ABSTRACT
Background
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide, and prevalence is still substantially higher in men than in women. Causative factors include smoking and alcohol use, while human papillomavirus (HPV) infection is causally related to a subset of oropharyngeal cancers. In this retrospective study, we aimed to provide estimates on the clinical and economic burden of HNSCC in Spain.
Methods
We used the discharge reports from the Spanish Minimum Basic Data Set (MBDS), to retrospectively analyze hospital discharge data in individuals with a diagnosis of HNSCC in any diagnostic position, based on the ICD coding system (ICD-9-CM and ICD10 CM), from 2009 to 2019.
Results
A total of 175,340 admissions and 14,498 deaths due to laryngeal, pharyngeal and oral cavity cancer were recorded in Spain, of which 85% occurred in men. The most prevalent diagnoses were laryngeal cancer in men (50.9%) and oral cavity cancer in women (49.1%). In general, the hospitalization and death rates for all major head and neck cancer sites decreased in men and increased or remained stable in women during the study period. However, the corresponding rates for tonsil cancer, strongly associated with HPV infection, increased significantly in men. Overall, the economic burden of HNSCC during the study period was estimated at 100 million euros per year on average.
Conclusion
HNSCC still places an important clinical and economic burden on the health system in Spain. Prevention strategies should be prioritized, and vaccination programs against HPV in both sexes should be reinforced.
Introduction
Head and neck cancer is a heterogeneous group of malignancies that usually begin in the squamous cells that line the mucosal surfaces of the upper aerodigestive tract, most commonly the oral cavity, pharynx and larynx. These cancers are often referred to as head and neck squamous cell carcinomas (HNSCC).Citation1,Citation2 In 2020, HNSCC was the seventh most common cancer worldwide, accounting for 878,348 new cases (670,146 in men and 208,202 women) and 444,347 new deaths (340,360 in men and 103,987 in women).Citation3 HNSCC is typically diagnosed in adult patients and, regardless of the etiology, men are at a substantially higher risk than women (2- to 4-fold) for developing all forms of HNSCC, especially laryngeal cancer.Citation1,Citation2
The most important risk factors for HNSCC are tobacco and alcohol consumption, the combined use of which has a synergistic effect that increases the risk of cancer development, particularly for oral cavity and laryngeal cancer.Citation4 Notably, heavy users of both substances have a ≥ 35 times greater risk of developing HNSCC.Citation5 However, growing evidence is showing that human papillomavirus (HPV) infection, predominantly type 16 (HPV-16), is causally related with a subset of oropharyngeal squamous cell carcinomas (OPSCC), especially in younger age groups.Citation6 The association between HPV infection and HNSCC development is strongest for tonsil cancer and weakest for oral cavity and laryngeal cancers.Citation7 Interestingly, HPV-positive HNSCCs comprise a distinct molecular, clinical, and pathological disease entity in a patient demographic that differs substantially from those of HPVnegative HNSCC, along with a markedly improved prognosis.Citation8–10 Individuals with HPV-positive HNSCC tend to be younger and healthier and have different risk factors related to sexual behavior.Citation11
HNSCC associated with smoking and alcohol consumption has been decreasing in incidence in recent decades, particularly among men, due to the substantial reduction in the prevalence of tobacco and alcohol consumption.Citation12,Citation13 Conversely, the incidence of HPV-positive OPSCC has been rising exponentially in the same period, predominantly among younger people in North America and EuropeCitation14–16 reaching up to 2.5 times that of HPV-negative OPSCC, and it is currently being more common than HPV-driven cervical cancer.Citation17 This increasing trend is likely to reflect the latency period of about 10 to 30 years after the onset of changes in sexual behaviors that are relevant to oral HPV exposure.Citation18,Citation19
In recent decades, HNSCC survival has improved only modestly. According to data from the Surveillance, Epidemiology and End Results Registry (SEER) in the United States, the overall 5-year relative survival rate increased from 55% in 1992–1996 to 66% in 2002–2006 in all age groups and all anatomical sites. However, this improvement is thought to be partially attributable to the emergence of HPV-associated HNSCC, rather than to advances in therapeutic interventions per se.Citation20
HNSCC is often preventable and can be cured when identified at early stages. Therefore, prevention strategies should be prioritized to help reduce the clinical burden of HNSCC, primarily by reducing or eliminating preventable risk factors, such as smoking and alcohol use, and secondly, by improving early detection of asymptomatic disease and subsequent interventions to prevent disease progression. Head and neck cancer screening programs have been hampered because many cancers appear de novo and HPV-related precancerous lesions are not well characterized.Citation21 Various screening programmes have reported detection rates for confirmed malignancy or premalignant pathology of less than 3%Citation22. Notably, the involvement of HPV infection in the development of HNSCC has important implications in this regard.Citation23 Prophylactic vaccination against HPV in both sexes holds great promise for reducing HPV-positive oropharyngeal cancers in the future, and efforts should be made to reinforce adherence to HPV vaccination programs.Citation24 In addition to its dire impact on morbidity and mortality, HNSCC places a substantial economic burden on the public health system. Unfortunately, many patients are diagnosed and treated at a late stage of the disease, conferring a poor prognosis and increased direct costs.Citation25,Citation26
In this retrospective epidemiological study, we aimed to provide estimates on the clinical and economic burden of HNSCC in Spain during the period 2009–2019. Information on trends in HNSCC incidence and mortality and its economic impact, may be useful when prioritizing prevention and medical intervention strategies in Spain.
Methods
Study design and data sources
We used discharge reports from the Minimum Basic Data Set (MBDS), published annually by the Spanish Ministry of Health, to retrospectively analyze hospital discharge data containing a diagnosis of malignant neoplasm of the head and neck in any diagnostic position over a 11-year period, from 1 January 2009, to 31 December 2019. The MBDS includes demographic data (age, sex, place of residence, place of hospitalization), dates of admission and discharge, primary and secondary diagnoses, diagnostic and therapeutic procedures, destination and situation at discharge, clinical and hospital history codes, and financing of care. This database reports more than 90% of admissions to both public and private acute care hospitals in Spain and is validated for data quality and overall methodology by the Spanish Ministry of Health.Citation27,Citation28
For each MBDS discharge record, we retrieved up to 41 diagnoses coded on the basis of the International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-9-CM, for cases collected between 2009 and 2015, and up to 45 diagnoses coded on the basis of the International Classification of Diseases, Tenth Revision, Clinical Modification, ICD-10-CM, for cases collected between 2016 and 2019. Records were grouped in oral cavity, pharynx, larynx, tonsils, base of tongue and other locations of oropharynx. For each record, the following variables were collected: diagnosis, sex, age, length of hospital stay, cost per hospital stay and outcome (discharge/death). Eligible malignant neoplasms of the head and neck are listed in .
