMD in China, 2010–2020.
N. meningitidis serogroup in the different periods
The results of N. meningitidis serogroups in IMD is shown in . The results showed an increasing proportion of NmB and others in China, while the proportion of serogroups A, C, and W decreased. In 2010–2014, NmC was the predominant circulating serogroup with 59.6% (95% CI: 43.8%–75.4%), followed by NmW with 24.4% (95% CI: 5.9%–42.9%) and others with 23.1% (95% CI: 6.4%–39.8%). However, after 2014, the proportion of NmB increased and reached to 52.4% (95% CI: 31.8%–73.1%), while NmC declined to 22.8% (95% CI: 18.2%–27.4%), followed by NmW and NmA with 16.8% (95% CI: 0%–36.9%) and 4.7% (95% CI: 2.6%–7.8%), respectively.
Table 4. Time trend of N. meningitidis serogroups in IMD in China, 2010–2020.
Publication bias
Publication bias analysis was conducted for the included studies in each serogroup, and funnel plots and Egger’$3 test linear regression were used for analysis. NmC had publication bias (P < .05), and the results are shown in and Figure S1 (in the supplementary material). For publication bias, the trim-and-fill method was used for correction. After filling in the missing studies, the proportion of NmC was 27.3% (95% CI: 13.2%–41.4%), which was lower than the original results.
Table 5. Bias test for each serogroup.
Discussion
The proportion of Nm serogroups in IMD varied in different countries. In this study, we systematically reviewed the prevalence of Nm serogroups causing IMD in China from 2010 to 2020. Meta-analysis indicated that the highest overall proportion in IMD was NmC with 49.7% in the last decade. Since 2015, IMD cases caused by NmB were increasing. Similarly, a meta-analysis based on global Nm serogroups in IMD in 2019 showed that the overall proportion of NmB in IMD was 48.5%, and NmB became the major Nm serogroup in IMD worldwide.Citation33 In Africa, since 2010, the conjugate vaccine MenAfriVac was deployed extensively for persons aged 1–29 years in all countries of the meningitis belt. The implementation of this programme led to nearly 99% decline in confirmed MenA cases during 2010–2015.Citation34,Citation35 Consequently, with the dramatic decline of MenA, the circulating serogroups have shifted and most disease is now caused by MenC, MenW and MenX.Citation34
Vaccination is regarded as the optimal strategy of preventing IMD. The first vaccines based on capsular polysaccharide against serogroups A, C, W and Y were successful for the prevention of IMD. However, polysaccharides are T-cell-independent antigens that result in short-lived immunity and could not induce immune memory.Citation36 And apart from group A polysaccharide, other vaccines components are poorly immunogenic in children younger than 2 years of age.Citation37 To overcome the problem of short duration protection and inability of memory response against the meningococcus, vaccines that conjugated the polysaccharide to a carrier protein were used.Citation38 In 1999, the UK first introduced the MenC polysaccharide conjugate vaccine into national immunization programs (NIPs) for routine infant immunization and as a booster vaccination for children and adolescents up to 18 years of age, then there was a marked decline in MenC disease.Citation38,Citation39 Furthermore, MenC conjugate vaccines also could decrease carriage among teenagers and induce herd protection.Citation38,Citation40 Polysaccharide-conjugate vaccines for serogroups A, C, W, and Y gradually replace polysaccharide vaccines and have been recommended or introduced into NIPs in many countries worldwide for years.Citation38 However, in Chinese routine national immunization programs, infants (6 months and 9 months) received two doses MenA polysaccharide vaccine and children (3 years and 6 years) received two doses MenAC polysaccharide vaccine, there was no longer booster for those over 6 years of age. In addition, polysaccharide conjugate vaccine was not introduced into EPI. But some conjugate vaccines could be available, including MenAC and MenACWY conjugate vaccine for infants older than 3 months of age, which should be paid for the cost by parents.
Polysaccharide vaccines could not be available for MenB vaccines because of their polysaccharide structure’$3 similarity to the N-acetylneuraminic acid structure of human nerve cells, which may lead to not only poor immunogenicity but also potential autoimmune antibodies.Citation41 The development of MenB vaccine was protein-based. Two MenB vaccine including 4CMenB (Bexsero, GSK) for infants, toddlers and children at 2–10 years of age or person aged 10–25 yearsCitation42,Citation43 and rLP2086 (Trumenba, Pfizer) for persons aged 10 to 25 years have been recommended for NIPs in many countries.Citation44,Citation45 MenB vaccines exhibited broad coverage and effectiveness. In the UK, the effectiveness of 4CmenB could reach 83% after two doses of inoculation, and the protective effect could persist for over 3 years among 75% of vaccinated individuals.Citation46,Citation47 In China, MenB became the predominant IMD serogroup, but no MenB vaccines are currently available.
In this study, we found the proportion of NmB, NmW and NmY are increasing, but the polysaccharide vaccines included in EPI currently cannot cover these serogroups. Vaccines targeting more serogroups should be introduced into EPI, and polysaccharide conjugate vaccines should gradually replace polysaccharide vaccines. Furthermore, new vaccines for NmB need to be developed in the future.
