ABSTRACT
In May 2021, while the immunization campaign was in progress, the emergence of new SARS-CoV-2 variants led us to assess attitudes toward participation in a COVID-19 vaccine clinical trial (VCT) in France. Between the 10th and the 23rd of May 2021, we conducted a cross-sectional online survey among a representative sample of the French population aged 18 and over and a specific sample of the French population over 65. Among the 3,056 respondents, 28.0% (856) would consider participation in a COVID-19 VCT. Factors independently negatively associated with willingness to participate in a COVID-19 VCT were female gender with an adjusted odd ratio (aOR) 0.42 and 95% confidence interval (95% CI) 0.35–0.51, and mistrust in health actors (in their own physician and pharmacists, health ministry, government, scientists in medias, medias and pharmaceutical companies) with aOR 0.86 (95% CI 0.84–0.88) by one-point increase in mistrust in health actors score. Factors positively associated with willingness to participate in a COVID-19 VCT were COVID-19 vaccination or intention to get vaccinated with aOR 4.89 (95% CI 3.15–7.61), being a healthcare worker with aOR 2.051 (95% CI 1.51–2.80), being at risk for severe COVID-19 with aOR 1.39 (95% CI 1.14–1.69) and altruism as the main reason for getting vaccination with aOR 1.56 (95% CI 1.29–1.88). In May 2021, despite COVID 19 vaccine availability, 28% of the French population would agree to participate in a COVID-19 VCT. Mistrust in health actors contributes to a reduction in the intention to participate. Attitudes toward COVID-19 vaccination predict attitudes toward participation in a COVID-19 VCT.
Effective vaccines against COVID-19 were made available only one year after the first reported cases of COVID-19 due to unprecedented efforts in vaccine development.Citation1 Consequently, a large number of vaccines simultaneously reached clinical development phases requiring the rapid recruitment of a large number of volunteers in vaccine clinical trials (VCTs). In April 2020, 48% of the respondents to a French survey were likely to agree to participate in a COVID-19 VCT if it was proposed.Citation2 Between October and December 2020, 50,000 participants joined the French national platform for COVID-19 vaccine trials COVIREIVAC.Citation3 Eagerness to be protected against COVID-19 may in part explain this enthusiasm. The emergence of variants of concern associated with immune escape, the waning effectiveness of available vaccines to prevent infection and transmission mean we must continue to evaluate 1st generation COVID-19 vaccines, evaluate upcoming COVID-19 vaccines and identify new effective vaccine strategies.Citation4 Moreover, additional data in specific populations such as immunocompromised patients, pregnant women and children, for example, were needed. In May 2021, 27.8% of the French Population had received at least one dose of COVID-19 vaccine, this proportion ranged from 0.2% in the 12–17 years age group and 83.2% in the 70–74 years age group.Citation5 Consequently, it is uncertain that willingness to participate in a COVID-19 VCT remained as high as previously observed before COVID-19 vaccines roll-out.
Recruitment in VCTs faces additional challenges compared to drug clinical trials. Likelihood of participating in a VCT was lower compared to a trial for a new medication or a medical device among diagnosed patients.Citation6 Vaccine hesitancy probably has an impact on willingness to participate in VCTs in general.Citation7 The COVID-19 vaccination campaign took place in a context of infodemic with the dissemination of fake news casting doubts on the vaccines’ safety. In parallel, trust in scientists may have been eroded during the COVID-19 pandemic.Citation8,Citation9 Consequently, the profile of potential participants in COVID-19 VCTs may have changed since the first pandemic wave and the vaccines roll-out.
Our aim was to evaluate willingness to participate in a COVID-19 VCT in May 2021 in France, recognized as a “par excellence vaccine-hesitant country”,Citation10 while COVID-19 vaccines had been available for more than 4 months. To our knowledge, attitudes toward participation in COVID-19 VCTs has not been evaluated since the authorization of the current vaccines in France.