Table 1. Definition of malignant neoplasms of the head and neck used according to the ICD-9-CM and ICD-10-CM codes.
Data analysis
The number of hospitalizations and deaths, examined by year, sex and age group, was expressed as absolute frequencies (n). The hospitalization rate, examined by anatomical site, year, sex and age group, and the death rate (defined as the ratio between in-hospital deaths and individuals in the Spanish population during the study period), examined by anatomical site, sex and age group, were expressed per 100,000 population and 95% confidence interval (CI). The fatality rate (defined as the number of deaths per inpatient population), examined by anatomical site, sex and age group, was expressed as percentage and 95% CI. Average hospital length of stay, examined by anatomical site and sex, was expressed as the median number of days (interquartile range, IQR). The cost per hospital stay (information included in the MBDS), examined by anatomical site and sex, was expressed in euros. We used the Chi-square test to assess significant differences in proportions, and analysis of variance (ANOVA) for multiple comparisons. To adjust statistical significance for multiple comparisons the post hoc Bonferroni correction was used. Poisson regression models were used to assess differences in the hospitalization and death rates between the study years and age groups. Corrected population data from municipal records, extracted from the Spanish National Institute of Statistics were used as the denominator for the hospitalization rates.Citation29 We assumed that the age distribution of the population registered in the MBDS was equivalent to the general Spanish population. In all tests, the level of significance was set at p < 0.05. For the statistical analysis, we used IBM SPSS Statistics for Windows, Version 19.0 (Armonk, NY: IBM Corp).
Results
Between 2009 and 2019, a total of 175,340 patients were hospitalized in Spain for malignant neoplasms of the head and neck. Of these, 84.7% were men. Among the major head and neck cancer sites, the most prevalent diagnosis in men was laryngeal cancer, which accounted for 50.9% of all hospitalizations, followed by pharyngeal cancer, 28.3% and oral cavity, 20.8%. In women, oral cavity cancer was the most prevalent malignancy, accounting for 49.1% of all hospitalizations, followed by pharyngeal cancer, 28.1% and laryngeal cancer, 22.9% (). The mean age at admission was 60.2 years (SD 12.2) for pharyngeal cancer (60.8 years [SD 11.5] in men and 57.0 years [SD 14.9] in women), 64.9 years (SD 10.7) for laryngeal cancer (65.2 years [SD 10.6] in men and 61.9 years [SD 12.0] in women), and 65.1 years (SD 13.2) for oral cavity cancer (64.0 years [SD 12.3] in men and 67.5 [SD 14.7] in women).
Table 2. Hospitalization rate, fatality rate, death rate, length of stay and direct cost associated with HNSCC in Spain by anatomical site and sex between 2009 and 2019.
Overall, the hospitalization rate for HNSCC during the study period was higher in men than in women. For laryngeal cancer, the mean annual hospitalization rate (per 100,000 population) was 29.96 (95% CI 29.75–30.17) in men and 2.36 (95% CI 2.3–2.42) in women. By age group, rates were highest among men aged 65–74 years and in women, among individuals aged 60–64 years. For pharyngeal cancer, hospitalization rates were 16.68 (95% CI 16.52–16.84) and 2.90 (95% CI 2.83–2.97), respectively, the highest rates being in men aged 60–69 years and in women aged 55–64 years. The corresponding rates for oral cavity cancer were 5.44 (95% CI 5.35–5.53) and 2.49 (2.43–2.55) per 100,000 population, respectively; in men, rates were highest in patients older than 60 years, while an age-dependent progressive increase, peaking at 8084 years, was observed in women ( and ).
Figure 1. Mean annual hospitalization rate (per 100,000 population) in men (A) and women (B) by anatomical site and age group in the general population of Spain between 2009 and 2019.
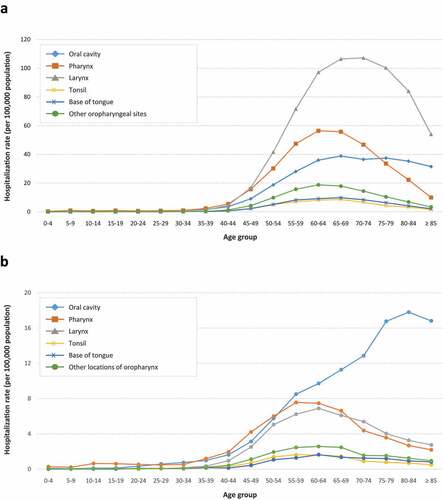
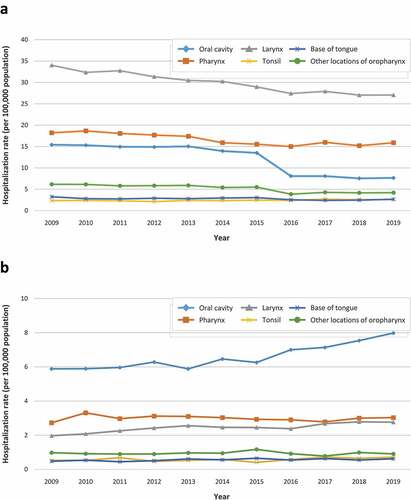
Among men, hospitalization rates showed a decreasing trend over the study period for all diagnoses except for tonsil cancer, while an increasing trend was observed in women. In men, the mean annual hospitalization rate (per 100,000 population) due to oral cavity cancer significantly decreased 2fold from 2009 to 2019 (15.41 vs. 7.66, p < 0.001); for cancers in other locations of oropharynx, rates decreased 1.5fold, from 6.15 to 4.19 (p < 0.001), and for laryngeal, pharyngeal and base of tongue cancers, rates decreased 1.3-fold (from 34.03 to 27.06 [p < 0.001]), 1.1-fold (from 18.19 to 15.89 [p < 0.001]), and 1.2-fold (from 3.25 to 2.65 [p = 0.018]), respectively. In contrast, hospitalization rates due to tonsil cancer increased 1.1-fold from 2.34 to 2.6 (p = 0.012). In women, hospitalization rates for oral cavity and laryngeal cancer increased significantly by 1.4 fold, from 5.88 to 7.98 and from 1.97 to 2.76, respectively (p < 0.001 for both comparisons); for base of tongue cancer, rates increased 1.3fold, from 0.48 to 0.62, (p = 0.027). Hospitalization rates for cancers of the pharynx, other locations of oropharynx and tonsils did not vary significantly throughout the study period ( and Supplemental Table S1).
