ABSTRACT
Vaccine-related immune responses are one of the causes of encephalitis. Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, COVID-19) have been administered worldwide due to the ongoing global pandemic; cases of SARS-CoV-2 vaccination-related encephalitis were scarcely reported. An 82-year-old female was diagnosed with acute encephalitis following her first dose of vaccination with mRNA-1273 against SARS-CoV-2. The patient presented with fever and headache five days after vaccination, followed by behavior change 17 days after vaccination. Electroencephalographic recordings revealed focal slow waves in the right frontoparietal regions. Brain MRI revealed the signal change in the right middle and posterior temporal lobe. Cerebrospinal fluid analysis showed mildly elevated protein. She responded well to steroid pulse therapy and made a full recovery. The severity of the immune response following COVID-19 vaccination may be alleviated if adequate treatment is achieved. Physicians must be alert for encephalitis after vaccination to help ensure a favorable outcome.
Introduction
Acute encephalitis is generally caused by an infection. Viral encephalitis and autoimmune encephalitis contribute to approximately three-quarters of diagnosed cases.Citation1 Other causes include various pathogens such as bacteria, fungi, rickettsiae, protozoa, and mycobacterial infections. Additionally, toxicity-related and post-infectious/immunization responses may precede this type of infection.Citation2 Diagnosis of encephalitis requires symptoms/signs including altered mental status for a least 24 hours associated with two or more of the following items: fever within 72 hours before or after the clinical presentation, seizures not attributable to a preexisting seizure disorder, new onset of focal neurological deficits, CSF pleocytosis, neuroimaging revealed new abnormalities and/or characterized electroencephalography (EEG) abnormalities.Citation1
Currently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are causing a global pandemic, and the prevalence of neurological complications of coronavirus disease 19 (COVID-19), such as COVID-19–associated encephalitis, is rising.Citation3–5 To combat the prevalence of SARS-CoV-2 infections, innovative mRNA vaccines have been developed, for which the efficacy and safety for preventing illness have been established.Citation6,Citation7 However, vaccination-associated serious adverse events such as myelitis and acute disseminated encephalomyelitis have been described but not well reported.Citation8 There has been a trend toward increased risk of rare neurological complications of Guillain–Barré syndrome and Bell’s palsy after the first dose of ChAdOx1nCoV-19 vaccines (Oxford-AstraZeneca) but not in mRNA COVID-19 vaccines.Citation9,Citation10
Recently, the major neurological complication subsequent to the first dose of mRNA COVID-19 vaccines and COVID-19 infection were reported.Citation9 However, few published reports of mRNA vaccination-related encephalitis and acute disseminated encephalomyelitis (ADEM).Citation9–14 This study aims to inform a rare case of acute encephalitis after the first dose of the mRNA COVID-19 vaccine and provide a review of the relevant literature to compare signs/symptoms and outcomes for postvaccinal encephalitis after different COVID-19 vaccines.
Case presentation
An 82-year-old female patient with diabetes, hypertension, and no previous seizure history or psychological disorder, presented with fever (38°C), malaise, and headache 5 days after receiving her first dose of the mRNA-1273 SARS-CoV-2 vaccine (Moderna). She initially visited the local public health center and a local medical doctor for help. After 3 days of treatment (acetaminophen), the patient’s symptoms subsided. However, 15 days after vaccination, the patient experienced general discomfort, palpitations, elevated blood pressure, and hand tremors. She went to the emergency room (ER) for first aid the next day.
At the ER, general routine laboratory tests, electrocardiography, and chest radiography were performed, but no abnormalities were found. The patient was taken to the neurological outpatient department 17 days after vaccination for progressive mental alteration with memory impairment, loss of attention and concentration, murmuring, and unsteadiness. Additionally, she complained of not being able to shower as well as previously, and aimless, repetitive behavior was noted ().
Figure 1. Summarizes the timeline of clinical course and manifestations of the patient with post-vaccination encephalitis.
