ABSTRACT
Amid subpar uptake of HPV vaccination in the United States, gender-generated disparities in HPV vaccination uptake have the potential to perpetuate existing disparities in HPV-associated cancers. Yet few studies have investigated the influence of parent–child gender on intentions to refuse HPV vaccination due to safety concerns/side effects. This study used nationally representative data, spanning 2010–2019, from the National Immunization Survey-Teen (NIS-Teen). NIS-Teen respondents are parents/guardians or primary caregivers of adolescents 13–17 years old living in the United States. Over the study period, intentions to refuse HPV vaccination due to safety concerns rose among all parent–child gender pairings but were highest among respondent mothers regarding their unvaccinated daughters. The results revealed a statistically significant increased likelihood of having intentions to refuse HPV vaccination due to safety concerns among all parent–child combinations compared with father–son pairs. These odds were consistently highest among mother–daughter pairs. In 2019, compared with father–son pairs, fathers were 1.94 (95% CI: 1.21–3.12) times more likely to report the intention to not vaccinate against HPV for their daughters, while mothers were 2.23 (95% CI: 1.57–3.17) and 2.87 (95% CI: 2.02–4.09) times more likely to report intentions to refuse HPV vaccination for their sons and daughters, respectively. These findings were persistent and constantly increased over the 10-year study period. Interventions aimed at correcting gender-based misperceptions and countering misinformation about the safety of the HPV vaccine are warranted.
Introduction
The human papillomavirus (HPV) vaccine was first approved in June 2006 by the US Food and Drug Administration and the Advisory Committee on Immunization Practices for use in females as a highly effective vaccine that confers protection against HPV infection and cervical cancer.Citation1 Hence, the vaccine was initially marketed solely for girls and young women. Over the years, cervical cancer rates in the US have gradually but significantly declined, while rates of oropharyngeal and other HPV-associated carcinomas have increased, especially among men.Citation2–4 The vaccine was made gender-neutral in October 2009, following its approval for males.Citation5 Yet, the female-focused introduction of the vaccines had already contributed to female child-centric misconceptions about the HPV vaccine and gender disparities in its uptake.Citation6
Predictors of HPV vaccination among adolescent boys differ from those of girls. Studies have found that parents are more likely to have safety concerns about the HPV vaccineCitation7,Citation8 for their female than for male adolescents, which informs their ultimate decision to refuse the vaccine. Safety concerns specific to the female gender include brief case reports linking the HPV vaccine to reduced fertility via induction of primary ovarian insufficiency,Citation9,Citation10 even though these reports have been debunked by a systematic review of the evidence.Citation11 On the other hand, despite evidence that has consistently identified provider recommendation as a strong predictor of HPV vaccination for boys,Citation8,Citation12 several studies have noted that providers are less likely to recommend vaccination for male children.Citation13–15 Disparities in provider recommendations for HPV vaccination could contribute to parental unawareness and culminate in gender disparities in HPV vaccination. With studies pointing to increasing rates of safety concerns for the HPV vaccine,Citation16,Citation17 as well as increasing physician recommendations for HPV vaccination,Citation15,Citation18 it is possible that gender disparities may be perpetuated further with an increase in vaccination refusals for females and an increase in acceptance rates for males.
Studies have found parent gender to be a significant predictor of child HPV vaccination.Citation12,Citation19 One study found that overall, parents were more likely to delay HPV vaccination for their female children relative to their male children.Citation19 Another study found thatfathers were less likely than mothers to allow HPV vaccination for their sons.Citation12 Gender disparities also exist in parental awareness of HPV—an important determinant of HPV vaccination. A study found that males (which include fathers) are less likely to be aware of HPV or the HPV vaccine.Citation20
Eliminating gender disparities in HPV vaccination is crucial given the cost-effectiveness of successful gender-neutral vaccination programs and the rising incidence of HPV-associated cancers.Citation21–23 Conversely, gender-generated disparities in HPV vaccination uptake have the potential to perpetuate existing disparities in HPV-associated cancers. Safety concerns are an important barrier to HPV vaccination and a key driver for parental vaccination refusal, particularly among parents of female children.Citation7,Citation8,Citation16 However, to our knowledge, no prior studies have examined this issue over time. Further, it is not known if these disparities persist with parental gender and if child gender is a predictor of parental evaluation of vaccine safety and subsequent refusal. Therefore, the goal of this study was to bridge existing gaps in the literature by examining the influence of parental and child gender on the decision to refuse the HPV vaccine due to safety concerns/side effects over a 10-year period.