Figure 2. Trend over time of mean annual hospitalization rate (per 100,000 population) in men (A) and women (B) by anatomical site in the general population in Spain between 2009 and 2019.
A total of 14,498 head and neck cancer-related in-hospital deaths were reported in Spain from 2009 to 2019, of which 87.4% occurred in men and 12.6% in women. Most deaths occurred in patients older than 35 years. Among men, 47.3% of all deaths were due to laryngeal cancer, with maximum figures recorded in patients aged 65–69 years, 40.6% due to pharyngeal cancer, with maximum figures recorded in patients aged 60–64 years, and only 12.2% due to oral cavity cancer, almost half of which occurred in men aged 55–69 years. In women, 43.2% of deaths were due to pharyngeal cancer, with maximum figures recorded in patients aged 55–59 years, 36.2% due to oral cavity cancer, 50% of which occurred in women older than 75 years, and 20.6% due to laryngeal cancer, more than half among patients aged 50–69 years ( and ).
Table 3. Death and fatality rates in men by anatomical location and age group in Spain from 2009 to 2019.
Table 4. Death and fatality rates in women by anatomical location and age group in Spain from 2009 to 2019.
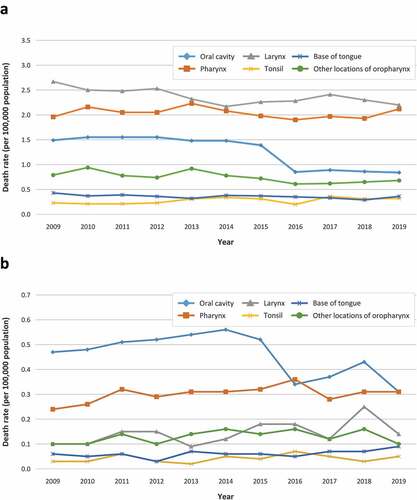
The estimated mean annual death rates (per 100,000 population) in men were 2.30 (95% CI 2.24–2.36) for laryngeal cancer, with the highest rates recorded in patients aged 75–79 years, 1.97 (95% CI 1.92–2.02) for pharyngeal cancer, with the highest rates recorded in patients aged 65–69 years, and 0.59 (95% CI 0.56–0.62) for oral cavity cancer, with the highest rates recorded in patients older than 80 years. Among women, the corresponding rates were 0.25 (95% CI 0.23–0.27) for oral cavity cancer, the highest rates being in patients older than 85 years, 0.30 (95% CI 0.28–0.32) for pharyngeal cancer, with the highest rates recorded in women aged 75–79 years, and 0.14 (95% CI 0.13–0.15) for laryngeal cancer, with the highest rates found in women older than 85 years ( and ). The annual death rate for oral cavity cancer decreased significantly during the study period both in men, from 1.49 in 2009 to 0.84 in 2019, and in women, from 0.47 to 0.31 (p < 0.001 for both sexes). In men, death rates due to laryngeal cancer and cancers in other locations of oropharynx also decreased significantly, from 2.67 to 2.20, and from 0.79 to 0.68, respectively, (p < 0.001 for both comparisons). In contrast, the corresponding rates for tonsil cancer increased significantly from 0.23 to 0.32 (p = 0.001) ().
Figure 3. Trend over time of mean annual death rate (per 100,000 population) in men (A) and women (B) by anatomical site in the general population in Spain between 2009 and 2019.
Fatality rates for pharyngeal cancer were estimated at 12.22% (95% CI 11.91–12.53) and 10.44% (95% CI 9.75–11.13) in men and women, respectively, 7.93% (95% CI 7.74–8.12) and 6.12% (95% CI 5.52–6.72) for laryngeal cancer, respectively, and 11.23% (95% CI 10.7–11.76) and 10.18% (95% CI 9.44–10.92) for oral cavity cancer, respectively (). In men, fatality rates showed a slight but significant increasing trend over the study period for all diagnoses except for base of tongue cancer: from 9.73% to 10.93% for oral cavity cancer (p = 0.037); from 10.83% to 13.36% for pharyngeal cancer (p < 0.0001); from 7.91% to 8.14% for laryngeal cancer (p = 0.015); from 9.87% to 12.25% for tonsil cancer (p = 0.019), and from 12.97% to 16.15% for cancers in other locations of oropharynx (p = 0.034). In women, no significant changes in the fatality rate were observed (Supplemental Table S2).
The median length of hospital stay for inpatients with pharyngeal cancer was 6 (IQR 10) days for men and 6 (IQR 8) days for women, with an estimated cost of 5,955 and 5,541 euros per patient, respectively, and a total cost of 29.2 million euros per year on average; for inpatients with laryngeal cancer, median length of stay were 6 (IQR 12) and 5 (IQR 12) days, with an estimated cost of 7,066 and 7,118 euros per patient, respectively, and a total cost of 57.8 million euros per year on average; for inpatients with oral cavity cancer, median length of stay were 7 (IQR 11) and 7 (IQR 10) days, with an estimated cost of 7,486 and 6,984 euros per patient, respectively, and a total cost of 14.8 million euros per year on average. The total cost of the hospitalizations for HNSCC during the 11 year study period was estimated to be around 101.8 million euros per year on average ().