Strengths and limitations
This study included almost all studies about the distribution of IMD serogroups in China in the past 10 years, and we systematically reviewed the prevalence of Nm serogroups causing IMD in China. It provided preliminary estimate of the current serogroup and age groups distribution of IMD cases in China.
The main obstacle for this study was that most studies calculated the incidence rate of IMD based on different regions, not age groups. With the limitation of data, we only calculated the proportion of each age group, rather than the incidence rates. In addition, in some studies, the Nm serogroups may not be reported due to lack of laboratory diagnostic kits or insufficient laboratory capacity for culture and PCR. The publication bias and heterogeneity detected in the present analysis cannot be ignored, and all of these factors must be considered when interpreting the results.
Conclusion
This systematic review showed that NmC was still the major prevail serogroup between 2010 and 2020, but the proportion of NmB in IMD was increasing rapidly since 2015. The current serogroup distribution in China highlights the need of replacing the meningococcal polysaccharide vaccines that are being used in the National Immunization Program with more appropriate vaccines.
Supplemental Material
Download TIFF Image (4.3 MB)Supplemental Material
Download MS Word (315 KB)Disclosure statement
Nopotential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2071077.
Additional information
Funding
References
- Hill DJ, Griffiths NJ, Borodina E, Virji M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin Sci (Lond). 2010;118(9):547–8. doi:10.1042/CS20090513.
- Schubert-Unkmeir A. Molecular mechanisms involved in the interaction of Neisseria meningitidis with cells of the human blood-cerebrospinal fluid barrier. Pathog Dis. 2017;75(2). doi:10.1093/femspd/ftx023.
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768–82. doi:10.1016/j.vaccine.2019.04.020.
- Olbrich KJ, Müller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–38. doi:10.1007/s40121-018-0213-2.
- World Health Organization. Meningococcal meningitis; 2018. https://www.who.int/news-room/fact-sheets/detail/meningitis.
- Harrison OB, Claus H, Jiang Y, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–73. doi:10.3201/eid1904.111799.
- Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi:10.1186/1478-7954-11-17.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- European Centre for Disease Prevention and Control (ECDC). Invasive meningococcal disease, annual epidemiological report for 2017. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf.
- Centre for Disease Prevention and Control. Meningococcal disease surveillance. 2021.https://www.cdc.gov/meningococcal/surveillance/.
- Lingani C, Bergeron-Caron C, Stuart JM, Fernandez K, Djingarey MH, Ronveaux O, Schnitzler JC, Perea WA. Meningococcal meningitis surveillance in the African meningitis belt, 2004–2013. Clin Infect Dis. 2015;61(Suppl 5):S410–5. doi:10.1093/cid/civ597.
- Li J, Wu D, Wen N, et al. Serogroup distribution of meningococcal meningitis in China,2015-2019. Chin J Vacc Immunization. 2020;26(2):241–44.
- Li J, Shao Z, Liu G, et al. Meningococcal disease and control in China: Findings and updates from the Global Meningococcal Initiative (GMI). J Infect. 2018;76(5):429–37. doi:10.1016/j.jinf.2018.01.007.
- Shao ZJ, Li W, Ren J, Liang XF, Xu L, Diao BW, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet. 2006;367(9508):419–23. doi:10.1016/S0140-6736(06)68141-5.
- Shao Z, Zhou H, Gao Y, Ren H, Xu L, Kan B, Xu J. Neisseria meningitidis Serogroup W135, China. Emerg Infect Dis. 2010;16(2):348–49. doi:10.3201/eid1602.090901.
- Chen C, Zhang TG, He JG, et al. A first meningococcal meningitis case caused by serogroup X Neisseria meningitidis strains in China. Chin Med J (Engl). 2008;121(7):664–66. doi:10.1097/00029330-200804010-00017.
- Guo LC, Liu XC, Xu QY, et al. Epidemiological analysis on serogroup Y neisseria meningitidis firstly isolated from patient in Tianjin. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50(9):825–27. doi:10.3760/cma.j.issn.0253-9624.2016.09.015.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi:10.1016/j.ijsu.2021.105906.
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–39. doi:10.1016/j.jclinepi.2011.11.014.
- Chen LP, Sun XY, Wei JJ, et al. Epidemiological characteristics of meningococcal meningitis inWenzhou, 2005-2018. Chin J PHM. 2019;35(5):670–78. CNKI:SUN:GGWS.0.2019-05-026.
- Wu JH, He TT, Guo TY, et al. Surveillance of cerebrospinal meningitis in Shaoxing from 2005 to 2011. Zhejiang Prevent Med. 2013;25(02):39–41. doi:10.3969/j.issn.1007-0931.2013.02.014.
- Shan XY, Bai AY, Liu ZY. Multi locus sequence typing of Neisseria meningitidis in Jinan, Shandong. Dis Surveillance. 2018;33(12):38–42. CNKI:SUN:JBJC.0.2018-12-012.