Methods
Design
The National Immunization Survey-Teen (NIS-Teen) is a population-based, nationally representative survey that collects teen vaccination data from parents of children 13–17 years of age via telephone interviews with parents/guardians and an immunization history questionnaire mailed to healthcare providers.Citation16 The NIS-Teen study design and its data collection methods were previously described in detail.Citation24
Sample
For this analysis, we extracted data for 2010–2019 from the NIS-Teen. The NIS-Teen was approved by the National Center for Health Statistics Research Ethics Review Board. Since NIS-Teen data are fully anonymized and available to the public, our study did not require ethical approval.
Study outcomes
We examined for variations in intentions to refuse HPV vaccination due to safety concerns/side effects caused by the gender of the responding parent and the gender of the child. Specifically, we evaluated reports of safety concerns/side effects as the main reason for intentions to refuse HPV vaccination by the responding parent’s gender (father vs. mother) and the gender of the pertinent child (boy vs. girl). We restricted our study sample to those respondents whose children had not received any HPV vaccine shot. These respondents were then asked: “How likely is it that [teen name] will receive HPV shots in the next 12 months?” Those who responded, “Not too likely,” “Not likely at all,” and “Not Sure/Don’t Know” were identified as respondents with no apparent intentions of vaccinating their child. These respondents were asked an additional follow-up question: “What is the MAIN reason [teen name] will not receive HPV shots in the next 12 months.” The response option of interest in this study was the selection of safety concerns/side effects as the main reason for the intent to refuse the HPV vaccine. We chose this outcome of interest because, of the reasons for HPV non-vaccination intent accounted for in the NIS-Teen, safety concerns/side effects were among the most predominantly reported and were the only factors found to have an increasing and statistically significant upward trend over the years.Citation16,Citation17
We compared responses based on the gender of the responding parent and pertinent child using the following combination pairs: i) respondent mother and teen daughter, ii) respondent mother and teen son, iii) respondent father and teen daughter, and iv) respondent father and teen son. Before 2010, data for father–son and mother–son combinations were unavailable.
Data analysis
Data were weighted to represent the US population. Weights were adjusted for non-response, non-resolution of telephone numbers, subsampling of one age-eligible child per household, and presence of multiple telephone lines in the home. Additional post-stratification adjustments were applied based on respondent sociodemographic characteristics, US state of residence, and missing provider data.Citation25
The weighted prevalence and associated 95% confidence intervals (CIs) of intentions to refuse HPV vaccination due to safety concerns/side effects were estimated by parental and child gender from 2010 to 2019. Survey-weighted multivariable logistic regression was conducted to examine associations between intentions to refuse HPV vaccination due to safety concerns/side effects with parental and child gender pairs, controlling for key sociodemographic variables including age, race/ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, and multiple/other races), and mother’s education level. All analyses were conducted using R version 4.0.3. For all tests, a P value <0.05 was considered statistically significant.
Results
shows the unweighted and survey-weighted study sample sizes, weighted percentages, and associated CIs for the respondent–child combinations and race/ethnicities for survey years 2008 to 2019. While intentions to refuse HPV vaccination due to safety concerns/side effects increased over the years, there were marked differences in parent/guardian gender and the gender of the pertinent child. This observation was consistent throughout the study period (). Over the 10-year period, reported intentions to refuse HPV vaccination due to safety concerns/side effects were the least frequent when fathers were responding to surveys about their unvaccinated sons (3.3% in 2010 and 14.6% in 2019), while this was highest when mothers were responding to surveys about their unvaccinated daughters (23.8% in 2010 and 33.2% in 2019).
Figure 1. Prevalence of safety concerns/side effects as the main reason for HPV non-vaccination intent by gender of parent and child.
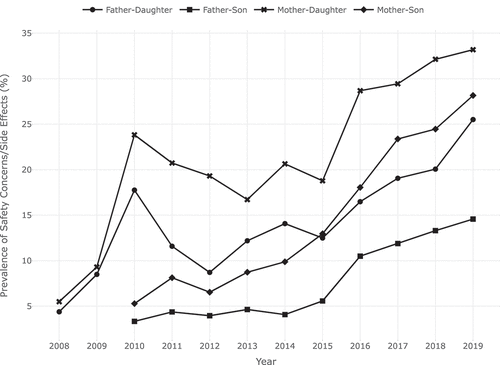
Table 1. Study demographics for respondent–child combinations and race/ethnicities for survey years 2008 to 2019.