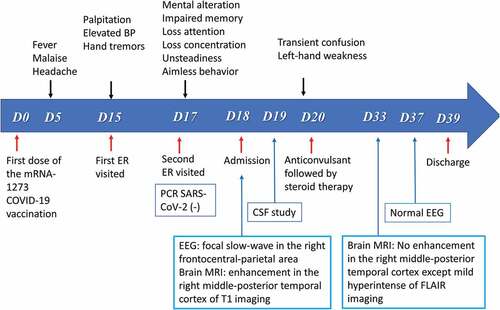
Neurological examination revealed a mask-like face, weak grasp in the left hand, and gait deviation to the right, which resulted in admission to our neurological ward via the ER 18 days after vaccination. Polymerase chain reaction (PCR) and real-time PCR for SARS-CoV-2 were negative. An emergency brain imaging study (magnetic resonance imaging [MRI]) revealed hyperintense-signal on fluid-attenuated inversion recovery (FLAIR) sequence imaging () and abnormal gyral enhancement on T1-weighted imaging in the right middle and posterior temporal lobe (). Cervical spinal MRI revealed markedly herniated intervertebral discs at the C3-C5 level with no evidence of myelitis. EEG recordings revealed intermittent focal slow waves in the right frontocentral to parietal regions (). On admission, the patient was alert and afebrile and scored 21/30 on the Mini-Mental State Examination. A lumbar puncture was performed immediately, and cerebrospinal fluid (CSF) analysis showed no pleocytosis but elevated CSF protein (Pandy’s CSF test 1 positive). CSF rapid plasma reagin (RPR), treponema pallidum hemagglutination (TPPA) immunoelectrophoresis, and cytology were negative. In addition, the CSF IgG index was within normal limits (). A multiplex PCR assay (BioFire FilmArray; BioFire Diagnostics, Salt Lake City, UT, USA) with a meningitis and encephalitis panel did not detect viruses, bacteria, or fungi in the central nervous system ().
Figure 2. Post-COVID-19 vaccination encephalitis: magnetic resonance imaging (MRI) of the brain demonstrates prominent T2 fluid attenuated inversion recovery (FLAIR)-hyperintense lesions in the right middle temporal (a) and posterior temporal (c) regions with gadolinium enhancement (b, d). The EEG recordings exhibit intermittent focal slow waves in the right frontocentral to parietal regions (e). Post-treatment brain MRI (at 20 days after admission) shows attenuated signal intensity on the T2 FLAIR image (f, h), in the absence of enhancement in the middle-posterior temporal gyri (g, i). EEG findings were normal (j).
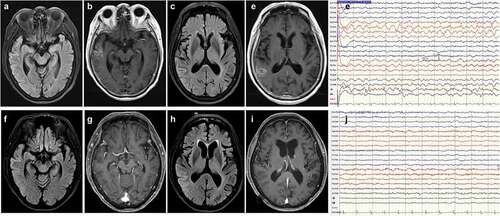
Table 1. Extensive study of Laboratory profile.
Table 2. Multiplex polymerase chain reaction assay.
On day 3 after admission, there was one episode of confusion associated with left-hand weakness while grasping, lasting around 15 min. Therefore, intravenous valproic acid (400 mg, per 8 h/day) was started after a loading dose of 800 mg. After an anticonvulsant was administered, the confusion improved, except for the recent memory impairment. The patient also received intravenous dexamethasone (5 mg per 6 h for 5 days). During admission, extensive diagnostic workup was performed, including biochemistry and measurement of serum rheumatoid factor, antinuclear antibody, anti-dsDNA, an Anti-Extractable nuclear antigen (Anti-ENA) screen, ANCA levels, homocysteine, C-reactive protein, erythrocyte sedimentation rate, copper, and thyroid hormone levels, all of which were within normal limits. Serum anti-aquaporin 4 (AQP4) antibodies, IgG index, tumor markers, anticardiolipin IgG/IgM, C3, C4, RPR, and TPPA, human immunodeficiency virus, cryoglobulin, and immunoelectrophoresis were all negative (). Electrophysiological studies, such as somatosensory evoked potentials and brainstem auditory evoked potentials, were all within normal limits. The visual evoked potential study revealed an absent P100 response in the right eye due to severe diabetic retinopathy. Furthermore, we sent the patient’s CSF and serum to Molecular Biology Lab (Uni Pharma Co, Taipei, Taiwan) for workup for limbic encephalitis (CSF) and paraneoplastic neurologic syndrome (serum), both of which were negative (). Accordingly, the patient was diagnosed with encephalitis with level II diagnostic certainty.Citation15 After 7 days of anticonvulsant treatment, the patient’s orientation returned to normal, except for retrograde amnesia of the previous events. After discharge, a repeat brain MRI (20 days after admission) and EEG both were significantly improved (). The whole clinical course is depicted in .
