ABSTRACT
Vaccinations against Streptococcus pneumoniae are included in infant immunization programs globally. However, a substantial burden due to pneumococcal disease (PD) remains. This study aimed to estimate the cost of emergency department (ED) visits and hospitalizations associated with invasive pneumococcal disease, all-cause pneumonia, and acute otitis media in children <15 years of age in the Liguria region of Italy between 2012 and 2018. The retrospective cohort study used data from the Liguria Region Administrative Health Databases and the Ligurian Chronic Condition Data Warehouse, which contain information on hospital stays, outpatient visits, laboratory/imaging techniques, surgical procedures, and pharmaceutical prescriptions. Patients with one or more ED or inpatient claim for PD (based on International Classification of Diseases, Ninth Revision, Clinical Modification codes) were included. Cost of ED visits and hospitalizations were estimated from the diagnosis-related group system and procedures performed in the ED. In Ligurian children <15 years of age during 2012–2018, the median annual number of hospitalizations plus ED visits due to PD was 4,009, and the median estimated annual cost was €3.6 million. All-cause pneumonia accounted for the majority of hospitalization costs during the study period. Number and costs of ED visits and hospitalizations increased from 2012 to 2018. Despite widespread infant immunization in Liguria, economic costs due to PD-associated ED visits and hospitalizations remained high in children 0–14 years of age.
Introduction
Streptococcus pneumoniae can cause a range of diseases, including invasive pneumococcal disease (IPD), noninvasive pneumonia, and acute otitis media (AOM).Citation1 Pneumococcal conjugate vaccines (PCVs) are currently included in vaccination programs in 146 countries;Citation2 however, there remains a residual burden of disease. In Europe, the incidence of IPD in 2017 was 14.5 per 100,000 in children aged <1 year of age with case fatality rates as high as 3% in children <15 years of age.Citation3Citation4 Incidence of pneumococcal pneumonia in Europe was 187 per 100,000 children <5 years of age in 2015,Citation5 and the incidence of AOM was approximately 3,000 per 100,000 children <4 years of age in 2014.Citation6 Furthermore, although the overall incidence of IPD has decreased significantly since the introduction of childhood PCVs, the severity and persistence of IPD cases have increased.Citation7–9 In addition, vaccine coverage in some regions is suboptimal.Citation10 Consequently, the costs attributable to pneumococcal disease (PD) remain high.Citation5Citation11–13 In Europe, total healthcare costs associated with community-acquired pneumonia were estimated at €10.1 billion annually; one-third of these costs were related to indirect costs such as loss of working days.Citation13
The pneumococcal immunization recommendations in Italy vary regionally due to regional autonomy in the organization of health services.Citation14 In general, the 7-valent PCV (PCV7) first introduced in the early 2000’s was replaced by the 13-valent PCV (PCV13) in the early 2010’s.Citation14 Comparing incidence rates of PD before and after the introduction of a new PCV has suggested that PCVs can reduce the burden of PD in Italian children. For example, in North-east Italy, annual pneumonia-associated hospitalization rates in children ≤4 years of age decreased significantly from 379.7 per 100,000 in 2004 to 211.9 per 100,000 in 2013.Citation15 In the Veneto region of Italy, the mean annual incidence rate of IPD and syndromic invasive disease following the introduction of PCV13 was reduced from 40.1 episodes per 100,000 person-years in 2010 to 31.2 episodes per 100,000 person-years in 2017, although this decrease was not significant.Citation16 Despite this, PD continues to cause substantial economic burden on the health system in Italy. In the Veneto region of Italy, the total regional expenditure in 2017 was €3.59 million due to all-cause pneumonia and €2.76 million due to AOM in children <15 years of age.Citation17,Citation18
New vaccines recently approved in adults are currently being investigated in the pediatric population.Citation19–21 These vaccines contain all serotypes in the currently licensed PCV13 and additional serotypes.Citation22,Citation23 To better understand the potential value of new vaccines in a given region of Italy, it is important to quantify the current burden of PD following the introduction of PCV13, but prior to the introduction of new PCVs in that region. Liguria is an administrative region of Italy with a pediatric population (<15 years of age) of approximately 170,000 children.Citation24–27 A large-scale infant vaccination program against S. pneumoniae with PCV7 was introduced in Liguria in 2003 and replaced by PCV13 in 2013.Citation24,Citation28 Infants receive three doses, at ages 3, 5, and 11 months, with vaccine uptake in Liguria being more than 90% since 2007.Citation24,Citation29 This retrospective cohort analysis estimated the number and cost of hospitalizations and emergency department (ED) visits associated with PD in children <15 years of age in the Liguria region from 2012 to 2018.