In analyses controlled for sociodemographic variables including age, race/ethnicity, and mother’s education level, results from the multivariable logistic regression conducted for each survey year revealed a consistent, statistically significant increased likelihood of intention to refuse HPV vaccination due to safety concerns/side effects among all parent–child combinations compared with the father–son combination (). In all survey years, the odds of non-vaccination intent due to safety concerns/side effects were consistently highest among mother–daughter pairs.
Table 2. Multivariable logistic regression results for association between non-vaccination intent due to safety concerns/side effects with parental and child gender combination pairs, controlling for key sociodemographic variables including age and race/ethnicity.
In 2010, mothers were more than 9.25 (95% CI: 6.34–13.51) times more likely to report intentions to refuse HPV vaccination due to safety concerns/side effects for their daughters compared with fathers who responded to the same question about their sons. In 2019, compared with father–son pairs, fathers were 1.94 (95% CI: 1.21–3.12) times more likely to report intentions to refuse HPV vaccination due to safety concerns/side effects for their daughters, while mothers were 2.23 (95% CI: 1.57–3.17) times more likely to report intentions to refuse HPV vaccination for their sons and 2.87 (95% CI: 2.02–4.09) times more likely for their daughters.
Discussion
Our findings highlight marked differences in intentions to refuse HPV vaccination due to safety concerns/side effects by the gender of the responding parent (father vs. mother) and pertinent child (son vs. daughter). Parents were far more likely (albeit higher among mothers) to report safety concerns/side effects as a reason for their intention to refuse HPV vaccination for their daughters compared with their sons. Despite the well-documented benefits of HPV vaccination, including protection against several cancers, it is concerning that our findings were persistent and increased consistently over a 10-year period. Several studies have pointed to a slowing of HPV vaccination uptake rates among girls,Citation26–28 and the findings of this study provide further evidence of how gender and gender-centric parental perceptions may play a contributory role.
Amid a paucity of prior published studies in this area, our findings call for additional studies to further define the role parent–child gender plays in vaccination decisions. For example, further studies are needed to determine if the underlying basis for the intent to refuse HPV vaccination due to safety concerns/side effects differs fundamentally between fathers and mothers, especially in light of studies showing that fathers are less informed about HPV and HPV vaccination than mothers.Citation20
Misconceptions about the HPV vaccine and the female child are wide-ranging.Citation6 They include claims that the HPV vaccine is associated with infertility,Citation9,Citation10 and that vaccinating young girls may be perceived as an endorsement for the initiation of sexual activity.Citation29,Citation30 These misconceptions may explain our findings, which are suggestive of an increased sense of misguided protectiveness toward female children by parents regarding HPV vaccination.
Furthermore, the misperception that the HPV vaccine is a vaccine for females as opposed to males persists,Citation6 despite the inception of gender-neutral HPV vaccination more than a decade ago. The initial marketing and portrayal of the vaccine to be specifically used for cervical cancer prevention in women may have played a role in the persistence of this misconception. The benefits of the vaccine for males are equally well documented, as it has been shown to be cost-effective and crucial in the prevention of non–gender-specific HPV-associated cancers, such as oropharyngeal and anal cancers. Citation4,Citation5,Citation21,Citation23
While our study findings are of significant concern, a lack of HPV awareness and a surge in vaccination misinformation remain potent mediators of gender-related misperceptions about HPV vaccination. Awareness of HPV and the HPV vaccine has been on the decline, especially among racial minority groups,Citation31 amid existing gender disparities in HPV awareness.Citation4,Citation20 Addressing all possible misconceptions about the vaccines in a gender-specific manner can bring about a slow but meaningful reversal of the current trend. Comprehensive educational campaigns are required to dispel widely held misconceptions and the spate of misinformation about the safety of the HPV vaccine, as well as to increase awareness about HPV vaccination. Existing policies aimed at boosting awareness of HPV vaccination should be enforced, with dedicated efforts committed to educating parents about the benefits of vaccination for both male and female children. Additionally, messaging projected to the public should be devoid of gender bias. Given the centrality of physician recommendation to male vaccination acceptance,Citation8,Citation12 conscious efforts by physicians must also be taken to promote this practice during wellness visits. In the same vein, as safety concerns are the predominant reason for non-vaccination of female children,Citation7,Citation8 these concerns should be consciously addressed using multiple media channels.