Discussion
This retrospective nationwide study provides data on the burden of hospitalizations and mortality due to head and neck malignant neoplasms in the general population in Spain from 2009 to 2019. More than 175,000 admissions for laryngeal, pharyngeal and oral cavity cancer, and almost 15,000 related in-hospital deaths were recorded during the 11-year period analyzed, representing a substantial clinical and economic burden for both patients and the health care system. The mean age of patients at admission was 63 years, and around 85% of them were men, corresponding to an overall male to female incidence and death rate ratio of approximately 6:1. These figures are similar to those reported in other studies conducted in Spain and in other countries.Citation30–35
Epidemiologic studies on cancer over decades have shown considerable differences between male and female subpopulations in terms of cancer incidence and mortality, with higher rates in males relative to females for most cancer types.Citation3,Citation36 Cancers of the lip, larynx, hypopharynx, tonsil and oropharynx, in particular, have been reported to be among the 10 types of cancers with the largest male-to-female incidence rate ratio.Citation37 Male to female hospitalization and death rate ratios were particularly high for laryngeal (13:1 and 16:1) and hypopharyngeal cancer (12:1 and 14:1), while more modest ratios were found for oral cavity cancer (2:1 for both). Cancer development at these sites is strongly related to tobacco exposure and to excessive alcohol intake.Citation38 Although smoking prevalence in Spain has decreased significantly in the last decades, even more drastically in men than in women (−42% and −10%, respectively, from 1993 to 2017),Citation39–41 it remains high among the population aged 15 years or more (22%), and is still higher in men than in women, 26% and 19%, respectively. This sex disparity is even more pronounced when only heavy smokers are considered, 6% vs 3%, respectively.Citation39 Comparable figures have been reported across the 27 EU member countries, estimated at 18% (22% in men and 15% in women) and 6.0% (9% in men and 3% in women), respectively.Citation42 Regular alcohol consumption is also higher in men than in women, 49% vs 25%, respectively, as is binge drinking, at 12% vs 4%, respectively.Citation39 Alcohol consumption in the WHO European Region has decreased by about 11% in the last 25 years, particularly in the Mediterranean countries, which have recorded a decline of up to 34%Citation13. The greater prevalence of tobacco and alcohol consumption in men, together with the latency period between exposure and cancer development,Citation43,Citation44 results in the currently higher tobacco- and alcohol-related head and neck cancer incidence in men than in women.Citation45 A recently published systematic review of population-based studies representing 18 countries and spanning almost fifty years showed that, in men, incidence trends for non-HPV-related head and neck cancers declined in all countries except Taiwan and England, while in women, incidence trends increased in both HPV-related and non-HPV-related cancers in different geographical areas, especially in Asian and European countries.Citation46
Compared with previous data for the period 1997–2008, the sex disparity in HNSCC hospitalization and death rates in Spain during the period 20,092,019 has narrowed by half, with the male to female ratios dropping from 13:1 and 14:1 to 6:1 and 7:1, respectively.Citation31 The narrowing sex differential reflects the overall decreasing trend in the hospitalization and death rates from all the major anatomic sites in men, along with a parallel upward or stable trend in women. We found that between 2009 and 2019, hospitalization rates for all major anatomic sites decreased significantly in men, −50% for oral cavity, −20% for laryngeal, and −12% for pharyngeal cancer, as did the death rates for oral cavity (−44%) and laryngeal cancer (−18%). In women, conversely, the hospitalization rates for these two major anatomic sites increased significantly, by 36% for oral cavity and 40% for laryngeal cancer. However, the death rate from oral cavity cancer decreased by 34%, particularly in the last 5 years of the study period, with no significant changes in the other major anatomic sites. The observed trends in hospitalization and death rates are consistent with those reported in this same population for the period 1997–2008, with generalized decreases in both rates in men and increases in women.Citation31 Compared to the continuous upward trend in oral cancer death rate in women reported for the entire period 1997–2008, the abrupt change in the trend from the middle of the 2009–2019 period is striking. Although we cannot rule out the contribution of other potential causal relationships, e.g., clinical or epidemiological, it is likely that the transition from ICD-9-CM to ICD-10-CM coding, starting in 2016, has had an effect on the apparent trend in the hospitalization and death rates during the study period.Citation47–49
The significant increase in hospitalization and/or death rates observed in both men and women for tumors arising from squamous cells in the oropharynx (i.e., tonsil cancer and base of tongue cancer) suggest that other risk factors apart from smoking and alcohol consumption, are involved in the current epidemiological pattern of this subset of head and neck cancers. There is enough evidence of the etiological role of HPV infection (mainly HPV 16) in oropharyngeal cancer, particularly for tumors arising from the tonsil, base of tongue and other oropharyngeal sites.Citation50 In recent decades, an increase in the proportion of HPVpositive oropharyngeal cancers (i.e., tonsil and base of tongue cancers) was reported in several economically developed countries, most notably among white, male, middle-aged individuals who often do not have a history of tobacco or alcohol use.Citation14,Citation51–54 This growing trend might reflect changes in sexual behaviors relevant to oral HPV exposure (e.g., oral sex and multiple sex partners).Citation18,Citation19 Data on the prevalence of HPVrelated head and neck cancer vary widely depending on the geographic distribution and the subsites of the oropharyngeal tissues. The highest prevalence of HPV-positive oropharyngeal cancer has been reported for United States and Canada (50–60%),Citation55 while in Europe, it has been reported to range from 24% to 57% in southern and northern countries, respectively.Citation56,Citation57 In Spain, several studies have also reported an increase in the prevalence of HPV-positive oropharyngeal cancers in recent decades. Rodrigo et al. reported a nonsignificant but increasing prevalence from 1.8% in the period 1990–1999 to 6.1% in 2000–2009 in northern Spain.Citation58 Another retrospective cohort study showed an increasing risk of HPV-related oropharyngeal cancer from 1991 to 2016 among people in the north-eastern region of Spain (5-year period increase of 30%; 31% for tonsil cancer, and 66% for base of tongue cancer), which was highest in the last 5 years of the study (2012 to 2016).Citation59 Previous results from our group for the period 1997–2008 showed that the hospitalization and death rates for tonsil cancer in Spain still showed a favorable downward trend in men, while in women, the rates for base of tongue cancer already showed a significant upward trend.Citation31
Many studies have suggested that the increasing incidence of HPV-positive oropharyngeal cancers is found almost exclusively among middle-aged individuals, but recent evidence suggests that HPV-positive cancers in elderly patients may be more common in current clinical practice than previously believed.Citation60,Citation61 In our study, the mean age at admission of patients with tonsil cancer was 61 years (61 for men and 60 for women) and for those with base of tongue cancer, 62 years (62 for men and 63 for women). Compared with previous results for the period 1997–2008, the mean age at admission of patients with oropharyngeal cancer—potentially associated with HPV infection—, has increased by around 5% in men and 7% in women,Citation31 suggesting that the patient demographics of the disease may be changing, as previously observed in other studies.Citation62–64 Using novel age-period-cohort projection methods, Tota et al. predicted that oropharyngeal cancer incidence rates will probably continue to increase in the near future in the United States, primarily in older (≥65 years) white individuals.Citation61 Although HPVrelated oropharyngeal cancers are associated with a much better prognosis and survival than HPV-unrelated cancers (hazard ratios for overall survival and disease free survival, 0.7 and 0.5, respectively), preventive strategies in the near future will be of radical