ABSTRACT
In recent years, advances in the treatment and management of patients with systemic lupus erythematosus (SLE) have improved their life expectancy and quality of life. However, lupus nephritis (LN) still represents a major life-threatening complication of the disease. Belimumab (BEL), a fully human monoclonal IgG1λ antibody neutralizing soluble B cell activating factor, was approved more than ten years ago as add-on therapy in adults and pediatric patients with a highly active, autoantibody-positive disease despite standard of care (SoC). Recently, the superiority of the addition of BEL to SoC was also demonstrated in LN. In this review, we provide a comprehensive overview of the study landscape, available therapeutic options for SLE (focusing on BEL in renal and non-renal SLE), and new perspectives in the treatment field of this disease. A personalized treatment approach will likely become available with the advent of novel therapeutic agents for SLE and LN.
Introduction
Systemic Lupus Erythematosus (SLE) is an autoimmune disease with a heterogeneous clinical presentation, affecting mainly women of childbearing age and minorities.Citation1 The condition may manifest in multiple organ systems, such as skin, mucous/serous membranes, and joints. In addition, it may result in life-threatening complications involving vital organs and tissues such as the brain, blood, and the kidney.Citation1 The production of multiple autoantibodies in chronic inflammation and subsequent immunological events are the leading cause of damage during the disease course.Citation2 Genetic, environmental, hormonal, epigenetic, and immunoregulatory factors act sequentially or simultaneously on the immune system through several partially elucidated mechanisms leading to a loss of self-tolerance.Citation1 Almost every immune cell within both the innate and adaptive immune arms is involved in the pathogenesis of the disease.
Immunological pathways involved
A crucial mechanism for triggering the activation of an aberrant immune response is the dysregulation of several cell death pathways, including apoptosis, necrosis, necroptosis, pyroptosis, and neutrophil death through the formation of neutrophil extracellular traps (NETs), and the incomplete clearance of debris which induces the accumulation of remnants into tissues.Citation3 In addition to other unknown factors, these mechanisms lead to a death-dependent immunogenic and non-tolerogenic immune response.Citation4 Furthermore, non-phagocytosed dead cells are presented as autoantigens eliciting B and T cell responses in SLE extrafollicular reactions and germinal centers (GCs).Citation5,Citation6 Consequently, the formation of immune complexes (ICs)-containing autoantibodies that recognize nuclear and cytoplasmic autoantigens released from dead cells induce a potent downstream of pro-inflammatory events, including the synthesis of type I interferons (IFNs).Citation7
Immunologic events in the pathogenesis of lupus nephritis
Lupus nephritis (LN), as defined by clinical and laboratory findings, is a common and severe manifestation of the disease occurring in about 40% of SLE patients, most commonly within the first five years after the diagnosis.Citation8 Clinically asymptomatic urinary sediment abnormalities, nephritic or nephrotic syndromes, and rapidly progressive renal failure represent the broad spectrum of its presentation.Citation9 Organ damage is the result of glomerular, tubulointerstitial, and vascular lesions.Citation10 In LN, the main aetiopathogenetic event is the deposition of ICs into the kidney associated with activating the complement system.Citation11 Autoantibodies can react with glomerular autoantigens, especially those within the glomerular basement membrane (GBM). Thus, the localization of ICs influences the clinical LN phenotype.Citation12
Subendothelial ICs cause endothelial dysfunction, complement system activation, and the enrollment of immune cells into crescents, also containing proliferating cells from the parietal layer of the Bowman’s capsule (“proliferative” variants). These proliferative variants with subendothelial immune deposits correspond to classes III and IV LN according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification, which is currently in use and is regularly revised.Citation13,Citation14
Moreover, subepithelial ICs, on the other hand, cause podocyte damage with only limited pro-inflammatory cell recruitment because the GBM prevents direct interaction with the intravascular space. As a result, there is less glomerular inflammation, but substantial glomerular filtration unit dysfunction causing significant proteinuria, corresponding to class V LN.Citation12,Citation13
In addition, increasing evidence suggests the tubulointerstitial compartment’s involvement in the pathogenesis of the more severe LN forms, which are associated with the formation of T and B cell aggregates and ectopic GC-like structures containing follicular dendritic cells.Citation15 The resulting inflammation, tubular atrophy, and interstitial inflammation/fibrosis influence the long-term prognosis of the disease.Citation10,Citation12,Citation14
Belimumab (BEL), a fully human monoclonal IgG1λ antibody neutralizing soluble B cell activating factor (BAFF), is the first biological drug approved for the treatment of active SLE despite standard of care (SoC) since 2011.Citation16,Citation17 First, BAFF inhibition effectively delayed SLE onset in experimental murine models.Citation18 Then, four randomized controlled trials—among them the BLISS-52 and -76 trials—demonstrated the effectiveness of BEL over SoC therapy in SLE patients.Citation19–22 Recently, BEL has been approved for the treatment of LN based on a phase III trial (BLISS-LN).Citation23
This product review on BEL aims to summarize the rationale for its design and mechanism of action and discusses its role within the spectrum of currently available and future SLE and LN therapies.
Key issues
Considerable clinical heterogeneity exists among patients with SLE
Lupus nephritis is associated with substantial morbidity and mortality
Belimumab was the first approved biological therapy for SLE
Anifrolumab, an interferon receptor antagonist, has recently been approved for non-renal SLE, and numerous other therapies are under investigation
For LN, standard treatment consists of corticosteroids in combination with either mycophenolate mofetil or cyclophosphamide
Belimumab has recently been approved in the United States and Europe for LN in addition to standard of care
Voclosporin has been FDA-approved for LN in addition to standard of care
Despite negative trials as the sole agent, rituximab is recommended for refractory SLE, and several recent studies have investigated it in combination with BEL
Current treatment options for systemic lupus erythematosus and lupus nephritis
Several therapeutic options are available for the treatment of SLE and LN, which are also supported by current guidelines.Citation24,Citation25
The use of the antimalarial drug hydroxychloroquine (HCQ) is considered standard of care (SoC) in all patients with SLE. HCQ is generally well tolerated but does require regular ophthalmological screening. In addition to this, many patients need glucocorticosteroid (CS) therapy to control SLE disease activity, the dose and route of administration depending on SLE severity and end-organ involvement.Citation24 However, CS contribute to long-term organ damage, and their optimal use is still a matter of debate.Citation26,Citation27 Even though there is little evidence of superior efficacy for methylprednisolone pulse therapy during the induction phase in LN, doses ranging from 250 to 1000 mg per day for three consecutive days are considered SoC and allow for lower starting doses and more rapid tapering of subsequent oral CS.Citation25 For long-term management, reducing the daily CS dose to below 7.5 mg or, ideally, discontinuation is optimal. However, if neither is tolerated, the use of additional immunosuppressive agents such as methotrexate (MTX), azathioprine (AZA), or mycophenolate mofetil (MMF) is recommended, all of which can and should be considered early if the initial presentation of SLE is organ-threatening.Citation24 Intravenous Cyclophosphamide (CYC) can also be used for severe and organ- or life-threatening SLE manifestations, such as LN.Citation25 Its use is currently disfavored for several reasons, such as the need for intravenous administration and potential toxicities, including hemorrhagic cystitis and female infertility. They can be, in part, avoided by prophylaxis with gonadotropin-releasing hormone agonistsCitation28and the use of lower cumulative CYC doses.Citation29 According to the latest European Alliance of Associations for Rheumatology (EULAR) guidelines, biologics should be considered in SLE patients with residual disease activity or frequent disease flares despite SoC.Citation24 Belimumab is the only biologic mentioned in the latest EULAR recommendations. Nevertheless, rituximab (RTX), an anti-CD20 antibody targeting circulating B cells, may also be considered in refractory diseaseCitation25,Citation30despite negative trial results in SLE.Citation31,Citation32 Rituximab is discussed as a therapeutic (off-label) option in the EULAR recommendations, but its use is heterogeneous throughout Europe.Citation33 Recently, RTX has gained interest in combination with BEL. The respective trials will be discussed later in this article. The overall treatment goals for SLE are long-term patient survival and the prevention of organ damage.