Methods
Design and sample
Between the 10th and the 23rd of May 2021, we conducted a cross-sectional online survey among the French population aged 18 and over, with participants who were randomly selected from an online research panel of more than 750,000 individuals, developed and maintained by Bilendi (a company specialized in data collection, established in 12 European countries, bilendi.fr), 55,900 participants were randomly selected. They were invited to complete an online questionnaire accessible through a secure link. As we used the quota method to match French official census statistics, these people were solicited in successive batches according to the completion rate of strata defined according to gender, age, occupational category, region and size of the municipality of residence. Participation in the survey was voluntary; people received incentives in the form of points toward a 20-euro voucher. We then weighted the data to obtain a sample representative for these variables. The ethics committee of the University Hospital Institute Méditerranée Infection approved the study (#2021–001).
Data collected
In addition to background socio-economic variables (gender, age, profession), we collected 1) intention or history of COVID-19 vaccination, 2) concerns about COVID-19, 3) risk factors for severe COVID-19, 4) fear of getting infected by SARS-CoV-2, 5) mistrust in their physician, pharmacist, Health Ministry, French government, pharmaceutical companies, traditional media, and physicians interviewed in traditional medias (TV, radio, newspapers), scientists and healthcare professionals, 6) opinion toward vaccines in general “Is your opinion toward vaccines in general favorable?”, and 7) attitudes toward participation to a COVID-19 vaccine clinical trial “If you were offered to participate in a COVID-19 vaccine clinical trials, would you accept to participate?”.
Statistical analysis
Willingness to participate in a COVID-19 VCT was merged into a binary outcome: ‘yes’ if participants answered “yes certainly”, and “Yes probably”, ‘No’, if participants answered “Certainly Not”, “Probably Not” and “Don’t know”. We first used bivariate analyses to investigate factors associated with willingness to participate in a COVID-19 VCT, using respondents’ socio-economic background, COVID-19 concern, COVID-19 vaccination status, mistrust in Health actors – in their own physician and pharmacists, health ministry, government, scientists in medias, medias and pharmaceutical companies- (computing a mistrust in health actors score ranging from 7 to 28 Cronbach’s index 0.87) as variables of interest. We performed a multiple logistic regression with variables associated with willingness to participate in a COVID-19 VCT if the p-value was lower than 0.2 in bivariate analysis.
Results
A total of 3,056 individuals answered the questionnaire. Overall, 47.6% (1,455) of respondents were male. In terms of vaccination, 37.8% (1,156) were willing to get vaccinated and 39.0% (1191) were already vaccinated. Twenty-eight percent (856) were willing to participate in a COVID-19 VCT if offered the opportunity. Characteristics of the respondents and factors associated with willingness to participate in a COVID-19 VCT in bivariate analysis are depicted in . The proportion of respondents willing to participate in a COVID-19 VCT ranged from 24.5% in the 35–49 age group to 33.5% in the over 65 age group (p < 0.005). Women were less willing than men to accept enrollment in a COVID-19 VCT (19.6% vs 37.3%, p < 0.005). The proportion of respondents willing to participate in a COVID-19 VCT was 14.5%, and 40.4% in the group with a score lower than 16-median mistrust in health actors score- (p < 0.005). Among the 290 respondents who were healthcare workers (HCWs), 34.1% (99) would be willing to participate in a COVID-19 VCT if they were asked.
Table 1. Factors associated with willingness to participate in a COVID-19 VCT if offered in univariate analysis (SD standard deviation, percentages are expressed in lines).
In multivariable analysis (), factors positively associated with willingness to participate in a COVID-19 VCT were intention to get COVID-19 vaccine or COVID-19 vaccination and being a HCW with respective adjusted Odds Ratio (aOR) 4.77 (95% Confidence interval 3.07–7.41), 2.05 (95% CI 1.5–2.78). Factors negatively associated with willingness to participate in a COVID-19 VCT were female gender (aOR 0.45 and 95% CI 0.37–0.54), mistrust in health actors score (aOR 0.86 and 95% CI0.84–0.88) per point-increase in Mistrust in heath actors score.
Table 2. Factors associated with willingness to participate in a COVID-19 VCT if offered in multivariate analysis (Ref reference, aOR :adjusted odds ratio CI: confidence interval), the regression model included all variables with a p value <0.2 in the univariate analysis.