To our knowledge, we are the first to examine the influence of parental and child gender in citing safety concerns/side effects as a reason for the intention to refuse HPV vaccination. However, this study has several limitations. First, the study data are cross-sectional; hence, we cannot draw causal inferences. Second is the low response rate associated with national surveys, which also predisposes our study to non-response bias. Nevertheless, the data included in this study are nationally representative, and these results can be generalized to the US population. Additionally, the data used in the study spanned a 10-year period and provided adequate sample size and power to detect differences in the analyses.
In conclusion, in this study, we found a persistent pattern of disparities in parental intentions to refuse HPV vaccination due to safety concerns/side effects depending on the gender of the pertinent child. We found that parents (with mothers having the highest odds) are more likely to have intentions to refuse the HPV vaccine for their daughters due to safety concerns/side effects. Education-based interventions aimed at correcting gender-based misconceptions related to HPV vaccination would be highly beneficial.
Abbreviations
| HPV7 | = | human papillomavirus |
| CDC | = | US Centers for Disease Control and Prevention |
Contributors’ statement
Dr. Chido-Amajuoyi contributed to the conceptualization and design of the study, drafted the initial manuscript, interpreted study data, and reviewed and revised the manuscript.
Dr. Talluri contributed to the conceptualization and design of the study, carried out all statistical analyses, interpreted study data, and reviewed and revised the manuscript.
Dr. Jackson contributed to the interpretation of study data and the manuscript drafting, reviewing, and revising the manuscript.
Mr. Shete contributed to the interpretation of study data and the manuscript drafting, reviewing and revising the manuscript.
Dr. Fokom-Domgue contributed to the interpretation of study data and reviewed and revised the manuscript.
Dr. Shete contributed to the conceptualization and design of the study, interpreted study data, reviewed and revised the manuscript, obtained funding, and supervised the study.
Role of funder/sponsor
The funders were not involved in the study design, analysis, interpretation of data, or manuscript writing.
Acknowledgements
Editorial support was provided by Bryan Tutt, Scientific Editor, Research Medical Library, MD Anderson Cancer Center.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chesson HW, Dunne EF, Lawson HW, Markowitz LE, Saraiya M, Unger ER Quadrivalent human papillomavirus vaccine; recommendations of the Advisory Committee on Immunization Practices (ACIP). 2007.
- Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. Jnci. 2013;105:1–7. doi:10.1093/jnci/djs491.
- Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus–associated cancers—United States, 1999–2015. Morbidity Mortality Weekly Report. 2018;67:918. doi:10.15585/mmwr.mm6733a2.
- Lechner M, Jones OS, Breeze CE, Gilson R. Gender-Neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect Dis. 2019;19:131–132. doi:10.1016/S1473-3099(18)30802-8.
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JJ, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity Mortality Weekly Report. 2014;63:1–30.
- Daley EM, Vamos CA, Thompson EL, Zimet GD, Rosberger Z, Merrell L, Kline NS. The feminization of HPV: how science, politics, economics and gender norms shaped US HPV vaccine implementation. Papillomavirus Res. 2017;3:142–148. doi:10.1016/j.pvr.2017.04.004.
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolescent Health. 2017;61:288–293. doi:10.1016/j.jadohealth.2017.05.015.
- Gilkey MB, Moss JL, McRee A-L, Brewer NT. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928–5934. doi:10.1016/j.vaccine.2012.07.045.
- Little DT, Ward HRG. Adolescent premature ovarian insufficiency following human papillomavirus vaccination: a case series seen in general practice. J Invest Med High Impact Case Rep. 2014;2:2324709614556129. doi:10.1177/2324709614556129.
- Colafrancesco S, Perricone C, Tomljenovic L, Shoenfeld Y. Human papilloma virus vaccine and primary ovarian failure: another facet of the autoimmune/inflammatory syndrome induced by adjuvants. Am J Reprod Immunol. 2013;70:309–316. doi:10.1111/aji.12151.
- Christianson MS, Wodi P, Talaat K, Halsey N. Primary ovarian insufficiency and human papilloma virus vaccines: a review of the current evidence. Am J Obstet Gynecol. 2020;222:239–244. doi:10.1016/j.ajog.2019.08.045.