- Feng L, Xiong P, Li MS, et al. Analysis on the epidem iological characteristics and the etiological surveillance for epidem ic cerebrospinal meningitis in Shandong province from 2006 to 2013. Chin J Epidemiol. 2014;35(12):1407–08. doi:10.3760/cma.j.issn.0254-6450.2014.12.021.
- Yang M, Zhou HJ, Xu XQ, et al. Serogroup distribution and characteristics of molecular subtyping for Neisseria meningitidis isolated from Jiangxi, 2005-2015. Chin J Zoonoses. 2018;34(11):35–39.
- Sun YQ, Ma HS, Jia ZY, et al. Analysis on characteristic and flora change of epidemic cerebrospinal meningitis in Hebei Province. J Med Pest Control. 2012;28(011):1184–87. CNKI:SUN:YXDZ.0.2012-11-001.
- He BH, Jia ZY, Wang YT, et al. Study on the surveillance of pathogens and molecular subtyping of meningococcal meningitis cases during 2012-2013 in Hebei province. Chin J Health Lab Tec. 2015;25(005):708–11. CNKI:SUN:ZWJZ.0.2015-05-033.
- Jiang F, Zhang L, Zhan W, et al. Epidemiological characteristics of epidemic cerebrospinal meningitis in Guizhou Province from 2011 to 2013. J Publ Health Preve Med. 2014;25(6):80–81. CNKI:SUN:FBYF.0.2014-06-023.
- Li J, Li Y, Shao Z, et al. Prevalence of meningococcal meningitis in China from 2005 to 2010. Vaccine. 2015;33(8):1092–97. doi:10.1016/j.vaccine.2014.10.072.
- Fang LL, Chen H, Xu DC, et al. Analysis of the monitoring of the etiology and serology of epidemic cerebrospinal meningitis from 2014-2016. J Pathogen Biol. 2017;12(09):875–78. doi:10.13350/j.cjpb.170916.
- Zhan YH, Luan L, Zhang MH. Analysis on molecular epidemiological characteristics of Neisseria meningitidis in Suzhou, Jiangsu, 2011–2016. Dis Surveillance. 2018;33:640–43.
- Wu SW, Liu CT, Wang YY, et al. Multilocus sequence typing of Neisseria meningitis in Guizhou province, 2006-2017. Chin J Vacc Immunization. 2020;26(06):647–65.
- Dai DF, Li FJ, Xia X, et al. Epidemiological features of meningococcal meningitis and trend of serogroup switching of Neisseria meningitidis strains in Hunan Province, 1951-2016. Pract Prev Med. 2017;24:1440–42. doi:10.3969/j.issn.1006-3110.2017.12.009.
- Purmohamad A, Abasi E, Azimi T, Hosseini S, Safari H, Nasiri MJ, Imani Fooladi AA. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. 2019;134:103571. doi:10.1016/j.micpath.2019.103571.
- Parikh SR, Campbell H, Bettinger JA, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–98. doi:10.1016/j.jinf.2020.05.079.
- Mustapha MM, Harrison LH. Vaccine prevention of meningococcal disease in Africa: major advances, remaining challenges. Hum Vaccin Immunother. 2018;14(5):1107–15. doi:10.1080/21645515.2017.1412020.
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–20. doi:10.1038/nri2494.
- Bilukha OO, Rosenstein N. National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54:1–21.
- Pizza M, Bekkat-Berkani R, Rappuoli R. Vaccines against Meningococcal diseases. Microorganisms. 2020 Oct 3;8(10):1521. doi:10.3390/microorganisms8101521.
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–67. doi:10.1016/s0264-410x(01)00299-7.
- Borrow R, Abad R, Trotter C, van der Klis FRM, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31(41):4477–86. doi:10.1016/j.vaccine.2013.07.083.
- Borrow R. Advances with vaccination against Neisseria meningitidis. Trop Med Int Health. 2012;17(12):1478–91. doi:10.1111/j.1365-3156.2012.03085.x.
- Toneatto D, Pizza M, Masignani V, Rappuoli R. Emerging experience with meningococcal serogroup B protein vaccines. Expert Rev Vaccines. 2017;16(5):433–51. doi:10.1080/14760584.2017.1308828.
- Masignani V, Pizza M, Moxon ER. The development of a vaccine against Meningococcus B using reverse vaccinology. Front Immunol. 2019;10:751. doi:10.3389/fimmu.2019.00751.
- Perez JL, Absalon J, Beeslaar J, et al. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines. 2018;17(6):461–77. doi:10.1080/14760584.2018.1483726.
- Beeslaar J, Absalon J, Balmer P, et al. Clinical data supporting a 2-dose schedule of MenB-FHbp, a bivalent meningococcal serogroup B vaccine, in adolescents and young adults. Vaccine. 2018;36(28):4004–13. doi:10.1016/j.vaccine.2018.05.060.
- Parikh SR, Andrews NJ, Beebeejaun K, et al. Effectiveness and impact of a reduced infant schedule of 4cmenb vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. doi:10.1016/S0140-6736(16)31921-3.
- Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with Meningococcal group B vaccine (4cmenbf) in England. N Engl J Med. 2020;382(4):309–17. doi:10.1056/NEJMoa1901229.