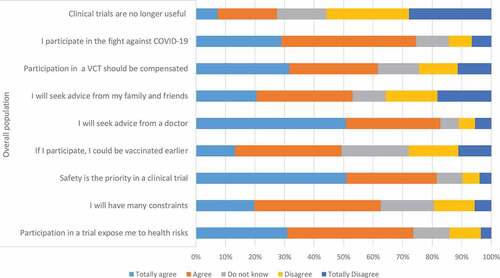
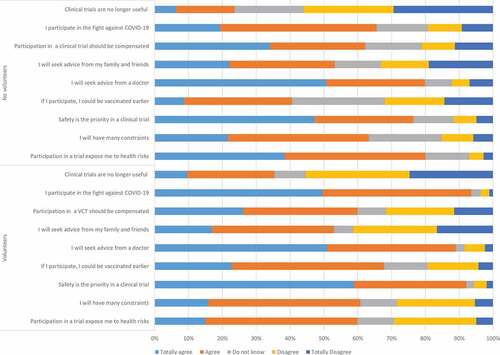
Concerns about potential vaccine candidate risks is probably one of the main barriers to participation in a COVID-19 VCT (). Sixty percent of the responders willing to participate in a COVID-19 VCT (514) and 79.9% (1758) of the responders unwilling to participate in a COVID-19 VCT considered that participation would expose them to health risks (p < 0.005). In the same vein, 92.1% (788) of the responders willing to participate knew that participants’ safety is a priority in a clinical trial and this proportion was only 76.5% (1683) in responders without willingness to participate. Sixty-two percent of the respondents (1,895) declared that participating in a COVID-19 VCT would add constraints, without significant difference between volunteers willing to participate and those unwilling to participate, 61.5% (1,879) respondents considered that participation in a VCT should be compensated. 78.4% (2,396) respondents would seek advice from their physicians if they were asked to participate in a COVID-19 VCT, while 53.6% (1,635) would seek advice from family and relatives. Having the opportunity to get vaccinated earlier in the VCT than in the regular vaccine roll-out was one of the motivations reported by 67.7% of the responders with willingness to participate in a VCT. Only 26.9% (822) respondents considered that conducting a COVID-19 VCT was useless while different vaccines were available. Participation in a COVID-19 VCT was considered as a contribution to the fight against COVID-19 by 70.9% (2,167) respondents, this proportion reached 93.4% in respondents with willingness to participate in a COVID-19 VCT ( and ).
Discussion
Public interest in participation in a COVID-19 vaccine clinical trials
In May 2021, about 4 months after the beginning of the COVID-19 vaccine campaign in France, 28% of the respondents would have considered participation in a COVID-19 VCT if they were asked. This proportion is close to the percentage previously observed in Michigan and in the United Kingdom before the COVID-19 vaccine roll-out. Intentions to participate ranged from 25% in Michigan to 41.4% in the UK.Citation11,Citation12 Willingness to participate in a clinical trial has eroded while several effective vaccines were available. This proportion was 48% in April 2020 in France.Citation2 However, it is noticeable that willingness to participate in a COVID-19 VCT did not disappear, even as COVID-19 vaccines became widely available in France. More rapid access to a vaccine, access to new generations of vaccines, potentially more effective, may explain public interest for VCTs.
Factors associated with willingness to participate in a COVID-19 VCT
We identified different factors associated with the willingness to participate in a COVID-19 VCT in the French population. The strong association between intention to get vaccinated against COVID-19 or previous vaccination and willingness to participate in a VCT may in part reflect vaccine confidence. Vaccine hesitancy was previously observed as a barrier to participation in a COVID-19 VCT.Citation2 In addition, mistrust in health actors is associated with rejection of COVID-19 VCT participation. This observation was also made in the USA.Citation11 Mistrust in doctors, scientists and politicians may contribute to negative attitudes toward clinical trials in France.Citation13 This point could become crucial as trust in scientists seems to have been eroding since the beginning of the pandemic, particularly in France.Citation8,Citation9
Women are less likely to participate in a COVID-19 VCT than men. This observation is consistent with previous observations.Citation2,Citation11,Citation12 A greater prevalence of COVID-19 vaccine hesitancy and of distrust in pharmaceutical companies in women may counterbalance the greater altruism of women.Citation2,Citation14,Citation15 Concerns about fertility or pregnancy during the clinical trials are not the only hypothesis to explain this association, as this association between gender and willingness to participate remained highly significant after adjustment on age.
