ABSTRACT
Background
A quadrivalent split influenza vaccine IIV4-W against both influenza A and B viruses is urgently needed.
Methods
To evaluate the safety and immunogenicity of IIV4-W in people aged 3–60 years, 2400 participants recruited in a double-blind phase III trial and were randomly assigned to the IIV4-W, TIV1 and TIV2 groups. The immunogenicity indicators were measured at 28 days postvaccination and for 180 days for safety follow-up.
Results
Adverse events (AEs) occurred in 162 (20.28%), 116 (14.55%) and 123 (15.41%) participants in the IIV4-W, TIV1 and TIV2 groups, respectively. All these AEs were mild and self-limiting, and no serious AEs related to the vaccines were observed. IIV4-W elicited a non-inferior immune response for matched strains (the lower limit of 95% CI for GMT ratio >0.67, for SCR and SPR difference >-10%) and superior immune response for the additional B strains (the lower limit of 95% CI for GMT ratio >1.5, for SCR difference >10%) versus TIVs. The lower limit of the 95% confidence interval of the GMT increase fold, the seroconversion rate and the seroprotection rate exceeded 2.5, 40% and 70% for the four strains in IIV4-W respectively.
Conclusions
IIV4-W was noninferior to the TIV-matched strains and was superior to the additional B strain. IIV4-W was safe in the participants and elicited high antibody titers.
Introduction
Circulation of various B strains increases human influenza infection in different regions and seasons.Citation1 As trivalent influenza vaccines (TIVs) contain two strains of influenza A lineage (A/H1N1 and A/H3N2) and one strain of influenza B (BV, B/Victoria or BY, B/Yamagata),Citation2 the limited cross-lineage protection of B strains of TIVs poses a long-term threat to human health. There is an urgent need for seasonal quadrivalent influenza vaccines that contain both A (H1N1, H3N2) and B (BV, BY) antigens to provide coverage against influenza.Citation3 Based on the influenza disease surveillance data from the previous year, the World Health Organization (WHO) recommends the dominant influenza stains used in vaccines for the next influenza season.Citation4 The reformulated seasonal quadrivalent influenza vaccine strains will replace the current existing influenza vaccines and may decrease the incidence of influenza-related consultations and hospitalizations in the upcoming influenza season.Citation3
Seasonal quadrivalent influenza vaccines were found to have noninferior immunogenicity and acceptable safety in several phase III trials in infants, children and adults compared to TIV.Citation5–7 IIV4-HL, which is produced by Hualan Biological Engineering, was the first available seasonal quadrivalent split influenza vaccine in China since the 2018/2019 influenza epidemic season for populations aged 3 years old or above (China Drug Approval No.: S20083016). However, there is still an urgent need for IIV4 in China. The phase III trial of IIV4-W by the Wuhan Institute of Biological Products Co. Ltd. was completed to evaluate the tolerability and immunogenicity in elderly individuals aged 60 years above in 2019.Citation8 To investigate the safety and immunogenicity of IIV4-W in children and older adults as a candidate influenza vaccine, we conducted a phase III noninferiority trial that compared IIV4-W with two controls (TIV1 and TIV2 produced by Changchun Institute of Biological Products Co. Ltd. and approved by the National Institutes for Food and Drug Control that included two influenza A strains, TIV1 containing influenza B/Yamagata and TIV2 containing B/Victoria) as the chosen influenza vaccine in China in participants aged 3–60 years.
Materials and methods
Study design
This randomized, double-blind, active-controlled, three-center trial was designed by the Wuhan Institute of Biological Products Co Ltd (WIBP). To evaluate the immunogenicity and safety of IIV4-W to two National Institutes for Food and Drug Control-licensed TIV in adults 3–60 years of age (Clinical approval number 2015L00649 and China clinical trial identifier:
CTR20160206),Citation9 the study was performed in three clinical centers during March 2016 and ended in July 2017: Chaoyang District, Beijing, Chingyuan and Quwo County of Shanxi Province in China. The safety set was evaluated for 180 days after vaccination, and the serological index of the participants was detected before immunization and 28 days postimmunization. The vaccine injection, safety and immunogenicity data collection was performed by the investigators of the Beijing City Centers for Disease Control and Prevention (CDC) and the Shanxi Province CDC. The Department of Health Statistics of the Fourth Military Medical University, as the independent data and safety monitoring board, was responsible for the safety and immunogenicity data monitoring and statistical analysis.