importance, especially considering the highrisk profile of older patients.Citation65 Prophylactic vaccination against HPV in both sexes holds great promise for reducing HPV-positive oropharyngeal cancers in the vaccine-eligible birth cohorts.Citation24 Although differences in the underlying molecular mechanisms are not expected, epidemiological differences such as age and gender distributions might suggest that the efficacy of the HPV vaccine shown in genital cancers may not be directly translatable to oropharyngeal cancer.Citation66 Promising evidence of the potential utility of HPV vaccination in the prevention of oral HPV infection has been published. A recent systematic review including 9 studies and almost 49,000 participants showed the significant and stable effectiveness of HPV vaccines on vaccine-type oral and oropharyngeal HPV infection, regardless of study design. The average relative prevention rate among all the studies was 83%. Furthermore, a significant percentage of participants developed IgG antibodies in oral fluids against vaccine-targeted HPV after vaccination, which could represent an alternative surrogate marker for vaccine effectiveness.Citation67 However, individuals who are currently experiencing an increasing incidence of oropharyngeal cancer (i.e., those born before 1970) are unlikely to benefit from vaccination in coming decades. According to the projections of a recently published population-based age-period-cohort study, the association of HPV vaccination with overall oropharyngeal cancer incidence through 2045 will remain modest (no vaccination vs vaccination: 14.3 vs 13.8 per 100,000 population in 2045), although reductions should occur among young and middle-aged adults.Citation68
In an important step forward in preventing HPV-related head and neck cancer, on 12 June 2020, the US Food and Drug Administration approved an expanded indication for the HPV 9-valent vaccine (Gardasil 9/Merck) to include the prevention of oropharyngeal and other cancers of the head and neck cancer caused by HPV, with the postmarketing requirement/commitment of demonstrating the efficacy of the vaccine in preventing oral persistent infection (i.e., > 6 months) with oncogenic HPV types in men aged 2045 years.Citation69,Citation70 The European Parliament insists that a gender-neutral and publicly-financed HPV vaccination programme be implemented in the Member States in order to ensure the elimination of all HPV-related cancers.Citation71,Citation72 In Spain, however, HPV vaccination has not yet been expanded to boys. The HPV 9-valent vaccine extends coverage for both genders.Citation73
Results from a recently published study showed that the cost of vaccinating a healthy individual throughout his entire life and in full compliance with national vaccination calendars in Spain ranges from 626 euros in men to 726 euros in women; the majority of this cost (67%) corresponds to vaccination during childhood and adolescence. In the case of people with underlying conditions, the cost ranges from 983 to 1,815 euros. The only difference in the vaccination schedule between men and women in this study was the HPV vaccine, which costs an estimated total of around 100 euros per healthy person for the 3 doses (29.16 euros per dose).Citation74 Comparable results have been reported for other Western European countries.Citation75
Our results clearly show the substantial economic burden associated with HNSCC, with a total direct cost estimated at around 100 million euros per year on average during the 11-year study period. Compared with previous data for the period 1997–2008, the average hospitalization length of stay throughout the study period decreased by about 20% in men, while it increased by 20% in women. However, the estimated mean cost per hospitalized patient slightly decreased by around 10% in both men (from 7,053 to 6275 euros) and women (from 6905 to 6212 euros), resulting in lower total health expenditure during the period.Citation31 The total direct costs of cancer in Spain have increased from 4.1 billion euros in 2009 (90 euros per capita)Citation76 to 5.2 billion euros in 2018 (112 euros per capita).Citation77 According to these estimates, the direct costs of HNSCC care would account for a small, but still considerable, proportion of the total cancer care expenditure in Spain. As in other countries such as the United States and France, direct and indirect costs in Spain contribute similarly to the overall societal cost of HNSCC, although direct costs are slightly higher than indirect costs, at around 55% and 45%, respectively.Citation26,Citation77 The economic impact of head and neck cancers is poorly documented and varies significantly according to national policies and healthcare strategies, making it very difficult to make reliable comparisons. However, our estimated average cost per patient of 6,692 euros is similar to that of other European countries such as France, which reported an estimated average cost per patient of 5,365 euros in 2007 (range 2,764 to 7,673 euros),Citation78 or the UK, where the estimated average cost per patient in the period between 2003 and 2009 was 4,287 GBP.Citation79 In north-eastern Italy, however, the average cost per patient in the period between 2007 and 2010 was somewhat higher, estimated at 10,092 euros.Citation80
Our study has several strengths. Its main advantage is derived from the use of the MBDS database, which, due to its large sample size, provides high statistical power and representativeness when analyzing clinical variables such as in-hospital mortality. Additionally, the MBDS is subject to a high-quality data audit process at the state level. Our study also has some limitations. The most important limitation of using the MBDS database is the potential undercoding of clinical variables, due, on the one hand, to the intrinsic characteristics of the system (limited number of coding for secondary diagnoses), and, on the other, to deficiencies in the preparation of hospital discharge reports (e.g., failure to include all diagnoses or procedures performed). Another important limitation is that some variables of interest for specific studies may be missing, such as the functional status of the patients, laboratory or treatment variables, or, in the case of this study, information on smoking or alcohol use or HPV infection. The MBDS encodes hospital admissions, so there can potentially be data redundancy for patients who have been hospitalized more than once during the period analyzed. This potential redundancy could affect the point estimates of the prevalence (overestimation) and lethality (underestimation) of the disease. However, due to the large number of records in the database, it is unlikely that a certain percentage of redundant data could alter the general trends in hospitalization and death rates in the long term. Despite this limitation, the MBDS is currently one of the most valuable tools available for clinical and epidemiological research. Head and neck cancer death rates were probably underestimated, since only inhospital deaths are recorded in the MBDS database. The MBDS database uses the coding scheme of the International Classification of Diseases. The transition from ICD-9-CM to ICD-10-CM coding, starting in 2016, probably had some impact on the epidemiological analysis, affecting the apparent hospitalization and mortality trends during the study period, although it is difficult to assess. A recent narrative literature review found significant gaps that limited the ability to draw reliable conclusions about the overall impact, positive or negative, of moving from ICD-9 to ICD-10 coding in the United States. It may be necessary to wait for longer-term epidemiological data to assess the true dimension of this transition.Citation81
In summary, our results show that, despite the slightly decreasing trend in the incidence and death rates of HNSCC in Spain in the last few decades, mainly due to the substantial reduction in the prevalence of smoking and alcohol use, HNSCC is still a clinical and economic burden on the Spanish health system. Although sex differences in hospitalization and death rates are gradually decreasing, trends in women remain stable or moderate compared to trends in men. On the other hand, the generalized increase in the incidence and death rates of head and neck cancer originating in anatomical sites more related to HPV infection, mainly oropharyngeal cancer, not only in young adults but also in older adults, is a cause for concern. In general, HNSCC survival has improved only modestly in the last decades. Prevention strategies should therefore be prioritized by discouraging smoking and alcohol use, reinforcing HPV vaccination programs, and improving early detection and treatment of asymptomatic disease to prevent disease progression.