As far as LN is concerned, the guidelines differ in some aspects:Citation25 Again, the SoC consists of HCQ and CS in varying doses. The latter is most frequently administered as initial intravenous pulse therapy for remission induction. In class III and IV LN, MMF (2–3 g per day) or low-dose CYC (500 mg every two weeks for six doses) are recommended to complete the remission induction regimen; high-dose CYC (0.5–1.0 g/m2 body surface area) is indicated in those patients at high risk of kidney failure (i.e. rapid-progressive glomerulonephritis [RPGN] at biopsy, reduced estimated glomerular filtration rate [eGFR], or severe inflammation). In class V LN with nephrotic range proteinuria, a combination of MMF with tacrolimus (TAC) can be considered as an alternative.Citation25,Citation34 Finally, the recommendations for maintenance therapy include MMF (especially when used for remission induction) or AZA (particularly when pregnancy is considered in the future), both in combination with low-dose CS if needed.Citation25
For refractory disease, RTX is recommended; as an add-on therapeutic approach, BEL is currently discussed and has recently been approved for the treatment of LN based on one large trial.Citation23,Citation25
Treatment goals in LN are the preservation or improvement of renal function, and a relevant reduction in proteinuria of at least 25% after three months of treatment, 50% after six months, and a urinary protein-to-creatinine ratio [uPCR] <0.7 g/g after one year (complete renal response, [CRR]).Citation25,Citation35 In addition, adjunctive therapy with inhibition of the renin-angiotensin-aldosterone-system (RAAS), statins, vitamin D, and calcium is also important.Citation25
An overview of the respective mechanisms of action of agents used or potentially used for the treatment of LN in the near future is given in .
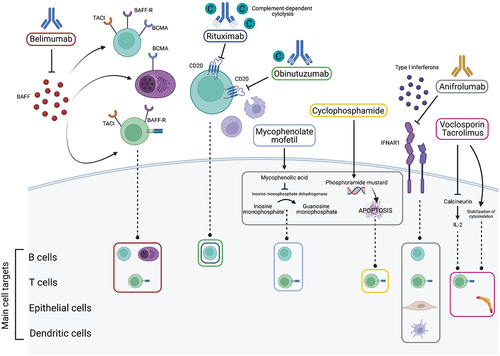
Figure 1. Main mechanisms of action of commonly used and selected promising drugs in Lupus nephritis. The upper part shows the extracellular mechanisms of action of the drugs, and the lower part the intracellular target structures and main cell types involved. Belimumab acts by blocking Bcell activating factor (BAFF) and subsequent inhibition of binding to its receptors (BAFF-R, TACI, BCMA) which are expressed on Band Tcells, thus decreasing antibody production and interfering with Tcell functions. Rituximab is achimeric mouse-human type Iantibody, and obinutuzumab is a humanized type II antibody that act by inhibition of cluster of differentiation (CD) 20 on B cells inducing cell death. They promote complement (C)-dependent cytotoxicity, antibody-dependent cellular toxicity, and antibody-dependent phagocytosis. The first mechanism is prevalent for rituximab, the others for obinutuzumab. Both traditional agents, mycophenolate mofetil and cyclophosphamide, are pro-drugs that are converted intracellularly to their active compounds with subsequent B and T cell apoptosis. Anifrolumab is anovel anti-interferon alpha receptor subunit 1 antibody (IFNAR1), which blocks downstream interferon pathways affecting B, T, epithelial, and dendritic cells. Voclosporin and tacrolimus act similarly as calcineurin inhibitors with subsequent effects on interleukin (IL)-2 inhibiting Tcell proliferation. Another effect is provided by the stabilization of the podocyte cytoskeleton. Created with biorender.com.
Recent developments
Several different and new agents have been investigated as treatment options for SLE and LN in the recent past.
Anifrolumab (ANI), a human monoclonal antibody against the receptor for type I interferons, has been tested as a treatment in active SLE in the TULIP-1Citation36 and -2 trials.Citation37 The mechanism of action of ANI is through blocking the interferon receptor and downstream pathogenic signaling pathways. The effects seem to be more pronounced in individuals with an increased interferon gene signature, which may be helpful as a biomarker of responsiveness.Citation38,Citation39 While the primary endpoint of SLE responder index (SRI)-4 after 52 weeks was not reached in TULIP-1, several secondary endpoints showed promising results and were re-investigated as primary composite endpoints in TULIP-2: Specifically, more patients treated with ANI showed a response after 52 weeks as measured by the BILAG-based composite Lupus assessment (BICLA); in addition, a significant reduction in CS dosage was possible in the ANI group.Citation37 The FDA has subsequently approved ANI to treat adults with moderate-to-severe SLE in 2021.Citation40 while European Medicines Agency (EMA) approval was obtained early in 2022. The issue of different endpoints in TULIP-1 and TULIP-2 is a matter of ongoing discussion, and the position of ANI in current practice has yet to be established. The reader is referred to other reviews for an in-depth discussion of this matter, which is beyond the scope of this review.Citation41
Another new agent is voclosporin (VCS), a novel calcineurin inhibitor (CNI) that has been shown to significantly improve the CRR rates in patients with LN when added to MMF and low-dose CS (AURORA-1 trial).Citation42 It has been approved for adults with active LN by the FDA,Citation43 whereas EMA approval is pending. The mechanism of action is thought to be a more potent inhibition of calcineurin and subsequent inhibition of T cell responses compared to cyclosporine A (CsA) or TAC, along with stabilization of the podocyte cytoskeleton, which is dysregulated in LN.Citation43–45 In addition, VCS has a more stable pharmacologic profile and a better metabolic profile than CsA, making monitoring blood levels unnecessary.Citation46
In the past few months, more evidence from phase II clinical trials in LN has been published: First, the NOBILITY trial, which investigated obinutuzumab, a type II B cell-depleting agent, in combination with MMF, has shown encouraging results on serological parameters, proteinuria, and CRR.Citation47 Second, ANI has been tested in LN patients and has beneficial effects on several renal endpoints, including CRR, with an intensified regimen but failed to reach the pre-specified primary endpoint.Citation48 Phase III clinical trial results for these agents are awaited.
Many more targets, some long-established while others are newly developed, are currently under research and investigation for the treatment of non-renal SLE, as summarized recently.Citation49 Among them are JAK-inhibitors such as tofacitinib or baricitinib; antibodies targeting B cells and plasma cells (e.g., ofatumumab, obexelimab, daratumumab), T cells, plasmacytoid dendritic cells, as well as co-stimulation mechanisms (e.g., belatacept, lulizumab). However, we will not review details on these drugs in this article as clinical trial data are awaited in the near future.