Ethics
The protocols of this trial were approved by the National Medical Products Administration (NMPA). The study was conducted following the principles of the Declaration of Helsinki and was consistent with the Good Clinical Practice (GCP) of China, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). All the participants and the guardians of young children (3–17 years old) signed written informed consent before enrollment.
Participants
Healthy participants aged 3–60 years old without a history of influenza vaccine injection with the last three years or influenza virus infection with the last three months according to inquiry were eligible for enrollment. Participants with an axillary temperature ≤37.0°C who abided by the clinical trial protocols were needed. For randomization, the recruited 2400 participants were sequentially assigned a number by SAS software to stratify them via block randomization, and the participants were randomly assigned to receive intramuscular injections of a single dose of 15 μg IIV4-W, TIV1 or TIV2 (1:1:1). The participants and investigators were both masked to the vaccine that was administered.
Vaccination
IIV4-W contained 60 μg (15 μg for each strain) HA antigen. The production process of IIV4-W includes the influenza vaccine strains grown in embryonated eggs, the harvest, concentration, inactivation, cleavage and purification of the viral liquid, then combination, dilution, and equal division of 0.5 mL inactivated viral liquid into a vial with standard techniques. The four influenza vaccine strains used for the 2015/2016 season (Northern Hemisphere) were recommended by the WHO, were approved by the State Food and Drug Administration (FDA) and were purchased from The National Institute for Biological Standards and Control (NIBSC). IIV4-W (batch number: 20151101) contains four vaccine strains (H1N1 lineage: NYMC X 179A reassortant derived from A/California/7/2009; the H3N2 lineage: NIB 88 reassortant derived from A/Switzerland/ 9715293/2013; the B/Victoria lineage: NYMC BX 51B reassortant derived from B/Massachusetts/2/2012; and the B/Yamagata lineage: NYMC BX 35 reassortant derived from B/Brisbane/60/2008). Licensed TIV1 (influenza vaccine, split virus, inactivated, 15 μg of each strain, batch number: 20150632) contains the same influenza A strains and one B strain (B/Yamagata lineage: NYMC BX 35 reassortant derived from B/Brisbane/60/2008), and TIV2 (influenza vaccine, split virus, inactivated, 15 μg of each strain, batch number: S20150801) contains the other influenza B strain (B/Victoria lineage: NYMC BX 51B reassortant derived from B/Massachusetts/2/2012) in addition to the same two influenza A strains.
Safety endpoints
Safety was assessed by the incidence, severity and duration of solicited systemic and injection-site adverse events (AEs), unsolicited AEs, and serious AEs (SAEs). All the participants were observed for 30 minutes after vaccination in case of acute AEs. Local AEs, including pain, redness, swelling, local inflammation, itching and systemic reactogenicity, including fever, fatigue, headache, gastrointestinal symptoms, etc., were solicited using memory aids (e.g., diary cards) during the week after vaccination. The adult participants or the children guardians reported any AEs by a contact card within 8–28 days. The unsolicited AEs included any other medical event in addition to the solicited AEs, such as oral herpes, upper respiratory tract infection, ear trauma, poisoning, and surgical complications. SAEs were recorded via a telephone interview 29 days to 6 months after vaccination.
The AEs were graded on a severity scale that ranged from grades 1 to 4. Grade 1 and 2 symptoms were mild and moderate and did not or only partially interfered with normal activity, while grade 3 AEs prevented the participant’s normal daily activity. Grade 4 AEs are life-threatening and require hospitalization.
General immunogenicity
For the immunogenicity subset, peripheral blood samples (5 mL/each) were collected and centrifuged for serum before the vaccination and 28 days after the vaccination. Hemagglutination inhibition (HI) assays were performed for the serological antibody assessments using the same procedure as previously reported.Citation8 Twenty-five microliters of serum was used for HI antibody detection of one subtype strain, and the HI titer was defined as the dilution factor of serum completely inhibiting hemagglutination.
Seroconversion rate (SCR) was defined as the proportion of the participants with either a prevaccination HI titer of <1:10 with a postvaccination titer ≥1:40 or the proportion of the participants with a prevaccination titer ≥1:10 with a ≥4 -fold increase in the antibody titers after vaccination, and seroprotection rate (SPR) was defined as the proportion of the participants with HI titers ≥1:40. According to the Committee for Human Medicinal Products (CBER) criteria, the immunogenicity indicators included that the lower limit of the two-sided 95% confidence interval (CI) of SCRs and SPRs should exceed 40% and 70% in the IIV4-W group, respectively. In addition, the lower limit of the 95% CI of geometric mean fold increase (GMFI) in the participants who received the vaccine exceeded 2.5-fold from baseline at 28 days postvaccination.