- Reiter PL, McRee A-L, Pepper JK, Gilkey MB, Galbraith KV, Brewer NT. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health. 2013;103:1419–1427. doi:10.2105/AJPH.2012.301189.
- Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi:10.1001/jamapediatrics.2013.2752.
- Gilkey MB, McRee A-L. Provider communication about HPV vaccination: a systematic review. Human Vaccines & Immunotherapeutics. 2016;12:1454–1468. doi:10.1080/21645515.2015.1129090.
- Sonawane K, Zhu Y, Lin Y-Y, Damgacioglu H, Lin Y, Montealegre JR, Deshmukh AA. HPV vaccine recommendations and parental intent. Pediatrics. 2021;147. doi:10.1542/peds.2020-026286.
- Chido-Amajuoyi OG, Talluri R, Shete SS, Shete S. Safety Concerns or Adverse Effects as the Main Reason for Human Papillomavirus Vaccine Refusal: National Immunization Survey–Teen, 2008 to 2019. JAMA Pediatr. 2021;175:1074–1076. doi:10.1001/jamapediatrics.2021.1585.
- Hirth JM, Fuchs EL, Chang M, Fernandez ME, Berenson AB. Variations in reason for intention not to vaccinate across time, region, and by race/ethnicity, NIS-Teen (2008–2016). Vaccine. 2019;37:595–601. doi:10.1016/j.vaccine.2018.12.017.
- Vadaparampil ST, Malo TL, Kahn JA, Salmon DA, Lee J-H, Quinn GP, Roetzheim RG, Bruder KL, Proveaux TM, Zhao X. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med. 2014;46:80–84. doi:10.1016/j.amepre.2013.07.009.
- Gilkey MB, Calo WA, Marciniak MW, Brewer NT. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Human Vaccines & Immunotherapeutics. 2017;13:680–686. doi:10.1080/21645515.2016.1247134.
- Adjei Boakye E, Tobo BB, Rojek RP, Mohammed KA, Geneus CJ, Osazuwa-Peters N. Approaching a decade since HPV vaccine licensure: racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Human Vaccines & Immunotherapeutics. 2017;13:2713–2722. doi:10.1080/21645515.2017.1363133.
- Chido-Amajuoyi OG, Domgue JF, Obi-Jeff C, Schmeler K, Shete S. A call for the introduction of gender-neutral HPV vaccination to national immunisation programmes in Africa. The Lancet Global Health. 2019;7:e20–e1. doi:10.1016/S2214-109X(18)30405-4.
- Qendri V, Bogaards JA, Baussano I, Lazzarato F, Vänskä S, Berkhof J. The cost-effectiveness profile of sex-neutral HPV immunisation in European tender-based settings: a model-based assessment. The Lancet Public Health. 2020;5:e592–e603. doi:10.1016/S2468-2667(20)30209-7.
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–8450. doi:10.1016/j.vaccine.2011.07.096.
- Centers for Disease Control and Prevention (CDC) National Opinion Research Center at the University of Chicago. National Immunization Survey-Teen: a User’s Guide for the 2016 Public-Use data file. Chicago, IL: National Opinion Research Center; 2016.
- Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Public Health Rep. 2009;124:642–651. doi:10.1177/003335490912400506.
- Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874. doi:10.15585/mmwr.mm6633a2.
- Moss JL. Evolving trends in human papillomavirus vaccination by sex and time: implications for clinicians and interventionists. J Adolescent Health. 2017;61:269–270. doi:10.1016/j.jadohealth.2017.06.004.
- Chido-Amajuoyi OG, Talluri R, Wonodi C, Shete S. Trends in HPV vaccination initiation and completion within ages 9–12 Years: 2008–2018. Pediatrics. 2021;147. doi:10.1542/peds.2020-012765.
- Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Human Vaccines & Immunotherapeutics. 2019;15:1628–1638. doi:10.1080/21645515.2019.1565267.
- Zimet GD, Rosberger Z, Fisher WA, Perez S, Stupiansky NW. Beliefs, behaviors and HPV vaccine: correcting the myths and the misinformation. Prev Med. 2013;57:414–418. doi:10.1016/j.ypmed.2013.05.013.
- Chido-Amajuoyi OG, Jackson I, Yu R, Shete S. Declining awareness of HPV and HPV vaccine within the general US population. Human Vaccines & Immunotherapeutics. 2021;17:420–427. doi:10.1080/21645515.2020.1783952.