Methods
Study design and population
This was a retrospective observational cohort analysis assessing the economic burden of IPD and syndromic invasive disease, all-cause pneumonia (including pneumococcal pneumonia), and AOM in children <15 years of age in Liguria, from October 2012 to September 2018.
Patients with one or more inpatient or ED claim for PD (as per International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis codes) were identified during the study period, using the codes described in Supplementary Table S1. A gap of 30 days between two inpatient or ED visits defined a new episode for all indications, with 6 months as the follow-up period for prescription drugs.
Data were collated for pneumococcal-specific ICD-9-CM codes (pneumococcal meningitis, pneumococcal bacteremia, and pneumococcal pneumonia) and for ICD codes for related diseases for which S. pneumoniae could be a potential causative pathogen (unspecified meningitis, unspecified bacteremia, all-cause pneumonia, and AOM). The former provides specific estimates with high certainty that episodes were caused by S. pneumoniae, and the latter provides more sensitive estimates that may capture additional pneumococcal episodes but may also include episodes caused by other pathogens.
Data sources
The study was conducted using data from the Liguria Regional Administrative Health Databases and the Ligurian Chronic Condition Data Warehouse (CCDWH). The administrative databases contain information on ED visits, outpatient visits, outpatient pharmaceutical dispensing records, and hospitalizations for the 1.6 million inhabitants in Liguria, such as the date of visit or admission, diagnoses, procedures, length of stay, discharge date, and discharge status (i.e., died or discharged alive). The CCDWH integrates four administrative healthcare data flows belonging to the regional health service and uses multiple Medicare data sources (hospital dispensing records, discharge diagnoses, medical fee exemptions, outpatient visits, and laboratory/imaging procedures) within a specified period using a predefined algorithm based on the codes assigned to specific diagnoses and procedures. These patient-level records are used to identify any comorbidities at the time of an ED visit or hospital admission. A record-linkage system then uses the unique personal identifiers to allow connections to be made across the two data sources described above. All data are archived in a relational database management system by means of big-data logic.
Costs of pneumococcal and related diseases
Costs of hospitalizations were based on diagnosis-related group (DRG) tariffs for hospital admissions.Citation30 Cost estimates for hospitalizations and ED visits, including admission for short-term observation and procedures performed in the ED, were estimated from the perspective of the government as the payer. Costs related to ED visits leading to hospitalization were estimated based on the DRG system only, including costs of prescriptions during the 6-month follow-up period.
Statistical analyses
The number and costs of ED visits and hospitalization associated with each manifestation were estimated for children <15 years of age overall, and then stratified by age groups (0–4 and 5–14 years) for the entire study period, as well as for each year. For each outcome, the 95% confidence interval (CI) range was estimated using the z-value of normal distribution method. Continuous variables were summarized as means and standard deviations (SDs), and categorical variables as frequency distributions and 95% CIs for patient demographics. Lastly, the per capita costs of ED visits (cost of cases each year in relation to the resident population of the same age group) were estimated for the entire observation period. Data were analyzed using the JMP software (Version 13.0.0; SAS Institute, Cary, NC, USA). Medians were used to evaluate economic data. The data provided in this study are descriptive in nature and no formal comparisons were made.
Results
Patient demographics
From 2012 to 2018, there were 9,750 episodes of all-cause pneumonia (including 287 episodes of pneumococcal pneumonia), 17,040 episodes of AOM, 695 episodes of bacteremia (629 pneumococcal and 66 unspecified), and 11 episodes of meningitis (five pneumococcal and six unspecified) in children <15 years of age in Liguria ().
Table 1. Patient demographics.