Noninferiority immunogenicity for the for the matched strains
The primary noninferior immunogenicity indicators for the H1N1, H3N2 and matched B strains included the lower limit of the two-sided 95% CI of the geometric mean HI antibody titer (GMT) ratio difference exceeding 0.67 for the new vaccine/registered vaccine and exceeding −10% of the SCR and the SPRs difference for the new vaccine-registered vaccine.
Inferiority immunogenicity for the additional B strains
The superior immunogenicity indicators of the additional B strains in IIV4-W were determined as the lower limit of the two-sided 95% CI of the GMT ratio >1.5, and the SCR difference was >10%.
Statistical analysis
Considering the participants drop-out rate and the number of invalid blood samples, the sample size was estimated to achieve at least 90% power to demonstrate noninferiority over six 6 statistical tests (GMT ratios and SPRs for the matched strains compared with two TIV controls) using a one-sided alpha of 0.03 for each comparison with PASS15 software.
The safety analyses were conducted with Fisher’s exact probability tests. The HI titer was fitted and log10 transformed, and the significant differences in GMT and the adjusted GMT ratio between the IIV4-W and control groups were analyzed with analysis of covariance (ANOVA) and t-tests, respectively. The 95% CIs of the SPRs and SCRs were calculated by the Clopper-Pearson test, and the statistical analysis was conducted using the chi-square and Fisher’s exact probability tests with SAS software.
Results
Participants
A total of 2400 participants were enrolled, of which 800 were assigned to the IIV4-W, TIV1, or TIV2 groups. Due to the withdrawal of several participants, 799, 797, and 798 participants in the IIV4-W, TIV1, and TIV2 groups completed the immunizations and scheduled visits, respectively. The safety results of the overall subjects (safety set, SS) were analyzed, and the safety data of the 3- to 8-year-old children were highlighted. Because of incomplete immunogenicity samples (pre- and post-immunization), only 792, 789, 789 were available for immunogenicity analysis in groups IIV4-W, TIV1 and TIV2, respectively (). There were no significant differences in age or sex among the three groups, and the baseline demographic characteristics of the participants are listed in .
Figure 1. Participant disposition.
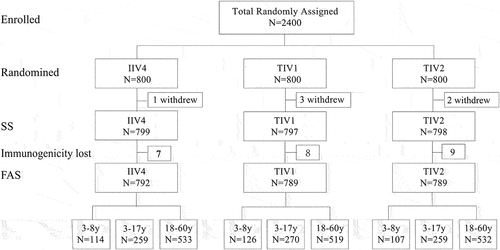
Table 1. Baseline characteristics of the participants.
Safety
All AEs (combined solicited and unsolicited AEs) occurred in 162 (20.28%), 116 (14.55%), and 123 (15.41%) participants in the IIV1, TIV1, and TIV2 cohorts during the follow-up, respectively. The most solicited local and systemic reactions were resolved within 7 days after immunization and were reported to be mild (grade 1) or moderate (grade 2) in severity after vaccination in the three groups; fewer than 0.50% or 0.88% of the patients reported grade 3 systemic or local AEs in the IIV4-W group (). Four, two, and two vaccine-unrelated SAEs were observed up to six months after the first vaccination in the IIV4-W, TIV1 and TIV2 groups, respectively, and no AEs led to withdrawal from the study.
Table 2. The severity of solicited AEs in all subjects.
Although there were no significant differences in the prevalence of total solicited (systemic or local) and unsolicited AEs among the IIV4-W, TIV1 and TIV2 vaccination groups (P > .05), fever as the most common solicited systemic AE was reported by 88 (10.89%), 43 (5.27%), 61 (7.64%) participants in the IIV4-W, TIV1 and TIV2 groups with a significant difference (p = .0002). Headache, as the second most common systematic AE, was reported by 16 (2.00%), 5 (0.63%), and 5 (0.63%) participants in the three vaccination groups, respectively (p = .0175) (). There were no differences in the incidence of fatigue, vomiting, diarrhea, myalgia, coughing, or hypersensitivity that were observed among the IIV4-W and the two control groups (P > .05). Pain was the most common local AE and was observed in 37 (4.63%), 18 (2.26%), and 28 (3.51%) participants in the IIV4-W, TIV1 and TIV2 groups, respectively (p < .05). Other injection-site AEs, such as redness, swelling, induration and itching, had similar incidences among the three groups (P > .05).