Ethical considerations
All data included in this study were obtained as part of routine clinical activity and were evaluated retrospectively and anonymously, in strict compliance with the current Spanish and European legislation. For this reason, the Research Ethics Committee of the Rey Juan Carlos University ruled that no formal ethics approval was required for this study.
Acknowledgements
The authors thank the Subdirección General del Instituto de Información Sanitaria for providing the information on which this study is based and Noelia López-Malpartida for critically reading the manuscript and her comments and suggestions. The authors received medical writing support in the preparation of this manuscript from Luis F. García-Fernández, PhD.
Data availability statement
All datasets underlying the current study are available on the Spanish National Health System (CMBD) website at: https://www.mscbs.gob.es/en/estadEstudios/estadisticas/cmbdhome.htm. The information contained in this repository is in the public domain and can be accessed without the need for any administrative permissions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):1–14. doi:10.1056/NEJMra1715715.
- Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6(1):92. doi:10.1038/s41572-020-00224-3.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi:10.1158/1055-9965.epi-08-0347.
- Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287.
- Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:299–309.
- Gotz C, Bischof C, Wolff KD, Kolk A. Detection of HPV infection in head and neck cancers: promise and pitfalls in the last ten years: a meta-analysis. Mol Clin Oncol. 2019;10(1):17–28. doi:10.3892/mco.2018.1749.
- Wagner S, Sharma SJ, Wuerdemann N, Knuth J, Reder H, Wittekindt C, Klussmann JP. Human papillomavirus-related head and neck cancer. Oncol Res Treat. 2017;40(6):334–340. doi:10.1159/000477252.
- Faraji F, Eisele DW, Fakhry C. Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2017;2(1):10–18. doi:10.1002/lio2.37.
- Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519–525. doi:10.1001/jamaoto.2018.0395.
- Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg (1979). 2014;151(3):375–380. doi:10.1177/0194599814538605.
- World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2000–2025, Second edition. [accessed 2021 Dec 21]. https://www.who.int/tobacco/publications/surveillance/trends-tobacco-smoking-second-edition/en/
- Centre for Addiction and Mental Health. Public health gains and missed opportunities. Trends in alcohol consumption and attributable mortality in the WHO European Region, 1990-2014; 2016.
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi:10.1200/jco.2011.36.4596.
- Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35(5):747–755. doi:10.1002/hed.22015.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS, Bray F, Gillison ML. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi:10.1200/jco.2013.50.3870.
- Mahal BA, Catalano PJ, Haddad RI, Hanna GJ, Kass JI, Schoenfeld JD, Tishler RB, Margalit DN. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660–1667. doi:10.1158/1055-9965.epi-19-0038.
- Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang D, Haugen TH, Turek LP. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108(5):766–772. doi:10.1002/ijc.11633.
- D’-Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263–1269. doi:10.1086/597755.
- Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994–1001. doi:10.1634/theoncologist.2009-0289.
- Gogarty DS, Shuman A, O’-Sullivan EM, Sheahan P, Kinsella J, Timon C, O’-Neill JP. Conceiving a national head and neck cancer screening programme. J Laryngol Otol. 2015;130(1):8–14. doi:10.1017/S0022215115003084.
- Shuman AG, Entezami P, Chernin AS, Wallace NE, Taylor JM, Hogikyan ND. Demographics and efficacy of head and neck cancer screening. Otolaryngol Head Neck Surg (1979). 2010;143(3):353–360. doi:10.1016/j.otohns.2010.05.029.
- Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A review of HPV-related head and neck cancer. J Clin Med. 2018;7(9):241. doi:10.3390/jcm7090241.
- Guo T, Eisele DW, Fakhry C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer. 2016;122(15):2313–2323. doi:10.1002/cncr.29992.
- Lee JM, Turini M, Botteman MF, Stephens JM, Pashos CL. Economic burden of head and neck cancer. A literature review. Eur J Health Econ. 2004;5(1):70–80. doi:10.1007/s10198-003-0204-3.
- Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865–882. doi:10.1007/s40273-014-0169-3.
- Ministerio de Sanidad y Consumo. Agencia de Calidad del Sistema Nacional de Salud. Instituto de Información Sanitaria. Metodología de análisis de la hospitalización en el sistema nacional de salud. Modelo de indicadores basado en el registro de altas (CMBD). [accessed 2021 Nov 26]. https://www.mscbs.gob.es/estadEstudios/estadisticas/docs/metod_modelo_cmbd_pub.pdf
- Rodríguez Del Águila M, Perea-Milla E, Librero J, BuzónBarrera M, Rivas Ruiz F Atlas VPM Nº 4. Análisis del control de calidad del Conjunto Mínimo de Datos Básicos de Andalucía en los años 2000 a 2003. Sevilla. [accessed 2021 Nov 26]. https://www.atlasvpm.org/atlas/atlas-no4-variaciones-en-la-hospitalizacion-por-problemas-y-procedimientos-cardiovasculares-en-el-sistema-nacional-de-salud/
- Instituto Nacional de Estadística (INE). Demography and population. [accessed 2021 Nov 26]. https://ine.es/dyngs/INEbase/en/categoria.htm?c=Estadistica_P&cid=1254734710984
- de Souza DL, Bxernal-Pérez MMB, Curado MP. Predicted incidence of oral cavity, oropharyngeal, laryngeal, and hypopharyngeal cancer in Spain and implications for cancer control. Cancer Epidemiol. 2011;35(6):510–514. doi:10.1016/j.canep.2011.02.012.