In total, 54.32% (95% CI: 53.74–54.91) of patients were male, and the mean (SD) age was 5.01 (3.55) years. In this study’s population, 11.51% (95% CI: 11.13–11.88) of children in total had comorbidities, including chronic respiratory disease in 7.54% (95% CI: 7.23–7.85), cardiovascular disease in 2.38% (95% CI: 2.20–2.56), neoplasia in 0.56% (95% CI: 0.47–0.65), diabetes in 0.10% (95% CI: 0.06–0.14), and renal failure in 0.07% (95% CI: 0.04–0.10) (). These characteristics were representative of those of the general population of children <15 years of age in Liguria during 2012–2018, with a total prevalence of comorbidities of 6.90% (95% CI: 6.78–7.02). Prevalence of specific comorbidities were as follows: chronic respiratory disease in 3.50% (95% CI: 3.41–3.58), cardiovascular disease in 1.29% (95% CI: 1.23–1.34), neoplasia in 0.30% (95% CI: 0.27–0.32), diabetes in 0.13% (95% CI: 0.11–0.14), and renal failure in 0.03% (95% CI: 0.02–0.04).
Total number and costs of ED visits and hospitalizations
The total annual numbers and costs of ED visits and hospitalizations for IPD and syndromic invasive disease, all-cause pneumonia, and AOM combined in children <15 years of age are shown in . The total median annual cost of ED visits and hospitalizations over the study period was €3,592,848, with ED visits followed by hospitalizations accounting for 97% of these costs, the remainder being ED visits that did not lead to hospitalization. The median cost per episode was €891 and the median cost per capita was €21.05. Both the total annual number of episodes and the associated costs appeared to remain stable between 2012 and 2016, showing a sudden drop trend in number of events and costs between 2017 and 2018, in particular according to the upward trend of the regional vaccine coverages at 24, 36 and 48 months ().
Figure 1. Total ED visits/hospitalization costs and vaccination coverage in Liguria at 24, 36 and 48 months of age.
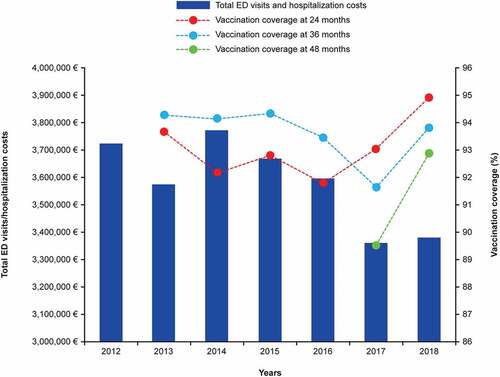
Table 2. Healthcare resource utilization and costs for invasive pneumococcal disease and syndromic invasive disease, all-cause pneumonia, and acute otitis media, in children <15 years of age in the Liguria region 2012–2018.
Number and costs of ED visits and hospitalizations for pneumonia, bacteremia, and AOM
The number and costs of ED visits and hospitalizations are shown separately for pneumonia, bacteremia, and AOM in .
Table 3. Healthcare resource utilization and costs for pneumonia, for children aged <15 years in the Liguria region 2012–2018.
Table 4. Healthcare resource utilization and costs for bacteremia, for children aged <15 years in the Liguria region 2012–2018.
Table 5. Healthcare resource utilization and costs for acute otitis media, for children aged <15 years in the Liguria region 2012–2018.
All-cause pneumonia accounted for most hospitalization costs (72.7%). The median annual cost of ED visits and hospitalizations associated with all-cause pneumonia was €2.61 million (). Annual total costs of ED visits and hospitalizations due to all-cause pneumonia fluctuated over time, with a substantial burden remaining in 2018 of €2,485,962. However, costs due to pneumococcal pneumonia showed a decreasing trend from €194,813 in 2012 to €73,111 in 2018.
The number of cases and costs of pneumococcal-specific bacteremia peaked at 119 and €580,673 in 2015 and then declined to 60 and €342,270 in 2018, respectively. The number of cases and costs of unspecified bacteremia remained stable over time, with cases ranging from 6 in 2015 and 2018 to 12 in 2012, 2013, and 2017, and costs ranging from €21,500 in 2015 to €52,583 in 2012 (). Throughout the study period, only five hospitalizations attributable to pneumococcal-specific meningitis (occurring in 2012 and 2013), and only six hospitalizations attributable to unspecified meningitis (in 2013, 2014, and 2018) were reported. Bacteremia and meningitis accounted for 11.3% and 0.25% of overall hospitalization costs, respectively (data not shown).