Table 3. The frequency and severity of solicited AEs in 3–8 years old cohorts.
In 3- to 8-year-old children, solicited systemic AEs were reported by 23.68%, 19.84% and 20.00% of participants in the IIV4-W, TIV1 and TIV2 groups, respectively, which was higher than in the overall cohort (IIV4-W, 16.52%; TIV1, 9.28%; TIV2, 10.78%). The proportion of participants with local AEs in 3- to 8-year-old children was similar to that in the overall cohort (). Although the solicited systemic AEs in the IIV4-W group seemed to be higher than those in the comparator TIV groups, the results of statistical analysis showed that there was no significant difference in the incidence of solicited AEs among the three groups (P > .05). Most of the solicited AEs were mild and moderate in intensity. Systemic fever and injection-site pain were the most common solicited AEs in 3- to 8-year-old children, the same as in the whole ago cohort.
General immunogenicity
A total of 2341 serological results were obtained. All the participants had similar prevaccination HI titers (P > .1) among the IIV4-W, TIV1 and TIV2 groups, and over 70% of the participants had detectable HI antibodies (HI > 1:10) at baseline (). The lower limit of the 2-sided 95% CI of the GMFI in the IIV4-W group and in the comparator TIV group exceeded 2.5-fold among the 3–17 and 18–60 years age cohorts for H1N1, H3N2 or the matched B lineage strains (). In the 3–17 year cohorts, the HI titer GMTs but not the GMFI was higher than that in the 18–60 year cohort for the four strains among the IIV4-W, comparator TIV1 and TIV2 groups (). The HI titer and GMFI induced by the IIV4-W additional B lineage strain were significantly higher than those elicited by the comparators TIV1 and TIV2 without the matched B strain (p = .0001) ().
Table 4. Immunogenicity measures in the participants.
Higher SCRs and SPRs of IIV4-W against H1N1, H3N2, BY and BV were observed in the cohorts aged 3–17 and 18–60 years. More than 60% of the participants had HI titers that seroconverted for the H1N1 and H3N2 strains, and more than 40% of the participants had HI titers that seroconverted for matched BV and BY strains in the IIV4-W, TIV1 and TIV2 strains, which exceeded the CBER criteria and required a lower limit of the two-sided 95% CI of SCRs (). The SCRs of IIV4-W, TIV1 and TIV2 for H1N1 and H3N2 in the 3–17 cohort were lower than those of the participants who were over 18 years old. The SCRs for the BY strain in the 18-60-year-old cohort were slightly higher than those in the 3-17-year-old cohort. The SCRs for the BV strain were similar in the two age cohorts.
The SPRs for the H1N1, H3N2 and BY strains were not significantly different among the 3-17- and 18-60-year age cohorts (). Higher SPRs for the BV strain were observed in the 3-17-year age cohort than in the 18-60-year age cohort. In the two age cohorts, the SRPs against the H1N1 and H3N2 subtypes exceeded 70% in the trial and in the control vaccine groups, which is the same as the SPRs against the BY strain in the IIV4-W and TIV1 groups and against the BV strain in the IIV1 and TIV2 groups. The SCRs and SPRs against the BY strain in the TIV2 group and against the BV strain in the TIV1 group were at low levels because of the lack of BV strain antigen in the TIV1 and BY strain antigen in the TIV2 vaccine.
Among the trial and control vaccine groups, there were no differences in the HI titer GMTs and GMFI for H1N1 and H3N2 in the 3-8-year cohort (p > .2). The GMFI exceeded 15.81 (12.31, 20.29), 10.39 (8.02, 13.46), 6.91 (5.55, 8.62) and 4.80 (3.89, 5.92) for the H1N1, H3N2, BY and BV strains of IIV4-W, respectively (). The SCRs for the BY strain for the 3-8-year-old cohort were statistically equivalent to those in the 18-60-year-old cohort and were slightly higher than those in the 3-17-year-old cohort.