Table 3. Laboratory assessment for autoimmune encephalitis during admission.
Discussion
We present the case of an 82-year-old female patient with acute encephalitis following a first dose of the mRNA-1273 vaccine for SARS-CoV-2. Her symptoms were relatively mild, and the brain parenchyma revealed focal cerebritis with increased CSF protein but without pleocytosis. Due to the typical symptoms and signs, combined prednisolone and anticonvulsant treatment were administered, resulting in relatively good outcomes. The patient presented with altered mental status and an unsteady gait. She also had abnormal behavior and repeated non-purposeful actions. EEG revealed regional slow waves in the right temporal-parietal region. Brain MRI revealed gadolinium enhancement in the right middle-posterior temporal gyrus. Extensive laboratory investigations were conducted during admission. FilmArray PCR tests for viruses, bacteria, tuberculosis, and fungal infections were all negative. Oligoclonal bands in the blood and CSF were also negative. The possibility of autoimmune encephalitis related to paraneoplastic neurological syndrome (i.e., anti-Hu, anti-Yo, anti-Ri, anti-PNMA2 (Ma2/Ta), anti-CV2, and anti-Amphiphsyin) and limbic encephalitis (i.e., antibody for NMDAR, AMPAR1, AMPAR2, CASPR2, LGI1 R, and GABAβR) was excluded. Additionally, the negative AQP4 antibodies and normal serum/CSF IgG index values ruled out the probability of AQP4-Antibody-Positive neuromyelitis optica spectrum disease and minimized the possibility of multiple sclerosis. According to the Brighton collaboration criteria for acute encephalitis, the diagnosis of our patient was categorized as having a level of certainty 2 (B1-b: altered of consciousness; B2-b: Inconsistent or absent response to other external stimuli; C5: Motor weakness; C8 cerebellar dysfunction; E3: EEG changes consistent with encephalitis; Acute inflammation or consistent with encephalitis; F1: MRI showed acute inflammation or consistent with encephalitis; X1: no evidence of neoplasm, vascular disorder, toxic/metabolic encephalopathy).Citation15
In a brief review of SARS-CoV-2 vaccine-associated encephalitis, four studies published between Jan 2021 and Jan 2022, involving a total of six patients, were found ().Citation10–14 Most cases were associated with the ChAdOx1 vaccine (3/6 cases), followed by the mRNA-1273 vaccine (2/6 cases) and BNT162b2 (Pfizer–BioNTech) (one case). Encephalitis was diagnosed in four cases, and two were diagnosed with ADEM. In our patient, the temporal association between COVID vaccination and neurological manifestations onset was 17 days, which was in line with a recent self-controlled case series study (approximately 1–28 days).Citation9 Pleocytosis was present in five cases (7-294/μL). No patients experienced seizures. Though, because our patient presented with episodic, unusual, repetitious behavior, we administered anticonvulsant treatment for suspected complex partial seizures. According to the diagnostic criteria of acute encephalitis, one of the minor criteria is “generalized or partial seizures not fully attributable to a preexisting seizure disorder.”Citation1,Citation16,Citation17However, our case only had symptoms indicative of a partial seizure. In post-COVID-19 vaccination encephalitis, only 1/7 of cases experience seizure (including the presenting case), which may be due to the lower severity of neuroinflammation in post-vaccination encephalitis than in pathogen-related infectious encephalitis. Five patients were treated with steroids, and one patient refused treatment with improved symptoms. Overall, the outcomes of these patients were relatively good. Nevertheless, death has been reported to occur in up to 32% of cases of COVID-19 infection-related encephalitis.Citation5
Table 4. Main features of post-COVID 19 vaccination encephalitis.