In contrast with previous observations for other vaccine candidates,Citation7 older age is not a barrier to participation in a COVID-19 VCT.Citation2,Citation11,Citation12 For non COVID-19 VCTs, the refusal rate to participate in a clinical trial is 2- to 3-fold greater in older people than in younger populations.Citation16 Older age was rapidly observed as a major risk factor for severe COVID-19Citation17 and was associated with a decrease in immunogenicity of vaccines.Citation18 In contrast, young adults were probably more complacent, and may have perceived a low personal benefit in participating in a COVID-19 VCT. In addition, individuals with risk factors for severe COVID-19 were significantly more prone to accept enrollment in a COVID-19 VCT. Both observations suggest that recruitment of at-risk individuals in a VCT might be facilitated in the context of a pandemic as the COVID-19 pandemic.Citation19
Intention to participate in a COVID-19 VCT was greater in HCWs than in the general population. HCWs have been at the forefront since the beginning of the pandemic.Citation20 Greater willingness to participate in a COVID-19 VCT in HCWs has already been observed.Citation2,Citation21 In Uganda in Autumn 2020, hope of being protected was one of the key motivators in HCWs.Citation21 Vaccine eagerness in healthcare workers may in part explain this observation. However, the present study was carried out while COVID-19 vaccines were widely available for healthcare workers in France. Perceptions of clinical research and or of COVID-19 may differ between healthcare workers and the general population, particularly in the COVID-19 context, where they were at the front-line. We can hypothesize that HCWs had greater knowledge about clinical trials than the general population.
Motivations and barriers to participate in a COVID-19 VCT
In addition to potential personal benefit, altruism is a great motivator for participation in a VCT in general.Citation7 In our study, a large number of potential volunteers considered their potential participation as their contribution to the fight against the pandemic. In addition, we observed that individuals for whom protecting others was their first motivation to get vaccinated against COVID-19, were more prone to participate in a COVID-19 VCT. Altruism was also described as a key motivator in HCWs.Citation21
Proposal of participation in a COVID-19 VCT may be perceived as offering vaccination in a secure environment. However, this point is not a great motivator in our study. Although 92% of the responders with willingness to participate in a COVID-19 VCT reported that participants’ safety is a priority in a clinical trial, a large majority of respondents considered that participation in a COVID-19 VCT would expose them to health risks. The development of vaccines based on new technologies might have exacerbated this fear.Citation22 Safety concerns are the most common barrier to participation in a VCTCitation13,Citation23 whatever the context. In a study carried out in May–June 2020 in France in the general population, whereas 72% of the respondents had a positive attitude toward clinical trials, 41% of the respondents agreed with the following statement: Participants in clinical trials are only “guinea pigs”.Citation13 As vaccines are preventive strategies, recruitment in a VCT might be more challenging than recruitment in a drug clinical trial.Citation6 This observation was previously made in the context of COVID-19; 60% of the respondents would definitely or probably agree to participate in a drug clinical trial if they were infected, this proportion dropped to 44% considering a COVID-19 VCT.Citation24
A majority of respondents considered that participation in a COVID-19 VCT will bring constraints and participation should be financially compensated. The effect of financial incentives in VCTs is unclear, they are barriers in some trials and motivators in others.Citation7,Citation23
A great majority of the respondents will seek advice from their physician before participating in a COVID-19 VCT. This result contrasts with a previous observation out of the context of a pandemic, where only 17.4% of the respondents would seek general practitioner’s advice before participation in a VCT.Citation7 When rapid development of vaccines is required, it is necessary to recruit a large number of participants in different settings, and alliance with physicians might be crucial especially since some healthcare workers are not comfortable giving advice to their patients about participation in a VCT.Citation25
Our work presents some limitations. First, we did not indicate in our survey, the type of clinical vaccine trials, the potential vaccine candidates, placebo use or not, and type of vaccine candidates. Intention to participate may change between early and later phases of clinical trials. Secondly, we cannot exclude an overestimation of willingness to participate in a COVID-19 VCT and of altruism due to a social desirability bias.Citation26 Social desirability bias might be considerable in the context of COVID-19 risk mitigation behaviors.Citation27 Additionally, recourse to online panels can be associated with biases in the recruitment of participants. For instance, it is possible that participation to clinical trials is slightly over-estimated since marginalized groups are often under-represented in online panels and tend to be more vaccine hesitant.