Noninferiority immunogenicity for the matched strains
The lower limit of the two-sided 95% CI of the GMT ratio of IIV4-W/TIV1 and IIV4-W/TIV2 exceeded 0.67 for the matched three strains in the overall participants. The lower limit of the two-sided 95% CI of the SCR and SPR differences of IIV4-W-TIV1 and IIV4-W-TIV2 for H1N1, H3N2 and matched B strains exceeded −10% in all of the participants, which met the CBER criteria for noninferiority ().
Table 5. The non-inferiority and superiority comparisons between IIV4-W and TIVs.
Superiority immunogenicity for the additional B strains
The lower limit of the two-sided 95% CI of the GMT ratio of IIV4-W/TIV1 for the BV strain and IIV4-W/TIV2 for the BY strain exceeded 1.5 in all the participants. The lower limit of the two-sided 95% CI of the SCR and SPR differences of IIV4-W-TIV1 for the BV strain and IIV4-W-TIV2 of the BY strain exceeded 10%, which met the superiority criterion ().
Discussion
In phase III clinical trials, IIV4-W elicited a robust amount of humoral antibodies for the additional B strains (B/Yamaga or B/Victoria) compared to TIVs, with noninferior immunogenicity (GMT ratios and differences in SCRs and SPRs) for the H1N1, H3N2 and matched B strain lineages and inferior immunogenicity for the additional B strain lineages in the 3-60-year-old population. Most injection-site and systemic AEs were mild to moderate and were self-limiting. Overall, IIV4-W had satisfactory immunogenicity and an acceptable safety profile.
The subjects received IIV4-W provided a strong serological response with serum HI titer GMFI, SCR, and SPR over 2.5, 40%, 70% for young children and adults, respectively. IIV4-W met the noninferiority criteria for the H1N1, H3N2, BY and BV strains for the GMT ratio, SCR and SPR difference in the participants, and these noninferior immunogenicity results are consistent with other clinical trials of IIV4s.Citation10,Citation11 All the above results indicated that IIV4-W could be used as a candidate seasonal tetravalent influenza vaccine.
Although the antibody levels of the four influenza strains were generally higher in 3-17-year-old participants than in the populations aged 18–60 years old, the GMFI was higher in the 18-60-year-old cohorts than in the younger cohorts, which is likely due to the low prevaccination HI titer in the elder adults.Citation12,Citation13 For children, influenza vaccines may be moderately immunogenic because of the limited previous exposure to vaccines and viruses, so two doses of influenza vaccine are recommended for 6-month- to 8-year-old children to achieve protective antibody titers.Citation14 The SCR of one does IIV4-W was closer to that of two does quadrivalent influenza vaccine in children 3–8 years of age, indicating that IIV4-W could be used not only in adults but also in children.Citation6
Increased HI titers for the BV strain in TIV1 and the BY strain in TIV2 suggest that cross-reactivity of the B strains in TIV may elicit antibodies to heterologous B strains, similar to the previous clinical trial of split-virion trivalent influenza vaccine.Citation15 Regardless, compared to the matched B strains, the extent of cross-reactivity elicited by the heterologous B strains was expected to be low, and there was uncertainty regarding its ability to produce sufficient immune protection; IIV4 could reduce annual cases, hospitalizations and deaths.Citation16
In this clinical trial, the safety profile of IIV4-W was characterized for up to 6 months after vaccination. Although the incidences of local pain, systemic fever and headache were higher in the participants who received IIV4-W than in the participants who received TIV1 and TIV2, the incidences of other AEs, such as local redness, swelling and systemic fatigue, were not significantly higher than those in the control group. The reason for these results may be related to the fact that IIV4-W has one more B lineage strain antigen than TIV, and the total protein content of IIV4-W was 25% higher than that in TIV. The incidence of grade 3 and above AEs in the IIV4-W group was not significantly higher than that in the comparator group, and IIV4-W was not considered to increase the incidence of AEs of a higher severity relative to the comparator vaccine, indicating that IIV4-W was safe and well tolerated.