AOM accounted for most episodes of PD overall (61.6%, ); however, more than 90% of AOM episodes were ED visits without hospitalization (), and AOM accounted for only 9% of hospital costs. Annual numbers and costs of AOM episodes fluctuated and remained substantial throughout the study period, with the number of cases ranging from 2,252 in 2014 to 2,593 in 2018, and costs ranging from €274,896 in 2016 to €376,694 in 2013.
Prescriptions during follow-up period
The numbers and costs of prescriptions and days of drug therapy during the 6-month follow-up period after ED visits or hospitalizations from 2012 to 2018 are shown in . The median annual number of prescriptions in the study period was 3,469, with a median annual cost of €199,810, including €42,320 for antibacterial agents. Number of prescriptions, days of therapy, and costs of prescriptions for antimicrobials and antibacterial agents remained constant during the study period. Numbers of prescriptions ranged from 3,228 in 2016 to 3,730 in 2014, and days of therapy ranged from 102,792 in 2016 to 137,212 in 2014. Costs of antimicrobials ranged from €67,891 in 2016 to €103,372 in 2015, and of antibacterial agents from €64,457 in 2016 to €92,906 in 2014.
Table 6. Pharmaceutical prescriptions (days of drug therapy), antibiotic prescriptions, and cost for children aged <15 years in the Liguria region 2012–2018.
Discussion
This is the first study describing the post-PCV13 economic burden of PD in children in the Liguria region of Italy. Overall, the study population was representative of the general population of children <15 years of age in Liguria during 2012–2018. In this study, we reported median costs rather than mean costs to reduce the influence of outliers from skewed distributions. All-cause pneumonia accounted for the majority of the total costs of ED visits and hospitalization, with a median cost of €2.61 million over the study period. AOM accounted for 90% of episodes recorded; however, it only accounted for 9% of overall hospital costs. There were very few cases of bacteremia or meningitis (pneumococcal or unspecified); however, as the cost per episode was high, these diagnoses led to high hospitalization costs. In addition, there was a median number of 3,469 prescriptions attributable to all-cause pneumonia, IPD, syndromic invasive disease, or AOM annually, with a median annual cost of €195,206. Overall, there remained a substantial economic burden of ED visits and hospitalizations associated with pneumococcal diseases in children aged <15 years in the Liguria region of Italy, despite a well-established infant pneumococcal vaccination program.
The results of the study showed a gradual decrease in the number of ED access/hospitalizations and costs for pneumococcal disease in children 0–15 years in Liguria; this could be linked to increased immunization coverage.
From 2012 to 2018, the number of pneumococcal pneumonia cases decreased, while the number of all-cause pneumonia cases fluctuated but remained relatively constant or even increased over time. These findings are consistent with other studies. A persisting burden of all-cause pneumonia has been documented in Veneto,Citation17 other regions in Italy,Citation15 elsewhere in Europe,Citation31,Citation32 and worldwide.Citation5
There are currently limited data on the economic burden of pneumococcal diseases for comparison with our estimates. In Spain, mean annual costs of hospitalization due to PD for all patients registered in a hospital discharge database were €104.2 million; 50% of these costs were associated with pneumonia, a smaller proportion than in the current study.Citation33 In the Veneto region of Italy, total regional expenditure due to AOM in children aged <15 years was €2.76 million in 2017.Citation18 In addition, the cost of PD was found to be especially high in children with comorbidities in the United States.Citation34,Citation35
Notably, cost-effectiveness analyses represent the majority of evidence on resource use, costs for PD, and associated interventions in Italy.Citation36 Therefore, our study fills a knowledge gap in this research field. Moreover, CCDWH, the data source used for this study, has been used effectively in a previous study of our working group.Citation37 There are several study limitations. First, this is a database study that utilized ICD codes to identify pneumococcal diseases. Clinical laboratory results that could provide information on the pathogens causing disease were not available. We addressed this limitation by including both pneumococcal-specific ICD codes and ICD codes for related diseases for which S. pneumoniae could be a potential causative pathogen. Consequently, the study provides two estimates of cost: one which is highly specific, with a high degree of certainty that episodes included in the analysis were caused by S. pneumoniae, and one that is more sensitive, with the potential to capture additional undetected pneumococcal episodes, but also episodes that may be caused by other pathogens. Second, non-medical costs and indirect medical costs, for example the caregiver’s time, transportation, and loss of earnings, were not accounted for in this study.