Design
B cell activating factor and rationale for the development of belimumab
Anti-double-stranded DNA (anti-dsDNA) and other anti-nuclear autoantibodies (ANA), produced by autoreactive plasma cells, are the hallmark laboratory finding of SLE.Citation50 Soluble B cell activating factor (BAFF), a member of the tumor necrosis factor superfamily, was first described in 1999 by several independent groups.Citation51–54 BAFF has a pivotal role in promoting B cell tolerance checkpoint defects, most likely occurring at the transitional stage between new bone marrow emigrants and extramedullary mature naïve B cells. Hence, an expansion of transitional B cells may be detected in the peripheral blood of SLE patients, along with increased levels of circulating BAFF.Citation55 Some studies have demonstrated a direct association between BAFF serum concentrations, disease activity, and the occurrence of LN.Citation56–58
The possible pathogenetic role of BAFF in SLE stems from the observation of a lupus-like illness characterized by B cell hyperplasia, anti-dsDNA, and intrarenal ICs, in two independently derived strains of BAFF transgenic mice.Citation59 Together with its primary function as a survival factor for transitional and mature B cells, BAFF increases B cell responses via complex interactions with the B cell receptor (BCR) and Toll-like receptor (TLR) pathways, promoting extrafollicular B cell activation that has a role in the production of class-switched autoantibodies in SLE.Citation60
Furthermore, autoreactive B cells show a downregulation of BCR during “learned ignorance” ruled out by autoantigen-BCR interactions.Citation61 Consequently, in an environment of increased serum BAFF levels, the survival and maturation of these lower affinity self-reactive clones are enhanced, escaping from deletion and anergic processes, as their survival is strictly dependent on BAFF/BAFF-R signaling.Citation62 Thus, BAFF levels play a role in maintaining long-lived humoral immunity, influencing plasma cell survival, and impacting IgM and IgG production.Citation63 Indeed, patients on BEL for longer than seven years possessed fewer autoreactive IgM-expressing B and plasma cells compared to non-BEL users, suggesting that activated autoreactive B cells undergo negative selection.Citation64
The presence of BAFF-R on T cells and BAFF-dependent T cell activation pathways have been demonstrated, even if its functional effect has to be elucidated.Citation65 In general, in SLE pathogenesis, T cells, particularly interleukin (IL)17-producing T helper cells (Th) and T follicular helper (Tfh) cells, play an essential role in helping B cells produce antibodies. Furthermore, they participate in tissue damage by synthesizing multiple soluble local and systemic mediators.Citation66 In addition to the breakdown of the self-tolerance mechanisms described above, intrinsic hyperactivity and hyperresponsiveness of lymphocytes, thus related to B and T cell receptor defects, are found in SLE.Citation67 This process could be sufficient to initiate spontaneous, autoimmune GC responses, resulting in a loss of T cell tolerance and epitope spreading, perpetuating systemic autoimmunity.Citation60
Interestingly, BAFF, which is secreted by hematopoietic cells, has also been shown to be produced locally in the kidney. In one study, BAFF was found in renal tubular epithelial cells and correlated with proliferative forms of LN and disease activity.Citation68 The increased local BAFF levels may thus contribute to a local pro-inflammatory environment in the kidneys of lupus-prone mice and human biopsies and correlate with histopathological activity scores.Citation68 Another group showed elevated BAFF expression in proliferative LN (class III/IV).Citation69 In class IV, BAFF expression was also demonstrated in the glomeruli.Citation69
Based on the mechanisms described above, BEL was developed by the company Human Genome Sciences (HGS) with considerable efforts and brought into phase I clinical trials by GlaxoSmithKline,Citation70 which later acquired HGS and is now the official vendor of BEL. Additional details on the preclinical and development of BEL were comprehensively reviewed by Stohl and Hilbert.Citation16
The product of interest
The fully human monoclonal IgG1λ antibody BEL is available in two formulations: an intravenous route administered every four weeks (the first three doses are given two weeks apart) at a dose of 10 mg/kg of body weight, and as a subcutaneous injection, designed for self-administration, at a dose of 200 mg per week for SLE. A considerable amount of evidence observing its effect has emerged over the past years. A timeline with all relevant clinical trials and studies is shown in .
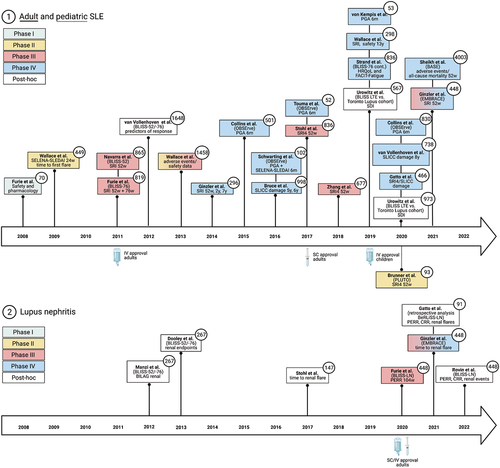
Figure 2. Timeline of milestone belimumab Phase I-IV trials, including post hoc analyses. The respective study phases are color-coded. The boxes show the first author and the name of the trial, if available. In addition, the main primary and secondary outcome measures are reported.
Evidence in non-renal SLE
Clinical trials assessing the efficacy and safety of BEL have been published for more than ten years, consisting of a portfolio of phase II, III, and IV clinical trial data. Early phase I and II trials showed that BEL, in addition to SoC (CS and antimalarial drugs with or without an immunosuppressant), was biologically active and well-tolerated in several different dosing regimens.Citation70,Citation71 Two major phase III trials were published in 2011: BLISS-52 and −76.Citation21,Citation22 The study design compared three treatment groups: SoC + BEL 1 mg/kg; SoC + BEL 10 mg/kg; and SoC + placebo (PBO). 865 and 819 patients were enrolled, respectively, and then analyzed regarding the same endpoint, once after 52 and after 76 weeks. Both analyses used the SLE Responder Index (SRI) as the primary endpoint, showing that numerically more patients receiving BEL + SoC reached at least a 4-point reduction in their SELENA-SLEDAI scores after 52 and 76 weeks, respectively. With the additional six months of follow-up provided by BLISS-76, the authors concluded that BEL also significantly reduced flares and was generally well-tolerated in the longterm. The rates of adverse events were similar when comparing the BEL and PBO groups.Citation21,Citation22 An increasing amount of “real world” evidence showing a clinically relevant benefit with BEL treatment is becoming available from international and long-term observation cohorts (OBSErve studies).Citation72–76
While the BLISS trials showed that many SLE patients benefited from BEL when added to standard treatment, they had excluded important end-organ manifestations of SLE, most notably patients with active LN. Several post hoc analyses of the BLISS trials have been published regarding possible renal endpoints under BEL.Citation19,Citation77 Manzi et al. showed that BEL mainly improved SLE activity in the musculoskeletal and mucocutaneous domains.Citation19 However, those patients without renal involvement at baseline showed a minor worsening in specific organ domains (as measured by the British Isles Lupus Assessment Group [BILAG] Score) under BEL than SoC alone. In addition, those patients with significant proteinuria at enrollment showed a more remarkable improvement with BEL than with PBO.Citation19
Dooley et al. established several renal endpoints for their post hoc analysis of the BLISS trial database, including renal flare rate, renal remission rate, as well as nephritic urine sediment; they were able to show that those 267 patients with some renal involvement at baseline showed a more noticeable improvement with BEL than with SoC alone.Citation77 The same observations were reported for those receiving MMF and those with serologically active disease.
Another study with at least one secondary endpoint concerning the renal domain was published by Stohl et al., although patients with active LN were also excluded.Citation20 Weekly subcutaneous BEL significantly improved SRI4 responses at week 52, and renal improvement favored BEL as well, although the time to the first renal flare among those patients with significant baseline proteinuria was shorter with BEL than with PBO.Citation20
Around the same time, several case reports and case series reported that patients who received BEL as an add-on treatment for SLE also showed significant renal benefits, most commonly reported by a reduction of proteinuria and markers of serological disease activity.Citation78–80 All relevant clinical trials and studies of BEL in SLE are summarized in .
Table 1. Study landscape of belimumab in systemic lupus erythematosus.
Table 2. Trials and studies of belimumab reporting renal endpoints.
Evidence in lupus nephritis
With LN being an important and often mortality-determining organ manifestation of SLE, these post hoc results of secondary endpoints and case series were welcomed. However, a randomized, controlled trial for LN itself was still eagerly awaited. BLISS-LN was then published in 2020: the primary endpoint for 448 patients was the primary efficacy renal response (PERR) at week 104, which was defined as uPCR <0.7 g/g, eGFR no worse than 20% below pre-study values, and no need for rescue therapy.Citation23 Furthermore, significantly more patients achieved PERR with BEL than PBO in BLISS-LN. Those patients treated with BEL showed a lower risk of a renal-related event (defined as doubling of serum creatinine or end-stage renal disease [ESRD]) and death.Citation23
More recently, Ginzler et al. have studied the efficacy and safety of BEL in patients of black African ancestry and included a secondary renal endpoint as well (EMBRACE).Citation93 Unfortunately, the EMBRACE study did not reach its primary endpoint of SRI improvement at week 52; however, there was a numerically lower risk of renal flare and a longer timespan until the first renal flare in the BEL group. In addition, patients with high disease activity or renal involvement at baseline benefited from BEL compared to PBO + SoC.Citation93 This result is particularly telling as LN is more common in SLE patients of African ancestry and progresses to ESRD more frequently, which is strongly influenced by the presence of risk alleles of the apolipoprotein 1 (APOL1) gene.Citation95,Citation96 Studies and trials of BEL reporting renal endpoints are shown in .
De-Novo lupus nephritis with belimumab
As mentioned above, Stohl et al. were among the first to note a shorter time to first renal flare with BEL, although the overall progression of renal involvement was ameliorated with the new treatment.Citation20 Indeed, several case reports and small studies are concerned with new-onset LN or a new LN flare during BEL treatment (). Those with the largest cohorts are Hui-Yuen et al. (3 new cases of LN among 195 subjects)Citation101 and Parodis et al. (3 de-novo LN out of 66 patients without prior LN; and 2 LN flares among 29 patients with known LN).Citation102
Table 3. De Novo lupus nephritis or lupus nephritis flares reported with belimumab.