Post-vaccination encephalitis is most often presented as ADEM, with a low incidence rate of around 0.1 to 0.2 per 100,000.Citation1 As vaccination is widely applied for the influenza virus and the current COVID-19 pandemic (SARS-CoV-2 infection), reports of post-vaccination encephalitis are starting to increase. The pathogenesis of post-vaccination encephalitis is due to molecular mimicry. The similarity between certain elements in the vaccine and specific human proteins leads to immune cross-reactivity.Citation18 One study of antigen cross-reactivity suggested mimicry of SARS-CoV-2 spike protein and myelin basic protein, which may cause autoimmunity.Citation19 In addition, cytokine production (i.e., interleukin [IL]-1b, tumor necrosis factor [TNF]-α, and G-CSF) with inactivated vaccines,Citation20 vaccine formulations, and adjuvants can stimulate the innate immune system and induce the production of inflammatory cytokines, which may be attributed to vaccine-related illness and possible neuroinflammatory disorders.Citation21
Because COVID-19 is a worldwide pandemic, acute encephalitis associated with SARS-CoV-2 infection rises.Citation9 The pathogenesis of COVID-19 associated encephalitis is not well understood. Para-infectious COVID-19 induction of an immune-mediated response, but not a post-infectious reaction, has been suggested.Citation22,Citation23 Recently, post-SARS-CoV-2 associated autoimmune encephalitis has been reviewed,Citation24 and neural IgG was not detected in any cases, either in CSF or serum, which is consistent with a previous report.Citation22,Citation24 Antigenic cross-reactivity between SARS-CoV-2 and human tissue is a possible mechanism of immunocompromise after SARS-CoV-2 infection.Citation19 Additionally, the para-infectious neuroinflammatory response to infection, such as cytokine-releasing syndrome, may play an important role.Citation25,Citation26 Nevertheless, post-vaccination immune responses result in the synthesis and release of pyrogenic cytokines (IL-1, IL-6, TNF-α, and prostaglandins) into the bloodstream, which could mimic the response to natural infection.Citation10 Since cytokine release is a crucial action of the immune response to SARS-CoV-2 infection and COVID-19 vaccination, and both are concomitant with encephalitis. Accordingly, we speculate that these events are likely to share common neuroimmune interactions, both long- and short-range.Citation27
Conclusion
In conclusion, we presented a rare case of acute encephalitis following COVID-19 vaccination and a brief review of cases of COVID-19 vaccination-related encephalitis. The treatment outcomes for the condition were favorable. There is no proven causal link, and that a review of case reports but only for clinical practice. No studies across the literature provided empirical evidence supporting a significantly increased risk of acute encephalitis post-vaccination; and need more post-marketing surveillance for the major neurological adverse effect of COVID-19 vaccination. However, because of the COVID-19 pandemic, various mutant coronavirus variants have been growing in prevalence, and the use of multiple doses, mixed COVID-19 vaccinations, or boosters has been suggested. Thus, we expect that cases of encephalitis following SARS-CoV-2 vaccination will become more prevalent. Physicians should be alert for the early symptoms/signs of encephalitis after vaccination; with timely treatment, favorable outcomes can be obtained.
Abbreviations
| COVID-19 | = | coronavirus disease 2019 |
| SARS-COV-2 | = | severe acute respiratory syndrome coronavirus 2 |
| CSF | = | cerebrospinal fluid |
| EEG | = | electroencephalography |
| ADEM | = | acute disseminated encephalomyelitis |
| ER | = | emergency room |
| PCR | = | polymerase chain reaction |
| MRI | = | magnetic resonance imaging |
| FLAIR | = | fluid-attenuated inversion recovery |
| Anti-ENA | = | Anti-Extractable nuclear antigen |
| ANCA | = | Anti-Neutrophil Cytoplasmic Antibody |
| RPR | = | rapid plasma regain |
| TPPA | = | treponema pallidum hemagglutination |
| NMDAR | = | N-methyl-D-aspartate receptor |
| AMPAR | = | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor |
| CAPSAR | = | Contactin-associated protein receptor |
| LGI1 R | = | Leucine-rich glioma-inactivated 1 receptor |
| GABAβR | = | gamma-amino butyric acid type β receptor |
| AQP4 | = | anti-aquaporin 4 |
| Ref | = | reference |
| Y | = | yes |
| no | = | N |
| SZ | = | seizure |
| Abs | = | antibodies |
| Tx | = | treatment |
| N/A | = | not available |
Authors’ contributions
Conceptualization, J.-J.G., and C.-H.L.; Investigation, J.-J.G., H.-P.T., C.-L.L., R.-F.H., M.-H.L., and C.-H.L; writing—original draft presentation, J.-J.G., and C.-H.L.; writing—review and editing, J.-J.G., H.-P.T., C.-L.L., R.-F.H., M.-H.L., and C.-H.L; visualization, M.-H.L.; supervision, C.-H.L.