Conclusions
In Liguria, there remained a significant economic burden of ED visits and hospitalization associated with pneumococcal diseases in children <15 years of age. The majority of costs were due to pneumonia. The impact of future PCVs on the burden of PD will depend on the proportion of diseases caused by S. pneumoniae and vaccine-type serotypes.
Author contributions
Conceptualization: Filippo Ansaldi, Francesca Senese, Gian Marco Prandi, Giancarlo Icardi, Tanaz Petigara, and Tianyan Hu; methodology: Matteo Astengo, Camilla Sticchi, Filippo Ansaldi, and Andrea Orsi; validation: Chiara Paganino, Federica Varlese, and Filippo Ansaldi; formal analysis: Matteo Astengo, Chiara Paganino, Daniela Amicizia, and Maria Francesca Piazza; data curation: Daniela Amicizia, Camilla Sticchi, Maria Francesca Piazza, and Andrea Orsi; writing, review, and editing: Francesca Senese, Gian Marco Prandi, Filippo Ansaldi, Chiara Paganino, Daniela Amicizia, Matteo Astengo, Maria Francesca Piazza, Andrea Orsi, Federica Varlese, Tianyan Hu, Tanaz Petigara, Camilla Sticchi, and Giancarlo Icardi; supervision: Giancarlo Icardi and Filippo Ansaldi.
All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (65.2 KB)Acknowledgements
Medical writing support, including assisting authors with the development of the outline and drafts, and incorporation of comments, was provided by Fiona Van, PhD, and Sarah Amir, PhD; editorial support, including fact checking, referencing, formatting, proofreading, and submission was provided by Ian Norton, PhD; all of Scion, London, UK, supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, according to Good Publication Practice guidelines (Link). The sponsor was involved in the study design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Disclosure statement
Tianyan Hu and Tanaz Petigara are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Francesca Senese and Gian Marco Prandi are employees of MSD, Rome, Italy; all may own stock in Merck & Co., Inc., Rahway, NJ, USA. Giancarlo Icardi and Andrea Orsi declare financial relationships with GSK, Janssen, MSD, Pfizer, Sanofi, and Seqirus. Filipo Ansaldi declares funding support from MSD. All other authors declare no conflicts of interests.
Data availability statement
The data used in this study cannot be made available in the manuscript, the supplemental files are in a public repository due to Italian data protection laws. The anonymized datasets generated during and/or analyzed during the current study can be provided on reasonable request, from the corresponding author, Daniela Amicizia ([email protected]).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2082205.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Pneumococcal disease - Types of infection. [accessed 2021 Sep 22]. https://www.cdc.gov/pneumococcal/about/infection-types.html.
- International Vaccine Access Center (IVAC). Johns Hopkins Bloomberg School of Public Health. VIEW-hub report: global vaccine introduction and implementation. [accessed 2021 Sep 22]. https://view-hub.org/sites/default/files/2020-08/VIEW-hub_Report_Jun2020_1.pdf.
- Georgalis L, Mozalevskis A, Martinez de Aragon MV, Garrido-Estepa M. Changes in the pneumococcal disease-related hospitalisations in Spain after the replacement of 7-valent by 13-valent conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2017;36(3):1–8. doi:10.1007/s10096-016-2834-2.
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease - annual epidemiological report for 2017. [accessed 2019 Oct 4]. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-pneumococcal-disease.pdf.
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Luksic I, Nair H, McAllister DA, Campbell H, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e757. doi:10.1016/S2214-109X(18)30247-X.
- Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36(11):1027–1031. doi:10.1097/INF.0000000000001654.
- Ricketson LJ, Conradi NG, Vanderkooi OG, Kellner JD. Changes in the nature and severity of invasive pneumococcal disease in children before and after the seven-valent and thirteen-valent pneumococcal conjugate vaccine programs in Calgary, Canada. Pediatr Infect Dis J. 2018;37(1):22–27. doi:10.1097/INF.0000000000001709.
- Syrogiannopoulos GA, Michoula AN, Tsimitselis G, Vassiou K, Chryssanthopoulou DC, Grivea IN. Pneumonia with empyema among children in the first five years of high coverage with 13-valent pneumococcal conjugate vaccine. Infect Dis (Lond). 2016;48(10):749–753. doi:10.1080/23744235.2016.1192720.