Table 4. Studies on sequential B cell-depleting therapies in systemic lupus erythematosus.
Most recently, Ginzler et al. discussed that among those patients without renal involvement at baseline, very few developed worsening renal function during BEL treatment (15 of 244 patients) in the EMBRACE trial, and those numbers were similar for the PBO group (9 of 115).Citation93 Importantly, these do not necessarily represent new-onset LN, as no renal biopsies were performed during the trial. In contrast to the above concerns about new-onset LN or flares during BEL treatment, a post hoc analysis of the BLISS-LN study cohort recently demonstrated fewer LN flares and a slower decline in eGFR during BLISS-LN, concluding that BEL might help preserve kidney function in LN.Citation100
Combination and sequential therapies including belimumab
As BEL became established as an add-on treatment for SLE and considering its mechanism of action in the B-cell domain of (auto)immunity, the potential benefits of a combination treatment with RTX were discussed. Rituximab is well-established in treating a plethora of hematological and rheumatological disease entities.
However, the role of RTX in SLE without renal involvement is ambivalent, as the largest trial investigating its effect in SLE (EXPLORER) found no statistically significant benefit neither in primary nor secondary endpoints.Citation32 After the LUNAR study found no statistically significant difference in complete or partial response rates in LN patients but noted a better reduction in anti-dsDNA levels,Citation31 RTX has regained some favor after the publication of RITUXILUP. This prospective cohort study combined RTX with MMF and achieved favorable outcomes and a notable CS-sparing capacity in LN patients.Citation108
Combining RTX as a B-cell depleting agent with BEL as a substance that hinders B-cell activation seemed a good fit. Indeed, multiple case reports highlighted the use of RTX treatment followed by BEL, which led to a reduction in proteinuria and enabled CS reduction.Citation109,Citation110 Several authors also reported a significant improvement with BEL after their patients had become refractory to RTX.Citation111,Citation112 However, a small study focusing explicitly on SLE patients with secondary non-depletion and non-response (2NDNR) to RTX found no benefit from switching to BEL but instead favored a shift to different anti-CD20 agents, such as ocrelizumab, ofatumumab, or obinutuzumab.Citation107
Seeing as a combination of B-cell targeting therapeutics was becoming a valid option, several small proof-of-concept studies were able to show a reduction of NET formationCitation113 as well as anti-dsDNA antibody titers and anti-C1q levels after RTX + BEL,Citation114 the last study comparing results with a group that received RTX monotherapy. Similarly, and most recently, the BEAT-Lupus trialCitation115 demonstrated that add-on BEL after RTX resulted in lower anti-dsDNA levels and fewer SLE flares than RTX (plus SoC) alone.
Regarding the use of sequential B-cell targeting therapy in LN more specifically, the SYNBIoSe-studyCitation113had a secondary renal endpoint and showed that out of 13 patients with LN, five achieved a CRR. Another phase II study investigated treatment with a re-induction regimen of CYC + RTX and added BEL as maintenance therapy in refractory LN patients, which was deemed safe but did not improve outcomes except in those patients with more severe renal involvement, particularly those with nephrotic-range proteinuria.Citation116
So far, the sequence of RTX first/BEL second has been used more frequently. However, several authors asked whether anti-BAFF treatment with BEL first and then finalizing B-cell depletion with RTX might be beneficial. Preliminary results of this combination showed no significant difference in SELENA-SLEDAI scores at week 52 and 104 compared with BEL alone; however, there were more serious adverse events such as severe infections.Citation117 Another study investigating sequential SLE treatment with BEL, then RTX, and then maintenance therapy again with BEL is currently recruiting (SYNBIoSe-2, NCT03747159). In light of novel type II B cell depleting agents in development or early phase clinical trials, we will have a clearer understanding of the use of combination therapies in the context of recently approved drugs soon. The trials investigating RTX/BEL combinations are summarized in detail in .
Safety
As a new drug among a plethora of more established treatment options, the safety of BEL has been monitored from the early phase II and III trials onwards. Wallace et al. reported that serious adverse events (AEs) were not statistically different between the three different dose groups of BEL and the PBO group in the phase II trial.Citation71 Still, urticaria was reported more frequently in the BEL group. There was one case of respiratory failure and one suicide in the BEL group, both of which were deemed unrelated to the study medication by the investigators. The BLISS-52 and -76 trials found statistically similar rates of AEs in the treatment and the PBO groups, too;Citation21,Citation22 the post hoc analysis of the pooled safety data confirmed that BEL was generally well tolerated.Citation82 Again, hypersensitivity with urticaria was rare but more frequent in the BEL group than in the PBO group. A similar safety profile as in the adult population was reported in pediatric SLE patients receiving BEL.Citation90 Dedicated studies of populations with AsianCitation85or Black AfricanCitation93ancestry also showed a similar safety profile compared with previous trials.
In addition, long-term safety data are available. For example, Ginzler et al.,Citation83 van Vollenhoven et al.,Citation91 and Wallace et al.Citation87reported no new AEs or other safety concerns after 7, 8, and 13 years of BEL use.
One concern that has been raised repeatedly by different authors is an increased rate of psychiatric AEs with BEL use: these mainly include insomnia, anxiety, and depression-related AEs.Citation82 Wallace et al. report that there was a doubling of the risk of developing a psychiatric disorder in all treatment groups if there was a previous history of psychiatric illness; however, severe depression was reported more frequently in the BEL than in the PBO groups, and two suicides occurred in the BEL cohort of the studies that were analyzed post hoc.Citation82 The most recent phase IV RCT, the BASE trial,Citation94 showed similar mortality and serious as well as opportunistic infections with BEL vs. PBO; however, also in the BASE trial, there was not only a higher rate of fatal infections with BEL but also of depression, suicidal ideation, and self-harm.Citation94 Although the absolute numbers were low, these results were discussed in detail. There is currently no known etiological link between BEL and an increased risk of suicide; however, a role of BAFF in neural cell survival has been stipulated.Citation120 The authors conclude that patients and clinicians should be aware of a higher incidence of depression and self-harming behavior in SLE patients than in the general population, whether or not they are treated with BEL, due to the high disease burden.
A large meta-analysis of 11 RCTs found no increased risk of psychiatric events with BEL treatment but does recommend caution when starting BEL in a patient with a previous medical history of depression or suicidal ideation.Citation121
Role of belimumab for the treatment of SLE and LN in the current treatment landscape
With the recent approvals of several agents for non-renal SLE and LN, physicians treating SLE patients are faced with the question of when to use these agents, in which patients, and for which organ manifestations. Belimumab has been in clinical use for over ten years now, and experience as well as published data have shown that it is a valuable add-on agent, especially in patients with high clinical disease activity (SLEDAI-2K > 10), serologic activity (high anti-dsDNA antibodies, low complement), and need for continued CS treatment.Citation81 Typically, one would use BEL in patients with active disease or failure to achieve remission despite therapy with CS at tolerable doses (<5 mg/d), antimalarials, and another immunosuppressive agent, such as AZA, MTX (non-renal SLE), or MMF (LN). In patients experiencing intolerable side effects from conventional immunosuppressives, BEL also has excellent efficacy in combination with antimalarials and low-dose CS in the authors’ experience. This latter situation could also be a setting where the recently approved ANI may have a role. Post hoc data from the TULIP trials show that ANI has relevant effects on musculoskeletal and skin disease in SLE.Citation122
In LN, one must distinguish BEL-treated from BEL-naïve patients: The former should continue their usual doses if a decision is made to continue BEL. However, as per the label, the latter should receive BEL at standard IV doses (10 mg/kg of body weight). For the SC route of administration, 400 mg per week (twice the standard dose) for four weeks is recommended for remission induction, and 200 mg per week (the standard dose) afterward.