The frequencies of fever induced by IIV4-W observed here were higher than those in other studies of IIV4, possibly due to the definition of fever grade (grade 1, 37.1–37.5°C grade 2, 37.6–39°C grade 3, ≥39.1°) based on the guiding principles for grading standards of adverse reactions in clinical trials of preventive vaccines issued by the State Food and Drug Administration being different from other studies (grade 1, 38.0–38.4°C grade 2, 38.5–38.9°C grade 3: ≥39.0°C or fever was defined as above 37.5 °C). The other safety results were consistent with the clinical study results of the other quadrivalent influenza vaccine, such as local pain;Citation5,Citation17–19 however, the incidence of redness and swelling was higher than that in other studies, just because of the different definitions of redness and swelling grades.Citation18,Citation19 The frequencies of systemic AEs decreased with increasing age, which was also observed previously.Citation17,Citation20
We took full account of the complexity of the participants in this study and excluded the factors affecting vaccination. In addition to dividing the participants into two age cohorts to assess the safety and immunogenicity of IIV4-W and comparator TIV so that the results were more detailed and reliable, we also analyzed the immunogenicity and safety of IIV4-W in people aged 3–8 years to determine whether IIV4-W is available for children. However, we did not evaluate the effectiveness of IIV4-W, which is not equivalent to its immunogenicity. The immunogenicity evaluation of a single epidemic season makes it challenging to assess the efficacy of IIV4-W against various influenza strains.
Conclusions
We found that the split influenza vaccine IIV4-W has a comparable safety profile with noninferior immunogenicity in individuals aged 3–60 years. IIV4-W containing both of the B lineage influenza strain antigens could decrease influenza hospitalization and could be considered a candidate influenza vaccine against seasonal influenza in children, adults and elderly individuals.Citation8
Abbreviations
| AE | = | Adverse Event |
| ANOVA | = | Analysis of Variance |
| CBER | = | The Center for Biologics Evaluation and Research |
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | Confidence Interval |
| FAS | = | Full Analysis Set |
| FDA | = | Food and Drug Administration |
| GCP | = | Good Clinical Practice |
| GMFI | = | Geometric Mean Fold Increase |
| GMT | = | Geometric Mean Titer |
| HI | = | Hemagglutination Inhibition |
| ICH | = | International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use |
| IIV4 | = | Quadrivalent Inactivated Influenza Vaccine |
| NMPA | = | National Medical Products Administration |
| QIV | = | Inactivated quadrivalent influenza vaccine |
| SAEs | = | Serious Adverse Events |
| SCR | = | Seroconversion Rate |
| SPR | = | Seroprotection Rate |
| SS | = | Safety Set |
| TEAE | = | Treatment Emergent Adverse Event |
| TIV | = | Trivalent Influenza Vaccine |
Authors’ contributions
Xiaoming Yang, Xiaoyuan Huang, Li Li, Ting Fan and Guohua Li were responsible for the study concept, design and statistical analysis. Xuanxuan Nian and Jiayou Zhang were responsible for the manuscript drafting and revision. Xuefen Gao, Wei Zhao, Wei Chen, Zhaoqing Zhang, Zhihao Yao, and Xixin Han were responsible for the clinical trial follow-up, and Jinrong Shi, Ying Wang, Haihe Bian, Nianmin Shi, Xinguo Li, and Kai Duan provided technical or material support.
Acknowledgments
The authors thank the investigators at the CDC of Beijjing City and the CDC of Shandong Province for their help with the trial implementation and the Department of Health Statistics of Fourth Military Medical University for the safety and immunogenicity data monitoring and analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003 [J]. J Virol. 2004;78(23):1–10. doi:10.1128/JVI.78.23.12817-12828.2004.
- Eichner M, Schwehm M, Hain J, Uphoff H, Salzberger B, Knuf M, Schmidt-Ott R. 4Flu - an individual based simulation tool to study the effects of quadrivalent vaccination on seasonal influenza in Germany [J]. BMC Infect Dis. 2014;14(1). doi:10.1186/1471-2334-14-365.
- Bianculli PM, Bellier L, Mangado IO, Pérez CG, Mieres G, Lazarov L, Petitjean A, Dibarboure H, Lopez JG. Switching from trivalent to quadrivalent inactivated influenza vaccines in Uruguay: a cost-effectiveness analysis [J]. Hum Vaccin Immunother. 2022;1–10. doi:10.1080/21645515.2022.2050653.
- Dunkle LM, Izikson R, Patriarca P, Goldenthal KL, Muse D, Callahan J, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older [J]. N Engl J Med. 2017;376(25):2427–36. doi:10.1056/NEJMoa1608862.
- Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, Santos J, Montalban MC, de Looze FJ, Eizenberg P, Hall S, Dupuy M, Hutagalung Y. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: a randomized, controlled, phase III trial [J]. Vaccine. 2015;33(21):2485–92. doi:10.1016/j.vaccine.2015.03.065.