In conclusion, although COVID-19 vaccines were available in France, the proportion of the general French population still willing to participate in a COVID-19 VCT remained high. The COVID-19 pandemic has resulted in an unprecedented number of volunteers recruited in VCTs in a short period. It is premature to consider how interest in vaccine clinical research will be maintained in the future, and if this interest may contribute to preparedness for a future pandemic. It is not known if it will ever be necessary to mobilize so many volunteers again. To increase recruitment in VCTs and public interest for clinical trials, it seems primordial to better communicate about safety in VCTs and to reduce constraints due to participation in a VCT. Then, restoring confidence in vaccines, scientists, and physicians may also help to increase willingness to participate in VCTs in the general population. Further studies focusing on individuals with willingness to participate in COVID-19 VCTs may help to address the impact of public interest for COVID-19 VCT on the future of the recruitment in VCTs in general. In particular, because they allow to take into account more contextual elements, qualitative approaches based on in-depth interviews or participant observations are necessary to better understand the mechanisms leading both to the formation of perceptions of VCT and to their translation into actions. Designing interventions to increase knowledge and trust in vaccine clinical research, building communities of volunteers could contribute to facilitate the recruitment in VCTs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54(8):1636‑51. doi:10.1016/j.immuni.2021.07.017.
- Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38(45):7002‑6. doi:10.1016/j.vaccine.2020.09.041.
- Sambourg J, Bonneton M, Luong Nguyen L, Trillou C, Dohou J, Saint-Lary O, et al. Demographic characteristics and COVID-19 risk factors of volunteers registered on COVIREIVAC, the French national platform for COVID-19 vaccine trials. 31st European Congress of Clinical Mircobiology and Infectious Diseases; 2021 Jul.
- Krause PR, Fleming TR, Longini IM, Peto R, Briand S, Heymann DL, Beral V, Snape MD, Rees H, Ropero A-M, et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385(2):179‑86. doi:10.1056/NEJMsr2105280.
- Géodes - Santé publique France - Indicateurs : cartes, données et graphiques [Internet]. [accessed 2021 Oct 18]. https://geodes.santepubliquefrance.fr/#c=indicator&i=vacsi12.couv_dose1&s=2021-07-01&t=a01&view=map2
- Cobb EM, Singer DC, Davis MM. Public interest in medical research participation: differences by volunteer status and study type. Clin Transl Sci. 2014;7(2):145‑9. doi:10.1111/cts.12142.
- Detoc M, Launay O, Dualé C, Mutter C, Le Huec J-C, Lenzi N, Lucht F, Gagneux-Brunon A, Botelho-Nevers E. Barriers and motivations for participation in preventive vaccine clinical trials: experience of 5 clinical research sites. Vaccine. 2019;37(44):6633‑9. doi:10.1016/j.vaccine.2019.09.048.
- Algan Y, Cohen D, Davoine E, Foucault M, Stantcheva S. Trust in scientists in times of pandemic: panel evidence from 12 countries. PNAS. 2021;118(40):e2108576118. [accessed 2021 Oct 1]. https://www.pnas.org/content/118/40/e2108576118
- Sondage national « Les Français et la science » | science-and-you.Com [Internet]. [accessed 2021 Dec 27]. http://www.science-and-you.com/fr/sondage2021
- The state of vaccine confidence in the EU: 2018: the vaccine confidence project [Internet]. [accessed 2019 oct 2]. https://www.vaccineconfidence.org/research/the-state-of-vaccine-confidence-in-the-eu-2018/
- Thompson HS, Manning M, Mitchell J, Kim S, Harper FWK, Cresswell S, Johns K, Pal S, Dowe B, Tariq M, et al. Factors associated with racial/ethnic group–based medical mistrust and perspectives on COVID-19 vaccine trial participation and vaccine uptake in the US. JAMA Netw Open. 2021;4(5):e2111629. doi:10.1001/jamanetworkopen.2021.11629.