- Oligbu G, Collins S, Andrews N, Sheppard CL, Fry NK, Slack MPE, Borrow R, Ladhani SN. Characteristics and serotype distribution of childhood cases of invasive pneumococcal disease following pneumococcal conjugate vaccination in England and Wales, 2006–2014. Clin Infect Dis. 2017;65(7):1191–1198. doi:10.1093/cid/cix418.
- Moreira M, Castro O, Palmieri M, Efklidou S, Castagna S, Hoet B. A reflection on invasive pneumococcal disease and pneumococcal conjugate vaccination coverage in children in Southern Europe (2009–2016). Hum Vaccin Immunother. 2017;13(6):1–12. doi:10.1080/21645515.2016.1263409.
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–3412. doi:10.1016/j.vaccine.2011.02.088.
- Ceyhan M, Ozsurekci Y, Aykac K, Hacibedel B, Ozbilgili E. Economic burden of pneumococcal infections in children under 5 years of age. Hum Vaccin Immunother. 2018;14(1):106–110. doi:10.1080/21645515.2017.1371378.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2013;20(Suppl 5):45–51. doi:10.1111/1469-0691.12461.
- Monali R, De Vita E, Mariottini F, Privitera G, Lopalco PL, Tavoschi L. Impact of vaccination on invasive pneumococcal disease in Italy 2007-2017: surveillance challenges and epidemiological changes. Epidemiol Infect. 2020;148:e187. doi:10.1017/S0950268820001077.
- Baldo V, Cocchio S, Gallo T, Furlan P, Clagnan E, Del Zotto S, Saia M, Bertoncello C, Buja A, Baldovin T. Impact of pneumococcal conjugate vaccination: a retrospective study of hospitalization for pneumonia in North-East Italy. J Prev Med Hyg. 2016;57:E61–68.
- Barbieri E, Porcu G, Cantarutti A, Hu T, Petigara T, Prandi G, Scamarcia A, Giaquinto C, Cantarutti L. A retrospective database analysis to estimate the burden of invasive pneumococcal disease and unspecified invasive disease in children <15 years in the Veneto region (Italy). International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); 2020; Virtual.
- Barbieri E, Porcu G, Cantarutti A, Hu T, Petigara T, Prandi G, Scamarcia A, Giaquinto C, Cantarutti L. A retrospective database analysis to estimate the burden of pneumonia in children <15 years in the Veneto Region (Italy). Poster presented at the 12th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); 2020; Virtual.
- Barbieri E, Porcu G, Cantarutti A, Hu T, Petigara T, Alimenti C, Prandi G, Scamarcia A, Giaquinto C, Cantarutti L. A retrospective database analysis to estimate the burden of acute otitis media in children <15 years in the Veneto region, Italy. European Society for Paediatric Infectious Diseases (ESPID) Annual Meeting; 2020; Virtual.
- Food and Drug Administration. VAXNEUVANCE™ (Pneumococcal 15-valent conjugate vaccine) prescribing information. [accessed 2021 July 21]. https://www.fda.gov/media/150819/download.
- Food and Drug Administration. PREVNAR 20 (Pneumococcal 20-valent conjugate vaccine) prescribing information. [accessed 2021 July 26]. https://www.fda.gov/media/149987/download.
- Senders S, Klein NP, Lamberth E, Thompson A, Drozd J, Trammel J, Peng Y, Giardina P, Jansen KU, Gruber WC, et al. Safety and immunogenicity of a 20-valent Pneumococcal Conjugate Vaccine (PCV20) in healthy infants in the United States. Open Forum Infect Dis. 2020;7:S637. doi:10.1093/ofid/ofaa439.1421.
- Rupp R, Hurley D, Grayson S, Li J, Nolan K, McFetridge RD, Hartzel J, Abeygunawardana C, Winters M, Pujar H, et al. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother. 2019;15(3):549–559. doi:10.1080/21645515.2019.1568159.
- Hurley D, Griffin C, Young M, Scott DA, Pride MW, Scully IL, Ginis J, Severs J, Jansen KU, Gruber WC, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2021;73(7):e1489–e1497. doi:10.1093/cid/ciaa1045.