Consent for publication
The patient signed written consent for publishing her information and photos.
Ethical considerations
The patient signed written consent for publishing her information and photos.
Acknowledgments
The authors would like to express their gratitude to the patient who signed written consent for publishing her information and photos.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Halperin JJ. Diagnosis and management of acute encephalitis. Handb Clin Neurol. 2017;140:1–7. doi:10.1016/B978-0-444-63600-3.00018-0.
- George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. Plos One. 2014;9(9):e104169. doi:10.1371/journal.pone.0104169.
- Vandervorst F, Guldolf K, Peeters I, Vanderhasselt T, Michiels K, Berends KJ, Van Laethem J, Pipeleers L, Vincken S, Seynaeve L, et al. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip Neurosurg. 2020;22:100821. doi:10.1016/j.inat.2020.100821.
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi:10.1016/j.ijid.2020.03.062.
- Manzano GS, McEntire CRS, Martinez-Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19: systematic review and meta-synthesis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1080. doi:10.1212/NXI.0000000000001080.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi:10.1056/NEJMoa2035389.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–857. doi:10.1002/ana.26065.
- Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, Hunt D, Mei XW, Dixon S, Zaccardi F, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. doi:10.1038/s41591-021-01556-7.
- Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal encephalitis after ChAdox1 nCov-19. Ann Neurol. 2021;90(3):506–511. doi:10.1002/ana.26182.
- Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D’-Agostini S, Fabris M, Valente M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi:10.1016/j.clineuro.2021.106839.
- Torrealba-Acosta G, Martin JC, Huttenbach Y, Garcia CR, Sohail MR, Agarwal SK, Wasko C, Bershad EM, Hirzallah MI. Acute encephalitis, myoclonus and sweet syndrome after mRNA-1273 vaccine. BMJ Case Rep. 2021 Jul 26;14(7):e243173. doi:10.1136/bcr-2021-243173.
- Kania K, Ambrosius W, Tokarz Kupczyk E, Kozubski W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann Clin Transl Neurol. 2021;8(10):2000–2003. doi:10.1002/acn3.51447.
- Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3–40. doi:10.1007/s10072-021-05662-9.
- Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, Gidudu J, Katikaneni L, Khuri-Bulos N, Oleske J, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007 Aug 1;25(31):5771–5792. doi:10.1016/j.vaccine.2007.04.060.
- Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-Vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15(12):1315–1322. doi:10.1016/j.jocn.2008.05.002.
- Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, Stahl JP, Mailles A, Drebot M, Rupprecht CE, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi:10.1093/cid/cit458.
- Segal Y, Shoenfeld Y. Vaccine-Induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–594. doi:10.1038/cmi.2017.151.
- Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi:10.1016/j.clim.2020.108480.
- Nakayama T. Causal relationship between immunological responses and adverse reactions following vaccination. Vaccine. 2019;37(2):366–371. doi:10.1016/j.vaccine.2018.11.045.
- Nakayama T. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine. 2016;34(47):5815–5818. doi:10.1016/j.vaccine.2016.08.051.
- Zambreanu L, Lightbody S, Bhandari M, Hoskote C, Kandil H, Houlihan CF, Lunn MP. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J Neurol Neurosurg Psychiatry. 2020;91(11):1229–1230. doi:10.1136/jnnp-2020-323839.
- Meshref M, Hewila IM, Abdel Mageed S, Morra ME. COVID-19 associated with encephalitis: case report and review of literature. Neurologist. 2021;26(6):268–270. doi:10.1097/NRL.0000000000000347.
- Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune encephalitis after SARS-CoV-2 infection: case frequency, findings, and outcomes. Neurology. 2021;97(23):e2262–e2268. doi:10.1212/WNL.0000000000012931.
- Correia AO, Feitosa PWG, Moreira JLS, Sár N, Fonseca RB, Nobre MEP. Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res. 2020;37:27–32. doi:10.1016/j.npbr.2020.05.008.
- Pilotto A, Masciocchi S, Volonghi I, De Giuli V, Caprioli F, Mariotto S, Ferrari S, Bozzetti S, Imarisio A, Risi B, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis. 2021 Nov 2;73(9):e3019–e3026. doi:10.1093/cid/ciaa1933.
- Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477–504. doi:10.1152/physrev.00039.2016.