In the BLISS-LN trial, around 60% of patients had LN classes III or IV, roughly 25% class III/IV in combination with V, and a minority only class V LN.Citation23 As of yet, it is not clear which patients with LN will likely benefit most. However, post hoc data suggest that pure class V LN patients do not benefit as much as class III/IV with or without class V.Citation100 In class V without III or IV LN, combination therapies of MMF + TAC are a reasonable choice for patients not responding to CS + MMF alone.Citation34 MMF + TAC has been studied mostly in Asian populations and trial results may thus not be generalizable to other populations.Citation34 In prominent class V LN, VCS is likely to have a strong role as a new agent.Citation123
The authors’ approach is to start remission induction therapy in LN with methylprednisone for three days (dose between 250–1000 mg per day) and tapering in combination with MMF (1 g/d for the first week, then 2 g/d from the second week onwards, dose slowly increased as tolerated up to 3 g/d). The response is monitored every month for the first three months, then every three months for the first year. If at least a partial renal response has not been achieved after three months, BEL is added to MMF rather than switching to CYC due to toxicity concerns and ease of use of BEL and MMF in an outpatient setting. It is, however, acknowledged that this approach may differ from other physicians’ practice.
In the future, combining agents may be an option. Currently, most evidence exists for BEL/RTX combinations, but this constitutes an off-label use and should be reserved for refractory cases until more trials become available.
Commercial and public-health issues
Currently, BEL is the only approved biological drug for treating both SLE and LN. Nevertheless, SLE experts have been using RTX in refractory cases for years and are convinced of its usefulness despite the lack of evidence in clinical trials. In many places, however, insurance companies deny the reimbursement for RTX treatment in SLE or LN because BEL is approved. Therefore, RTX is likely to become a third-line agent in LN. Furthermore, the fact that BEL is approved both by the FDA and EMA resolves prescribing issues and thus ensures availability for patients who require BEL. However, it remains unclear if patients with diverse ethnic backgrounds and LN benefit equally well from the add-on treatment with BEL.
Soon, it is expected that patient selection will become more of an issue with more available and licensed therapies, such as ANI (for non-renal SLE) and VCS (for LN). Based on the available published data, ANI is likely to be used primarily in patients with dominant musculoskeletal or skin disease. Voclosporin, as a calcineurin inhibitor, will initially likely be administered to patients with nephrotic or sub-nephrotic range proteinuria due to the existing experience with TAC or CsA.
A subcutaneous formulation of BEL is available, which is a significant advantage for many SLE patients, who are typically young and have to accommodate their treatments with their daily lives. Nevertheless, some patients appreciate the advantage of being seen by healthcare professionals every four weeks with the IV administration.
The yearly costs for BEL are significant at about 10,000–15,000 € per year. These must be balanced against indirect costs (e.g., loss of productivity, unemployment, disability) for insurers and society in general.
Conclusions
The field of rheumatology, specifically SLE, has seen major advances in the last few years. Of note, two new drugs (ANI and VCS) have been approved recently for SLE and LN, respectively. In addition, BEL has an established role in SLE and a promising new role in LN. The coming years will be exciting for scientists, patients, and physicians to develop and test new and potent immunotherapeutics. Still, it will also be challenging to find the right place in future therapeutic algorithms and recommendations. It is expected that additional combination therapies will be tested and may allow for a considerable reduction of overall CS doses and their well-known side effects. However, drug development is expensive, and new drugs will be costly for several years. Future trials and clinical experience will tell if the benefit of these therapies justifies their costs. Nevertheless, SLE affects many people worldwide and has not seen any significant advancement regarding approved therapies between the 1950s and 2011. Therefore, the development of new candidate drugs is reason enough to look confidently into the future of the therapeutic landscape in SLE and LN.
Disclosure statement
M.P. reports no conflicts of interest. S.P. has received honoraria or travel support from Abbvie, Astra Zeneca, Bristol-Myers-Squibb, Galapagos, Janssen-Cilag, and Pfizer, all unrelated to this paper. B.T. reports no conflicts of interest. A.H.J.K. participated in consulting, advisory board, or speaker’s bureau for Alexion Pharmaceuticals, AstraZeneca, Aurinia Pharmaceuticals, Exagen Diagnostics, Inc., GlaxoSmithKline, and Pfizer and received funding under a sponsored research agreement unrelated to this review from GlaxoSmithKline and Foghorn Therapeutics. P.K. has received honoraria or travel support from Abbvie, Amgen, Biogen, Boehringer Ingelheim, Bristol-Myers-Squibb, Chugai, Gilead, GlaxoSmithKline, Janssen-Cilag, Lilly, Pfizer, and Sanofi-Aventis, all unrelated to this paper. P.K. received research grants from GlaxoSmithKline and Diamed Medizintechnik GmbH, unrelated to this paper. P.K. also discloses his participation as an investigator in the BLISS-LN trial. GlaxoSmithKline, the manufacturer of belimumab, had no role in the conceptualization, data acquisition, data interpretation, or writing of this paper.
Additional information
Funding
References
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–17. doi:10.1056/NEJMra1100359.
- Olsen NJ, Karp DR. Autoantibodies and SLE—the threshold for disease. Nat Rev Rheumatol. 2014;10(3):181–86. doi:10.1038/nrrheum.2013.184.
- Munoz LE, van Bavel C, Franz S, Berden J, Herrmann M, van der Vlag J. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus. 2008;17(5):371–75. doi:10.1177/0961203308089990.
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–30. doi:10.1016/j.cell.2010.02.014.
- Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46(1):191–201. doi:10.1002/1529-0131(200201)46:1<191:AID-ART10027>3.0.CO;2-K.
- Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–65. doi:10.1038/ni.3175.
- Leonard D, Eloranta M-L, Hagberg N, Berggren O, Tandre K, Alm G, Rönnblom L. Activated T cells enhance interferon-α production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes. Ann Rheum Dis. 2016;75(9):1728–34. doi:10.1136/annrheumdis-2015-208055.
- Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int. 2016;90(3):487–92. doi:10.1016/j.kint.2016.03.042.
- Moroni G, Depetri F, Ponticelli C. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun. 2016;74:27–40. doi:10.1016/j.jaut.2016.06.006.
- Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol. 2016;12(3):143–53. doi:10.1038/nrrheum.2015.159.
- Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G, Hughes G. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2(1):16039. doi:10.1038/nrdp.2016.39.
- Tamirou F, Houssiau FA. Management of lupus nephritis. J Clin Med. 2021;10(4):670. doi:10.3390/jcm10040670.
- Weening JJ, D’-Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JANA, Cook T, Ferrario F, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–30. doi:10.1111/j.1523-1755.2004.00443.x.
- Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D’-Agati VD, Ferrario F, Haas M, Jennette JC, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–96. doi:10.1016/j.kint.2017.11.023.
- Hong S, Healy H, Kassianos AJ. The emerging role of renal tubular epithelial cells in the immunological pathophysiology of lupus nephritis. Front Immunol. 2020;11:578952. doi:10.3389/fimmu.2020.578952.
- Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS–lupus connection. Nat Biotechnol. 2012;30(1):69–77. doi:10.1038/nbt.2076.
- Ruiz-Irastorza G, Bertsias G. Treating systemic lupus erythematosus in the 21st century: new drugs and new perspectives on old drugs. Rheumatology. 2020;59:v69–81. doi:10.1093/rheumatology/keaa403.
- Davidson A. The rationale for BAFF inhibition in systemic lupus erythematosus. Curr Rheumatol Rep. 2012;14(4):295–302. doi:10.1007/s11926-012-0258-2.
- Manzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, Ginzler EM, D’-Cruz DP, Doria A, Cooper S, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71(11):1833–38. doi:10.1136/annrheumdis-2011-200831.
- Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, Kleoudis C, Groark J, Bass D, Fox NL, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two–week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69(5):1016–27. doi:10.1002/art.40049.
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–30. doi:10.1002/art.30613.
- Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EKM, Thomas M, Kim H-Y, León MG, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31. doi:10.1016/S0140-6736(10)61354-2.
- Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, Amoura Z, Yu X, Mok C-C, Santiago MB, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–28. doi:10.1056/NEJMoa2001180.
- Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–45. doi:10.1136/annrheumdis-2019-215089.
- Fanouriakis A, Kostopoulou M, Cheema K, Anders H-J, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, et al. 2019 update of the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–23. doi:10.1136/annrheumdis-2020-216924.