- Gil-Prieto R, Viguera-Ester P, Álvaro-Meca A, San-Martín-Rodriguez M, Gil de Miguel Á. The burden of hospitalizations for head and neck neoplasm in Spain (1997-2008): an epidemiologic study. Hum Vaccin Immunother. 2012;8(6):788–798. doi:10.4161/hv.19819.
- Monteiro LS, Antunes L, Bento MJ, Warnakulasuriya S. Incidence rates and trends of lip, oral and oro-pharyngeal cancers in Portugal. J Oral Pathol Med. 2013;42(4):345–351. doi:10.1111/jop.12010.
- Schernberg A, Sagaon-Teyssier L, Schwarzinger M. Clinical and economic burden of head and neck cancer: a nationwide retrospective cohort study from France. Clinicoecon Outcomes Res. 2019;11:441–451. doi:10.2147/ceor.s198312.
- Boscolo-Rizzo P, Zorzi M, Del Mistro A, Da Mosto MC, Tirelli G, Buzzoni C, Rugge M, Polesel J, Guzzinati S, Group AW. The evolution of the epidemiological landscape of head and neck cancer in Italy: is there evidence for an increase in the incidence of potentially HPV-related carcinomas? PLoS One. 2018;13(2):e0192621. doi:10.1371/journal.pone.0192621.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654.
- Zhu Y, Shao X, Wang X, Liu L, Liang H. Sex disparities in cancer. Cancer Lett. 2019;466:35–38. doi:10.1016/j.canlet.2019.08.017.
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174–1182. doi:10.1158/1055-9965.EPI-08-1118.
- Lubin JH, Gaudet MM, Olshan AF, Kelsey K, Boffetta P, Brennan P, Castellsague X, Chen C, Curado MP, Dal Maso L, et al. Body mass index, cigarette smoking, and alcohol consumption and cancers of the oral cavity, pharynx, and larynx: modeling odds ratios in pooled case-control data. Am J Epidemiol. 2010;171(12):1250–1261. doi:10.1093/aje/kwq088.
- Ministerio de Sanidad CyBS. Informe Anual del Sistema Nacional de Salud 2019. [accessed 2021 Dec 20]. https://www.mscbs.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnualSNS2019/Informe_SNS_2019.pdf
- García-Mayor J, Moreno-Llamas A, De la Cruz-Sánchez E. Prevalencia de tabaquismo y hábitos de vida relacionados con la salud en función del uso del tabaco tras la implantación de la Ley 42/2010: análisis de encuestas de salud en España 2009-2017. Rev Esp Salud Pública. 2019;93:e201907042.
- GBD 2019. Tobacco collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. doi:10.1016/s0140-6736(21)01169-7.
- Eurostat. Daily smokers of cigarettes by sex, age and income quintile. [accessed 2021 Dec 21]. https://ec.europa.eu/eurostat/databrowser/view/hlth_ehis_sk3i/default/table?lang=en
- Altieri A, Bosetti C, Talamini R, Gallus S, Franceschi S, Levi F, Dal Maso L, Negri E, La Vecchia C. Cessation of smoking and drinking and the risk of laryngeal cancer. Br J Cancer. 2002;87(11):1227–1229. doi:10.1038/sj.bjc.6600638.
- Marron M, Boffetta P, Zhang ZF, Zaridze D, Wunsch-Filho V, Winn DM, Wei Q, Talamini R, Szeszenia-Dabrowska N, Sturgis EM, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182–196. doi:10.1093/ije/dyp291.
- Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4):e214708. doi:10.1001/jamanetworkopen.2021.4708.
- Menezes FDS, Fernandes GA, Antunes JLF, Villa LL, Toporcov TN. Global incidence trends in head and neck cancer for HPV-related and -unrelated subsites: a systematic review of population-based studies. Oral Oncol. 2021;115:105177. doi:10.1016/j.oraloncology.2020.105177.
- Sebastião YV, Metzger GA, Chisolm DJ, Xiang H, Cooper JN. Impact of ICD-9-CM to ICD-10-CM coding transition on trauma hospitalization trends among young adults in 12 states. Inj Epidemiol. 2021;8(1):4. doi:10.1186/s40621-021-00298-x.
- Hamedani AG, Blank L, Thibault DP, Willis AW. Impact of ICD-9 to ICD-10 coding transition on prevalence trends in neurology. Neurol Clin Pract. 2021;11(5):e612–e9. doi:10.1212/cpj.0000000000001046.
- Grief SN, Patel J, Kochendorfer KM, Green LA, Lussier YA, Li J, Burton M, Boyd AD. Simulation of ICD-9 to ICD-10-CM transition for family medicine: simple or convoluted? J Am Board Fam Med. 2016;29(1):29–36. doi:10.3122/jabfm.2016.01.150146.
- Marur S, D’-Souza G, Westra WH, Forastiere AA. HPV-Associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi:10.1016/s1470-2045(10)70017-6.
- Conway DI, Stockton DL, Warnakulasuriya KA, Ogden G, Macpherson LM. Incidence of oral and oropharyngeal cancer in United Kingdom (1990-1999) - recent trends and regional variation. Oral Oncol. 2006;42(6):586–592. doi:10.1016/j.oraloncology.2005.10.018.
- Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–366. doi:10.1002/ijc.24339.
- Rietbergen MM, Leemans CR, Bloemena E, Heideman DA, Braakhuis BJ, Hesselink AT, Witte BI, Baatenburg de Jong RJ, Meijer CJ, Snijders PJ, et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132(7):1565–1571. doi:10.1002/ijc.27821.
- Del Mistro A, Frayle H, Menegaldo A, Favaretto N, Gori S, Nicolai P, Spinato G, Romeo S, Tirelli G, da Mosto MC, et al. Age-Independent increasing prevalence of human papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci Rep. 2020;10(1):9320. doi:10.1038/s41598-020-66323-z.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi:10.1002/ijc.30716.
- Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA E6/E7 mRNA, and p16ink4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. doi:10.1016/s1470-2045(14)70471-1.
- Diz P, Meleti M, Diniz-Freitas M, Vescovi P, Warnakulasuriya S, Johnson NW, Kerr AR. Oral and pharyngeal cancer in Europe: Incidence, mortality and trends as presented to the Global Oral Cancer Forum. Transl Res Oral Oncol. 2017;2:2057178X17701517. doi:10.1177/2057178X17701517.