- Pepin S, Szymanski H, Rochín Kobashi IA, Villagomez Martinez S, González Zamora JF, Brzostek J, Huang L-M, Chiu C-H, Chen P-Y, Ahonen A. Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: a phase III randomized controlled study [J]. Hum Vaccin Immunother. 2016;12(12):3072–78. doi:10.1080/21645515.2016.1212143.
- Thiem VD, Chabanon AL, Fournier M, Lavis N, Quang ND, Ha VH, Sanicas M. Safety of a quadrivalent influenza vaccine in Vietnamese healthy subjects aged 6 months and older [J]. Hum Vaccin Immunother. 2021;17(3):690–93. doi:10.1080/21645515.2020.1795477.
- Fan R, Huang X, Nian X, Ou Z, Zhou J, Zhang J, et al. Safety and immunogenicity of a quadrivalent influenza vaccine in adults aged 60 years or above: a phase III randomized controlled clinical study [J]. Hum Vaccines Immunother. 2022;18(1):1–9.
- NMPA. Influenza vaccine (split virion). Inactivated; 2021 [accessed Jul 7].http://app1.nmpa.gov.cn/data_nmpa/face3/base.jsp?tableId=25&tableName=TABLE25&title=%E5%9B%BD%E4%BA%A7%E8%8D%AF%E5%93%81&bcId=152904713761213296322795806604&CbSlDlH0=qAk7racmf3Kmf3KmfKJzH8SG.zGAeIHwe4nC9ZxjqRZqqEV.
- Bart S, Cannon K, Herrington D, Mills R, Forleo-Neto E, Lindert K, Abdul Mateen A. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: a phase III, double-blind, multicenter, randomized, non-inferiority study [J]. Hum Vaccin Immunother. 2016;12(9):2278–88. doi:10.1080/21645515.2016.1182270.
- Moa AM, Chughtai AA, Muscatello DJ, Turner RM, Macintyre CR. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: a systematic review and meta-analysis of randomised controlled trials [J]. Vaccine. 2016;34(35):4092–102. doi:10.1016/j.vaccine.2016.06.064.
- Choi WS, Noh JY, Lee J, Choi JY, Lee JS, Kim MS, Kim HS, Bang J, Lavis N, Kim WJ. Immunogenicity and safety of a split-virion quadrivalent influenza vaccine in adults 18–60 years of age in the Republic of Korea. Hum Vaccin Immunother. 2018;14(3):587–92. doi:10.1080/21645515.2017.1381808.
- Basu I, Agarwal M, Shah V, Shukla V, Naik S, Supe PD, Srivastava MK, Giriraja KV, Pinjar P, Mishra PK. Immunogenicity and safety of two quadrivalent influenza vaccines in healthy adult and elderly participants in India - a phase III, active-controlled, randomized clinical study [J]. Hum Vaccin Immunother. 2022;18(1):1–10. doi:10.1080/21645515.2021.1885278.
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–97.
- Gorse GJ, Falsey AR, Ozol-Godfrey A, Landolfi V, Tsang PH. Safety and immunogenicity of a quadrivalent intradermal influenza vaccine in adults [J]. Vaccine. 2015;33(9):1151–59. doi:10.1016/j.vaccine.2015.01.025.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine [J]. Vaccine. 2012;30(11):1993–98. doi:10.1016/j.vaccine.2011.12.098.
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults [J]. Vaccine. 2013;31(5):770–76. doi:10.1016/j.vaccine.2012.11.074.
- Greenberg DP, Robertson CA, Talbot HK, Decker MD. Safety and immunogenicity of a quadrivalent influenza vaccine in adults 65 y of age and older [J]. Hum Vaccin Immunother. 2017;13(9):2058–64. doi:10.1080/21645515.2017.1344375.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewé W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: a phase III, randomized trial [J]. Vaccine. 2014;32(13):1480–87. doi:10.1016/j.vaccine.2014.01.022.
- Haber P, Moro PL, Lewis P, Woo EJ, Jankosky C, Cano M. Post-licensure surveillance of quadrivalent inactivated influenza (IIV4) vaccine in the United States, Vaccine Adverse Event Reporting System (VAERS), July 1, 2013-May 31, 2015 [J]. Vaccine. 2016;34(22):2507–12. doi:10.1016/j.vaccine.2016.03.048.