- Sethi S, Kumar A, Mandal A, Shaikh M, Hall CA, Kirk JMW, Moss P, Brookes MJ, Basu S. The UPTAKE study: implications for the future of COVID-19 vaccination trial recruitment in UK and beyond. Trials. 2021;22(1):296. doi:10.1186/s13063-021-05250-4.
- Schultz É, Ward JK, Atlani-Duault L, Holmes SM, Mancini J. French public familiarity and attitudes toward clinical research during the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(5):2611. doi:10.3390/ijerph18052611.
- Pahus L, Suehs CM, Halimi L, Bourdin A, Chanez P, Jaffuel D, Marciano J, Gamez A-S, Vachier I, Molinari N, et al. Patient distrust in pharmaceutical companies: an explanation for women under-representation in respiratory clinical trials? BMC Med Ethics. 2020;21(1):72. doi:10.1186/s12910-020-00509-y.
- Sisco MR, Weber EU. Examining charitable giving in real-world online donations. Nat Commun. 2019;10:3968. doi:10.1038/s41467-019-11852-z.
- Ridda I, MacIntyre CR, Lindley RI, Tan TC. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. 2010;28(4):901‑6. doi:10.1016/j.vaccine.2009.10.081.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‑43. doi:10.1001/jamainternmed.2020.0994.
- Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6). doi:10.2807/1560-7917.ES.2021.26.6.2100096
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403‑16. doi:10.1056/NEJMoa2035389.
- Labetoulle R, Detoc M, Gagnaire J, Berthelot P, Pelissier C, Fontana L, Botelho-Nevers E, Gagneux-Brunon A. COVID-19 in health-care workers: lessons from SARS and MERS epidemics and perspectives for chemoprophylaxis and vaccines. Expert Rev Vaccines. 2020;19(10):937‑47. doi:10.1080/14760584.2020.1843432.
- Kitonsa J, Kamacooko O, Bahemuka UM, Kibengo F, Kakande A, Wajja A, Basajja V, Lumala A, Ssemwanga E, Asaba R, et al. Willingness to participate in COVID-19 vaccine trials; a survey among a population of healthcare workers in Uganda. Plos One. 2021;16(5):e0251992. doi:10.1371/journal.pone.0251992.
- Ward JK, Alleaume C, Peretti-Watel P, Peretti-Watel P, Seror V, Cortaredona S, Launay O, Raude J, Verger P, Beck F, et al. The French public’s attitudes to a future COVID-19 vaccine: the politicization of a public health issue. Soc Sci Med. 2020;265:113414. doi:10.1016/j.socscimed.2020.113414.
- Detoc M, Gagneux-Brunon A, Lucht F, Botelho-Nevers E. Barriers and motivations to volunteers’ participation in preventive vaccine trials: a systematic review. Expert Rev Vaccines. 2017 ;16(5):467‑77. doi:10.1080/14760584.2017.1297706.
- Abdelhafiz AS, Abd ElHafeez S, Khalil MA, Shahrouri M, Alosaim B, Salem RO, Alorabi M, Abdelgawad F, Ahram M. Factors influencing participation in COVID-19 clinical trials: a multi-national study. Front Med. 2021;8:155. doi:10.3389/fmed.2021.608959.
- Detoc M, Touche C, Charles R, Lucht F, Gagneux-Brunon A, Botelho-Nevers E. Primary physicians’ attitudes toward their patients receiving a proposal to participate in a vaccine trial. Hum Vaccin Immunother. 2019;15(12):2969‑79. doi:10.1080/21645515.2019.1625646.
- Grimm P. Social desirability bias. In: Wiley International Encyclopedia of Marketing [Internet]. John Wiley & Sons, Ltd; 2010. [accessed 2021 Dec 28]. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781444316568.wiem02057
- Timmons S, McGinnity F, Belton C, Barjaková M, Lunn P. It depends on how you ask: measuring bias in population surveys of compliance with COVID-19 public health guidance. J Epidemiol Community Health. 2021;75(4):387‑9. doi:10.1136/jech-2020-215256.