- Rodrigo JP, Heideman DA, García-Pedrero JM, Fresno MF, Brakenhoff RH, Díaz Molina JP, Snijders PJ, Hermsen MA. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990-2009). Int J Cancer. 2014;134(2):487–492. doi:10.1002/ijc.28355.
- Mena M, Frias-Gomez J, Taberna M, Quirós B, Marquez S, Clavero O, Baena A, Lloveras B, Alejo M, León X, et al. Epidemiology of human papillomavirus-related oropharyngeal cancer in a classically low-burden region of southern Europe. Sci Rep. 2020;10(1):13219. doi:10.1038/s41598-020-70118-7.
- Zumsteg ZS, Cook-Wiens G, Yoshida E, Shiao SL, Lee NY, Mita A, Jeon C, Goodman MT, Ho AS. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2(12):1617–1623. doi:10.1001/jamaoncol.2016.1804.
- Tota JE, Best AF, Zumsteg ZS, Gillison ML, Rosenberg PS, Chaturvedi AK. Evolution of the Oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538–1546. doi:10.1200/jco.19.00370.
- Thompson JD, Harari PM, Hartig GK. Is HPV-associated oropharyngeal cancer becoming more common in older patients? Laryngoscope Invest Otolaryngol. 2018;3(6):446–449. doi:10.1002/lio2.181.
- Rettig EM, Zaidi M, Faraji F, Eisele DW, El Asmar M, Fung N, D’-Souza G, Fakhry C. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: analysis of the National Cancer Database. Oral Oncol. 2018;83:147–153. doi:10.1016/j.oraloncology.2018.06.013.
- Cline BJ, Simpson MC, Gropler M, Bukatko AR, Adjei Boakye E, Mohammed KA, Osazuwa-Peters N. Change in age at diagnosis of oropharyngeal cancer in the United States, 1975–2016. Cancers (Basel). 2020;12(11):3191. doi:10.3390/cancers12113191.
- Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–1820. doi:10.1002/ijc.22851.
- Gillison M. HPV vaccines and potential prevention of HPV-positive head and neck cancer. In: Group I, editor. Primary end-points for prophylactic HPV vaccine trials. Lyon, France: IARC Working Group Reports, No. 7. IARC HPV Working Group; 2014.
- Nielsen KJ, Jakobsen KK, Jensen JS, Gronhoj C, Von Buchwald C. The effect of prophylactic HPV vaccines on oral and oropharyngeal HPV infection-a systematic review. Viruses. 2021;13(7):1339. doi:10.3390/v13071339.
- Zhang Y, Fakhry C, D’-Souza G. Projected association of human papillomavirus vaccination with Oropharynx cancer incidence in the US, 2020-2045. JAMA Oncol. 2021;7(10):e212907. doi:10.1001/jamaoncol.2021.2907.
- US Food and Drug Administration. Supplement accelerated approval. [accessed 2021 Dec 22]. https://www.fda.gov/media/138949/download
- Osazuwa-Peters N, Graboyes EM, Khariwala SS. Expanding indications for the human papillomavirus vaccine: one small step for the prevention of head and neck cancer, but one giant leap remains. JAMA Otolaryngol Head Neck Surg. 2020;146(12):1099–1101. doi:10.1001/jamaoto.2020.4068.
- Colzani E, Johansen K, Johnson H, Pastore Celentano L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: a moral dilemma. Euro Surveill. 2021;26(50). doi:10.2807/1560-7917.es.2021.26.50.2001659.
- Parliament E Report on strengthening Europe in the fight against cancer – towards a comprehensive and coordinated strategy (2020/2267(INI)). [accessed 2022 April 06]. https://www.europarl.europa.eu/doceo/document/A-9-2022-0001_EN.pdf
- Alvarez Garcia FJ, Cilleruelo Ortega MJ, Alvarez Aldean J, Garces-Sanchez M, Garcia Sanchez N, Garrote Llanos E, Hernandez Merino A, Iofrio de Arce A, Montesdeoca Melian A, Navarro Gomez ML, et al. Immunisation schedule of the Pediatric Spanish Association: 2021 recommendations. An Pediatr (Engl Ed). 2021;94(1): 53 e1- e10. doi:10.1016/j.anpedi.2020.10.002.
- Soler-Soneira M, Olmedo-Lucerón C, Sánchez-Cambronero Cejudo L, Cantero-Gudino E, Limia-Sánchez A. El Coste de vacunar a lo largo de toda la vida en España. Rev Esp Salud Publica. 2020 Internet;94(11):e1–12.
- Ethgen O, Cornier M, Chriv E, Baron-Papillon F. The cost of vaccination throughout life: a western European overview. Hum Vaccin Immunother. 2016;12(8):2029–2037. doi:10.1080/21645515.2016.1154649.
- Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–1174. doi:10.1016/s1470-2045(13)70442-x.
- Hofmarcher T, Lindgren P, Wilking N, Jonsson B. The cost of cancer in Europe 2018. Eur J Cancer. 2020;129:41–49. doi:10.1016/j.ejca.2020.01.011.
- St Guily JL, Borget I, Vainchtock A, Rémy V, Takizawa C. Head and neck cancers in France: an analysis of the hospital medical information system (PMSI) database. Head Neck Oncol. 2010;2:22. doi:10.1186/1758-3284-2-22.
- Kim K, Amonkar MM, Högberg D, Kasteng F. Economic burden of resected squamous cell carcinoma of the head and neck in an incident cohort of patients in the UK. Head Neck Oncol. 2011;3:47. doi:10.1186/1758-3284-3-47.
- Polesel J, Lupato V, Collarile P, Vaccher E, Fanetti G, Giacomarra V, Palazzari E, Furlan C, Matrone F, Navarria F, et al. Direct health-care cost of head and neck cancers: a population-based study in north-eastern Italy. Med Oncol. 2019;36(4):31. doi:10.1007/s12032-019-1256-2.
- Kusnoor SV, Blasingame MN, Williams AM, DesAutels SJ, Su J, Giuse NB. A narrative review of the impact of the transition to ICD-10 and ICD-10-CM/PCS. JAMIA Open. 2020;3(1):126–131. doi:10.1093/jamiaopen/ooz066.