- Ugarte-Gil MF, Mak A, Leong J, Dharmadhikari B, Kow NY, Reátegui-Sokolova C, Elera-Fitzcarrald C, Aranow C, Arnaud L, Askanase AD, et al. Impact of glucocorticoids on the incidence of lupus-related major organ damage: a systematic literature review and meta-regression analysis of longitudinal observational studies. Lupus Sci Med. 2021;8(1):e000590. doi:10.1136/lupus-2021-000590.
- Porta SV, Ugarte-Gil MF, García-de la Torre I, Bonfá E, Gómez-Puerta JA, Arnaud L, Cardiel MH, Alarcón GS, Pons-Estel BA, Pons-Estel G. Controversies in systemic lupus erythematosus: are we treating our patients adequately? J Clin Rheumatol. 2022;28(2):e651–e658. doi:10.1097/RHU.0000000000001803.
- Kado R, McCune WJ. Ovarian protection with gonadotropin-releasing hormone agonists during cyclophosphamide therapy in systemic lupus erythematosus. Best Pract Res Clin Obstet Gynaecol. 2020;64:97–106. doi:10.1016/j.bpobgyn.2019.10.008.
- Houssiau FA, Vasconcelos C, D’-Cruz D, Sebastiani GD, Garrido Ed EDR, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, et al. Immunosuppressive therapy in lupus nephritis: the Euro-lupus nephritis trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–31. doi:10.1002/art.10461.
- González RF, Abida R, Gisca E, Duarte L, Isenberg DA. Can we predict if patients will require more than one cycle of rituximab? Rheumatology (Oxford). 2021:keab527. doi:10.1093/rheumatology/keab527.
- Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–26. doi:10.1002/art.34359.
- Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh H-J, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–33. doi:10.1002/art.27233.
- Rydén-Aulin M, Boumpas D, Bultink I, Callejas Rubio JL, Caminal-Montero L, Castro A, Colodro Ruiz A, Doria A, Dörner T, Gonzalez-Echavarri C, et al. Off-Label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med. 2016;3(1):e000163. doi:10.1136/lupus-2016-000163.
- Kraaij T, Bredewold OW, Trompet S, Huizinga TWJ, Rabelink TJ, de Craen AJM, Teng YKO. TAC-TIC use of tacrolimus-based regimens in lupus nephritis. Lupus Sci Med. 2016;3(1):e000169. doi:10.1136/lupus-2016-000169.
- Tamirou F, Lauwerys BR, Dall’-Era M, Mackay M, Rovin B, Cervera R, Houssiau FA. MAINTAIN nephritis trial investigators. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN nephritis trial. Lupus Sci Med. 2015;2(1):e000123. doi:10.1136/lupus-2015-000123.
- Furie RA, Morand EF, Bruce IN, Manzi S, Kalunian KC, Vital EM, Ford TL, Gupta R, Hiepe F, Santiago M, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:e208–19. doi:10.1016/S2665-9913(19)30076-1.
- Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, Bae S-C, Brohawn PZ, Pineda L, Berglind A, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382(3):211–21. doi:10.1056/NEJMoa1912196.
- Cooles FAH, Isaacs JD. The interferon gene signature as a clinically relevant biomarker in autoimmune rheumatic disease. Lancet Rheumatol. 2022;4(1):e61–72. doi:10.1016/S2665-9913(21)00254-X.
- Vital EM, Merrill JT, Morand EF, Furie RA, Bruce IN, Tanaka Y, Manzi S, Kalunian KC, Kalyani RN, Streicher K, et al. Anifrolumab efficacy and safety by type I interferon gene signature and clinical subgroups in patients with SLE: post hoc analysis of pooled data from two phase III trials. Ann Rheum Dis. 2022: annrheumdis-2021-221425. doi:10.1136/annrheumdis-2021-221425.
- Deeks ED. Anifrolumab: first approval. Drugs. 2021;81(15):1795–802. doi:10.1007/s40265-021-01604-z.
- Tanaka Y, Tummala R. Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Mod Rheumatol. 2021;31(1):1–12. doi:10.1080/14397595.2020.1812201.
- Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, Gibson K, Kaplan J, Lisk L, Navarra S, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397(10289):2070–80. doi:10.1016/S0140-6736(21)00578-X.
- Heo Y-A. Voclosporin: first Approval. Drugs. 2021;81(5):605–10. doi:10.1007/s40265-021-01488-z.
- Rovin BH, Solomons N, Pendergraft WF, Dooley MA, Tumlin J, Romero-Diaz J, Lysenko L, Navarra SV, Huizinga RB, Adzerikho I, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;95(1):219–31. doi:10.1016/j.kint.2018.08.025.
- Bîrsan T, Dambrin C, Freitag DG, Yatscoff RW, Morris RE. The novel calcineurin inhibitor ISA247: a more potent immunosuppressant than cyclosporine in vitro. Transpl Int. 2005;17(12):767–71. doi:10.1111/j.1432-2277.2004.tb00509.x.
- Li Y, Palmisano M, Sun D, Zhou S. Pharmacokinetic disposition difference between cyclosporine and voclosporin drives their distinct efficacy and safety profiles in clinical studies. Clin Pharmacol. 2020;12:83–96. doi:10.2147/CPAA.S255789.
- Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, Fragoso-Loyo H, Zuta-Santillan E, Schindler T, Brunetta P, et al. B-Cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81(1):100–07. doi:10.1136/annrheumdis-2021-220920.
- Jayne D, Rovin B, Mysler EF, Furie RA, Houssiau FA, Trasieva T, Knagenhjelm J, Schwetje E, Chia YL, Tummala R, et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann Rheum Dis. 2022;81(4):496–506. doi:10.1136/annrheumdis-2021-221478.
- Liossis SN, Staveri C. What’s new in the treatment of systemic lupus erythematosus. Front Med (Lausanne). 2021;8:655100. doi:10.3389/fmed.2021.655100.
- Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey‐goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71(9):1400–12. doi:10.1002/art.40930.
- Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–56. doi:10.1084/jem.189.11.1747.
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–63. doi:10.1126/science.285.5425.260.
- Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999;65(5):680–83. doi:10.1002/jlb.65.5.680.
- Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. Identification and characterization of a novel cytokine, THANK, aTNF homologue that activates apoptosis, nuclear factor-κB, and c-Jun NH2-terminal kinase. J Biol Chem. 1999;274(23):15978–81. doi:10.1074/jbc.274.23.15978.
- Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-Cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237(1):205–25. doi:10.1111/j.1600-065X.2010.00944.x.
- Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus. 2013;22(9):873–84. doi:10.1177/0961203313496302.
- Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, Recta V, Zhong J, Freimuth W. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58(8):2453–59. doi:10.1002/art.23678.
- Friebus-Kardash J, Branco L, Ribi C, Chizzolini C, Huynh-Do U, Dubler D, Roux-Lombard P, Dolff S, Kribben A, Eisenberger U, et al. Immune complexes containing serum B-cell activating factor and immunoglobulin G correlate with disease activity in systemic lupus erythematosus. Nephrol Dial Transplant. 2018;33(1):54–64. doi:10.1093/ndt/gfx220.
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–710. doi:10.1084/jem.190.11.1697.
- Rawlings DJ, Metzler G, Wray-Dutra M, Jackson SW. Altered B cell signalling in autoimmunity. Nat Rev Immunol. 2017;17(7):421–36. doi:10.1038/nri.2017.24.
- Heltemes-Harris L, Liu X, Manser T. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J Immunol. 2004;172(2):823–33. doi:10.4049/jimmunol.172.2.823.
- Jackson SW, Davidson A. BAFF inhibition in SLE—Is tolerance restored? Immunol Rev. 2019;292(1):102–19. doi:10.1111/imr.12810.
- Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam K-P, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180(6):3655–59. doi:10.4049/jimmunol.180.6.3655.
- Huang W, Quach TD, Dascalu C, Liu Z, Leung T, Byrne-Steele M, Pan W, Yang Q, Han J, Lesser M, et al. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. JCI Insight. 2018;3(17). doi:10.1172/jci.insight.122525.
- Ng LG, Sutherland APR, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173(2):807–17. doi:10.4049/jimmunol.173.2.807.
- Rother N, van der Vlag J. Disturbed T cell signaling and altered Th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front Immunol. 2015;6:610. doi:10.3389/fimmu.2015.00610.