- Durando P, Crovari P, Ansaldi F, Sticchi L, Sticchi C, Turello V, Marensi L, Giacchino R, Timitilli A, Carloni R, et al., Collaborative Group for Pneumococcal Vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine. 2009;27(25–26):3459–3462. doi:10.1016/j.vaccine.2009.01.052.
- Ansaldi F, de Florentis D, Canepa P, Bandettini R, Diana MC, Martini M, Durando P, Icardi G. Epidemiological changes after PCV7 implementation in Italy: perspective for new vaccines. Hum Vaccin. 2011;7(sup1):211–216. doi:10.4161/hv.7.0.14602.
- Ansaldi F, Sticchi L, Durando P, Carloni R, Oreste P, Vercelli M, Crovari P, Icardi G. Decline in pneumonia and acute otitis media after the introduction of childhood pneumococcal vaccination in Liguria, Italy. J Int Med Res. 2008;36(6):1255–1260. doi:10.1177/147323000803600612.
- Orsi A, Ansaldi F, Trucchi C, Rosselli R, Icardi G. Pneumococcus and the elderly in Italy: a summary of available evidence regarding carriage, clinical burden of lower respiratory tract infections and on-field effectiveness of PCV13 vaccination. Int J Mol Sci. 2016;17(7):1140. doi:10.3390/ijms17071140.
- Orsi A, Ansaldi F, Durando P, Turello V, Icardi G. Gruppo di studio ligure sullo pneumococco. [Immunization campaign with 13-valent pneumococcal conjugate vaccine in adults in Liguria Region, Italy: one year post-introduction preliminary results]. Epidemiol Prev. 2014;38:66–72.
- Ministero della Salute. DGPRV 0024720-P-27/05/2010. Indicazioni in merito alla somministrazione del vaccino antipneumococcico Prevenar 13 in età pediatrica. [accessed 2021 Sep 22]. https://www.fimpcalabria.org/public/vaccinazioni/indicazioni%20in%20merito%20alla%20somministrazione%20del%20vaccino%20antipneumococcico%20prevenar%2013%20in%20et%C3%A0%20pediatrica%20(2).pdf.
- Ministero della Salute. Supplemento ordinario n. 8 alla GAZZETTA UFFICIALE 28.01.2013 - Serie generale - n. 23. [accessed 2021 Sep 22]. https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=28/01/2013&redaz=13A00528&artp=1&art=1&subart=1&subart1=10&vers=1&prog=001.
- Lau WCY, Bielicki J, Tersigni C, Saxena S, Wong ICK, Sharland M, Hsia Y. All-Cause pneumonia in children after the introduction of pneumococcal vaccines in the United Kingdom: a population-based study. Pharmacoepidemiol Drug Saf. 2019;28(6):821–829. doi:10.1002/pds.4770.
- Nair H, Watts AT, Williams LJ, Omer SB, Simpson CR, Willocks LJ, Cameron JC, Campbell H. Pneumonia hospitalisations in Scotland following the introduction of pneumococcal conjugate vaccination in young children. BMC Infect Dis. 2016;16(1):390. doi:10.1186/s12879-016-1693-x.
- Darba J, Marsa A. Hospital incidence, in-hospital mortality and medical costs of pneumococcal disease in Spain (2008–2017): a retrospective multicentre study. Curr Med Res Opin. 2021;37(3):523–530. doi:10.1080/03007995.2021.1876007.
- Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16(1):182. doi:10.1186/s12913-016-1432-4.
- Wilson KM, Torok MR, Localio R, McLeod L, Srivastava R, Luan X, Mohamad Z, Shah SS, Pediatric Research in Inpatient Settings N. Hospitalization for community-acquired pneumonia in children: effect of an asthma codiagnosis. Hosp Pediatr. 2015;5(8):415–422. doi:10.1542/hpeds.2015-0007.
- Shiri T, Khan K, Keaney K, Mukherjee G, McCarthy ND, Petrou S. Pneumococcal disease: a systematic review of health utilities, resource use, costs, and economic evaluations of interventions. Value Health. 2019;22(11):1329–1344. doi:10.1016/j.jval.2019.06.011.
- Trucchi C, Paganino C, Orsi A, Amicizia D, Tisa V, Piazza MF, Gallo D, Simonetti S, Buonopane B, Icardi G, et al. Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults ≥50 years-old. BMC Health Serv Res. 2019;19(1):585. doi:10.1186/s12913-019-4412-7.