- Peng SL. Altered T and B lymphocyte signaling pathways in lupus. Autoimmun Rev. 2009;8(3):179–83. doi:10.1016/j.autrev.2008.07.040.
- Schwarting A, Relle M, Meineck M, Föhr B, Triantafyllias K, Weinmann A, Roth W, Weinmann-Menke J. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus. 2018;27(2):243–56. doi:10.1177/0961203317717083.
- Suso JP, Posso-Osorio I, Jiménez CA, Naranjo-Escobar J, Ospina FE, Sánchez A, Cañas CA, Tobón GJ. Profile of BAFF and its receptors’ expression in lupus nephritis is associated with pathological classes. Lupus. 2018;27(5):708–15. doi:10.1177/0961203317739132.
- Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, Merrill JT, Weinstein A, McCune WJ, Zhong J, et al. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10(5):R109. doi:10.1186/ar2506.
- Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61(9):1168–78. doi:10.1002/art.24699.
- Collins CE, Dall’-Era M, Kan H, Macahilig C, Molta C, Koscielny V, Chang DJ. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3(1):e000118. doi:10.1136/lupus-2015-000118.
- Collins CE, Cortes-Hernández J, Garcia MA, von Kempis J, Schwarting A, Touma Z, Kurtinecz M, Gairy K. Real-world effectiveness of belimumab in the treatment of systemic lupus erythematosus: pooled analysis of multi-country data from the OBSErve studies. Rheumatol Ther. 2020;7(4):949–65. doi:10.1007/s40744-020-00243-2.
- Schwarting A, Schroeder JO, Alexander T, Schmalzing M, Fiehn C, Specker C, Perna A, Cholmakow-Bodechtel C, Koscielny VB, Carnarius H. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther. 2016;3(2):271–90. doi:10.1007/s40744-016-0047-x.
- von Kempis J, Duetsch S, Reuschling N, Villiger R, Villiger PM, Vallelian F, Schaer DJ, Mueller RB. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Medical Weekly [Internet]. 2019 [accessed 2022 Mar 22]. https://smw.ch/article/doi/smw.2019.20022.
- Touma Z, Sayani A, Pineau CA, Fortin I, Matsos M, Ecker GA, Chow A, Iczkovitz S. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada study. Rheumatol Int. 2017;37(6):865–73. doi:10.1007/s00296-017-3682-9.
- Dooley MA, Houssiau F, Aranow C, D’-Cruz DP, Askanase A, Roth DA, Zhong ZJ, Cooper S, Freimuth WW, Ginzler EM, et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus. 2013;22(1):63–72. doi:10.1177/0961203312465781.
- Fließer EE, Korsten P, Koziolek MJ, Niewold TB, Patschan D, Müller GA, Patschan SA. Successful treatment of a mycophenolate mofetil-refractory proliferative lupus nephritis with belimumab in a 19-year-old woman. Lupus. 2013;22(14):1523–25. doi:10.1177/0961203313504145.
- Plüß M, Tampe B, Niebusch N, Zeisberg M, Müller GA, Korsten P. Clinical efficacy of routinely administered belimumab on proteinuria and neuropsychiatric lupus. Front Med (Lausanne). 2020;7:222. doi:10.3389/fmed.2020.00222.
- Margiotta DPE, Basta F, Batani V, Afeltra A. Belimumab and low-doses of mycophenolate mofetil as induction therapy of class IV lupus nephritis: case series and literature review. BMC Nephrol. 2018;19(1):54. doi:10.1186/s12882-018-0847-z.
- van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, Zhong ZJ, Freimuth W. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. 2012;71(8):1343–49. doi:10.1136/annrheumdis-2011-200937.
- Wallace D, Navarra S, Petri M, Gallacher A, Thomas M, Furie R, Levy R, van Vollenhoven R, Cooper S, Zhong Z, et al. Safety profile of belimumab: pooled data from placebo-controlled phase 2 and 3 studies in patients with systemic lupus erythematosus. Lupus. 2013;22(2):144–54. doi:10.1177/0961203312469259.
- Ginzler EM, Wallace DJ, Merrill JT, Furie RA, Stohl W, Chatham WW, Weinstein A, McKay JD, McCune WJ, Zhong ZJ, et al. Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. J Rheumatol. 2014;41(2):300–09. doi:10.3899/jrheum.121368.
- Bruce IN, Urowitz M, van Vollenhoven R, Aranow C, Fettiplace J, Oldham M, Wilson B, Molta C, Roth D, Gordon D. Long-Term organ damage accrual and safety in patients with SLE treated with belimumab plus standard of care. Lupus. 2016;25(7):699–709. doi:10.1177/0961203315625119.
- Zhang F, Bae S-C, Bass D, Chu M, Egginton S, Gordon D, Roth DA, Zheng J, Tanaka Y. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77(3):355–63. doi:10.1136/annrheumdis-2017-211631.
- Strand V, Berry P, Lin X, Asukai Y, Punwaney R, Ramachandran S. Long‐term impact of belimumab on health‐related quality of life and fatigue in patients with systemic lupus erythematosus: six years of treatment. Arthritis Care Res. 2019;71(6):829–38. doi:10.1002/acr.23788.
- Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, Weinstein A, McKay JD, McCune WJ, Petri M, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(7):1125–34. doi:10.1002/art.40861.
- Urowitz MB, Ohsfeldt RL, Wielage RC, Kelton KA, Asukai Y, Ramachandran S. Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis. Ann Rheum Dis. 2019;78(3):372–79. doi:10.1136/annrheumdis-2018-214043.
- Gatto M, Saccon F, Zen M, Regola F, Fredi M, Andreoli L, Tincani A, Urban ML, Emmi G, Ceccarelli F, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real‐life setting. Arthritis Rheumatol. 2020;72(8):1314–24. doi:10.1002/art.41253.
- Brunner HI, Abud-Mendoza C, Viola DO, Calvo Penades I, Levy D, Anton J, Calderon JE, Chasnyk VG, Ferrandiz MA, Keltsev V, et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis. 2020;79(10):1340–48. doi:10.1136/annrheumdis-2020-217101.
- van Vollenhoven RF, Navarra SV, Levy RA, Thomas M, Heath A, Lustine T, Adamkovic A, Fettiplace J, Wang M-L, Ji B, et al. Long-Term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension. Rheumatology. 2020;59(2):281–91. doi:10.1093/rheumatology/kez279.
- Urowitz MB, Ohsfeldt RL, Wielage RC, Dever JJ, Zakerifar M, Asukai Y, Ramachandran S, Joshi AV. Comparative analysis of long-term organ damage in patients with systemic lupus erythematosus using belimumab versus standard therapy: a post hoc longitudinal study. Lupus Sci Med. 2020;7(1):e000412. doi:10.1136/lupus-2020-000412.
- Ginzler E, Guedes Barbosa LS, D’-Cruz D, Furie R, Maksimowicz-McKinnon K, Oates J, Santiago MB, Saxena A, Sheikh S, Bass DL, et al. Phase III/IV, randomized, fifty-two-week study of the efficacy and safety of belimumab in patients of black African ancestry with systemic lupus erythematosus. Arthritis Rheumatol. 2022;74:112–23.
- Sheikh SZ, Scheinberg MA, Wei J-C, Tegzova D, Stohl W, de Toledo RA, Mucenic T, Banfi MRA, Maksimowicz-McKinnon K, Abud-Mendoza C, et al. Mortality and adverse events of special interest with intravenous belimumab for adults with active, autoantibody-positive systemic lupus erythematosus (BASE): a multicentre, double-blind, randomised, placebo-controlled, phase 4 trial. Lancet Rheumatol. 2021;3(2):e122–30. doi:10.1016/S2665-9913(20)30355-6.
- Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014;66(2):390–96. doi:10.1002/art.38220.
- Lea JP. Lupus nephritis in African Americans. Am J Med Sci. 2002;323(2):85–89. doi:10.1097/00000441-200202000-00005.
- De Scheerder M-A, Boey O, Mahieu E, Vanuytsel J, Bogaert A-M. Case report: successful treatment of membranous lupus nephritis with belimumab in an African female immigrant. Clin Rheumatol. 2016;35(6):1649–53. doi:10.1007/s10067-015-3153-1.
- Fontana F, Alfano G, Leonelli M, Cerami C, Ligabue G, Spinella A, Citriniti G, Manzini CU, Ferri C, Cappelli G. Efficacy of Belimumab for active lupus nephritis in a young Hispanic woman intolerant to standard treatment: a case report. BMC Nephrol. 2018;19(1):276. doi:10.1186/s12882-018-1066-3.
- Binda V, Trezzi B, Del Papa N, Beretta L, Frontini G, Porata G, Fabbrini P, Pozzi MR, Messa P, Sinico RA, et al. Belimumab may decrease flare rate and allow glucocorticoid withdrawal in lupus nephritis (including dialysis and transplanted patient). J Nephrol. 2020;33(5):1019–25. doi:10.1007/s40620-020-00706-3.
- Rovin BH, Furie R, Teng YKO, Contreras G, Malvar A, Yu X, Ji B, Green Y, Gonzalez-Rivera T, Bass D, et al. A secondary analysis of the belimumab international study in lupus nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2022;101(2):403–13. doi:10.1016/j.kint.2021.08.027.
- Hui-Yuen JS, Reddy A, Taylor J, Li X, Eichenfield AH, Bermudez LM, Starr AJ, Imundo LF, Buyon J, Furie RA, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol. 2015;42(12):2288–95. doi:10.3899/jrheum.150470.
- Parodis I, Vital EM, Hassan S-U, Jönsen A, Bengtsson AA, Eriksson P, Leonard D, Gunnarsson I, Rönnblom L, Sjöwall C. De Novo lupus nephritis during treatment with belimumab. Rheumatology (Oxford). 2021;60(9):4348–54. doi:10.1093/rheumatology/keaa796.
- Sjöwall C, Cöster L. Belimumab may not prevent lupus nephritis in serologically active patients with ongoing non-renal disease activity. Scand J Rheumatol. 2014;43(5):428–30. doi:10.3109/03009742.2014.887769.
- Staveri C, Karokis D, Liossis S-N. New onset of lupus nephritis in two patients with SLE shortly after initiation of treatment with belimumab. Semin Arthritis Rheum. 2017;46(6):788–90. doi:10.1016/j.semarthrit.2016.09.006.
- Anjo C, Mascaró J-M, Espinosa G, Cervera R. Effectiveness and safety of belimumab in patients with systemic lupus erythematosus in a real-world setting. Scand J Rheumatol. 2019;48(6):469–73. doi:10.1080/03009742.2019.1603324.
- Riancho-Zarrabeitia L, Villa Blanco I, Santos-Gómez M, Aurrecoechea E. Belimumab in systemic lupus erythematosus: experience in clinical practice settings in a regional hospital. Reumatol Clin (Engl Ed). 2020;16:188–89. doi:10.1016/j.reuma.2018.02.004.
- Hassan SU, Md Yusof MY, Emery P, Dass S, Vital EM. Biologic sequencing in systemic lupus erythematosus: after secondary non-response to rituximab, switching to humanised anti-CD20 agent is more effective than belimumab. Front Med (Lausanne). 2020;7:498. doi:10.3389/fmed.2020.00498.
- Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, Cairns TD, Lightstone L. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280–86. doi:10.1136/annrheumdis-2012-202844.
- Kraaij T, Huizinga TWJ, Rabelink TJ, Teng YKO. Belimumab after rituximab as maintenance therapy in lupus nephritis. Rheumatology (Oxford). 2014;53(11):2122–24. doi:10.1093/rheumatology/keu369.
- Gualtierotti R, Borghi MO, Gerosa M, Schioppo T, Larghi P, Geginat J, Meroni PL. Successful sequential therapy with rituximab and belimumab in patients with active systemic lupus erythematosus: a case series. Clin Exp Rheumatol. 2018;36:643–47.
- Gonzalez-Echavarri C, Ugarte A, Ruiz-Irastorza G. Rituximab-Refractory lupus nephritis successfully treated with belimumab. Clin Exp Rheumatol. 2016;34:355–56.
- Simonetta F, Allali D, Roux-Lombard P, Chizzolini C. Successful treatment of refractory lupus nephritis by the sequential use of rituximab and belimumab. Joint Bone Spine. 2017;84(2):235–36. doi:10.1016/j.jbspin.2016.01.008.
- Kraaij T, Kamerling SWA, de Rooij ENM, van Daele PLA, Bredewold OW, Bakker JA, Bajema IM, Scherer HU, Toes REM, Huizinga TJW, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. 2018;91:45–54. doi:10.1016/j.jaut.2018.03.003.
- van Dam LS, Osmani Z, Kamerling SWA, Kraaij T, Bakker JA, Scherer HU, Rabelink TJ, Voll RE, Alexander T, Isenberg DA, et al. A reverse translational study on the effect of rituximab, rituximab plus belimumab, or bortezomib on the humoral autoimmune response in SLE. Rheumatology (Oxford). 2020;59(10):2734–45. doi:10.1093/rheumatology/kez623.
- Shipa M, Embleton-Thirsk A, Parvaz M, Santos LR, Muller P, Chowdhury K, Isenberg DA, Doré CJ, Gordon C, Ehrenstein MR, et al. Effectiveness of belimumab after rituximab in systemic lupus erythematosus. Annals of Internal Medicine [Internet]. 2021 [accessed 2021 Nov 30]. https://www.acpjournals.org/doi/abs/10.7326/M21-2078.
- Atisha-Fregoso Y, Malkiel S, Harris KM, Byron M, Ding L, Kanaparthi S, Barry WT, Gao W, Ryker K, Tosta P, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. 2021;73(1):121–31. doi:10.1002/art.41466.
- Aranow C, Allart C, Amoura Z, Bruce IN, Cagnoli P, Furie R, Tak PP, Urowitz M, van Vollenhoven R, Clark KL, et al. Efficacy and safety of Subcutaneous Belimumab (BEL) and Rituximab (RTX) sequential therapy in patients with systemic lupus erythematosus: the Phase 3, randomized, placebo-controlled BLISS-BELIEVE study [Internet]. ACR Meeting Abstracts; 2021 [accessed 2021 Dec 30]. https://acrabstracts.org/abstract/efficacy-and-safety-of-subcutaneous-belimumab-bel-and-rituximab-rtx-sequential-therapy-in-patients-with-systemic-lupus-erythematosus-the-phase-3-randomized-placebo-controlled-bliss-believe-stud/.
- Petricca L, Gigante MR, Paglionico A, Costanzi S, Vischini G, Di Mario C, Varriano V, Tanti G, Tolusso B, Alivernini S, et al. Rituximab followed by belimumab controls severe lupus nephritis and bullous pemphigoid in systemic lupus erythematosus refractory to several combination therapies. Front Med (Lausanne). 2020;7:553075. doi:10.3389/fmed.2020.553075.
- Kraaij T, Arends EJ, van Dam LS, Kamerling SWA, van Daele PLA, Bredewold OW, Ray A, Bakker JA, Scherer HU, Huizinga TJW, et al. Long-Term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant. 2021;36(8):1474–83. doi:10.1093/ndt/gfaa117.
- Tada S, Yasui T, Nakatsuji Y, Okuno T, Koda T, Mochizuki H, Sakoda S, Kikutani H. BAFF controls neural cell survival through BAFF receptor. Plos One. 2013;8:e70924. doi:10.1371/journal.pone.0070924.
- Xie W, Huang H, Zhan S, Zhang Z. Risk of psychiatric disorders and all-cause mortality with belimumab therapy in patients with systemic lupus erythematosus: a meta-analysis of randomised controlled trials. Lupus Sci Med. 2021;8(1):e000534. doi:10.1136/lupus-2021-000534.
- Morand EF, Furie RA, Bruce IN, Vital EM, Dall’-Era M, Maho E, Pineda L, Tummala R. Efficacy of anifrolumab across organ domains in patients with moderate-to-severe systemic lupus erythematosus: a post-hoc analysis of pooled data from the TULIP-1 and TULIP-2 trials. Lancet Rheumatol. 2022;4(4):e282–92. doi:10.1016/S2665-9913(21)00317-9.
- Mejia-Vilet JM, Malvar A, Arazi A, Rovin BH. The lupus nephritis management renaissance. Kidney Int. 2022;101(2):242–55. doi:10.1016/j.kint.2021.09.012.
