ABSTRACT
A lack of confidence on the vaccination drive hinders the management of the COVID-19 pandemic. We aimed to assess the antibody response to the SARS-CoV-2 vaccine among hospitalized patients in China. This case-control study was based on SARS-CoV-2 sero-surveillance during hospitalization. From April to June 2021, hospitalized patients without documented COVID-19 infection from the Department of Urology were routinely assayed for anti-SARS-CoV-2 antibodies. The SARS-CoV-2 vaccination history of each participant was obtained from their vaccination records. Of the 405 participants, there were 37 seropositive participants (case group) and 368 seronegative participants (control group); 68 participants (16.8%) had received the inactivated SARS-CoV-2 vaccine, including 54 who received the Sinovac-CoronaVac vaccine and 14 received the Sinopharm vaccine. All seropositive participants who had received one or two doses of the SARS-CoV-2 vaccine were assessed for at least 16 days, while 31 (8.4%) of 368 seronegative controls who had received the vaccine were tested for 1–94 days. The overall seroconversion rate was 54.4% (37/68) in the vaccinated participants who received the inactivated SARS-CoV-2 vaccine. The odds ratio (OR) and confidence interval (CI) for seropositivity was 6.20 (95% CI: 2.05–18.71) in those received full vaccination with two doses versus those partially vaccinated participants with one dose after adjusting for sex and age. These findings imply that the inactivated SARS-CoV-2 vaccine could have a protective antibody response.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global public health issue that has impacted human lives and global financial conditions.Citation1 Vaccination is an important strategy for preventing and controlling pandemics. However, some people are still hesitant, or even refuse to get vaccinated against SARS-CoV-2 due to the lack of confidence in the safety and efficacy of vaccines.Citation2,Citation3 To advance the vaccination strategy and improve the vaccination coverage rates as soon as possible, more pragmatic evidence for the effectiveness and safety of the vaccines needs to be established and delivered to the public.
The entry of SARS-CoV-2 into its target cells depends on the binding between its cellular receptor angiotensin-converting enzyme 2 (ACE2) and the receptor binding domain (RBD) of the virus spike protein. The spike protein is highly immunogenic and is the target of neutralizing antibodies, which are considered to be clinically significant protective antibodies against SARS-CoV-2.Citation4,Citation5 Elicitation of host cellular and humoral immune reactions is important for the development and evaluation of vaccines. Previous studies have shown that the inactivated vaccine successfully induces SARS-CoV-2-specific neutralizing antibodies in mice and non-human primates.Citation6
Beyond the reverse transcriptase-polymerase chain reaction (RT-PCR) for identifying SARS-CoV-2,Citation7,Citation8 several serological tests have been developedCitation9,Citation10 for rapid screening and accurate detection of SARS-CoV-2. The detection of SARS-CoV-2 nucleic acid and serum anti-SARS-CoV-2 antibodies has been applied in hospitals in China.Citation11 Although the serological response after viral infection or vaccination is composed of a mixture of antibodies, detection of serum total antibodies, including IgM and IgG, is also interesting because of their strong correlation with neutralizing antibodies against SARS-CoV-2.Citation12 In addition, serological assays can support the determination of individuals with intense antibody responses, who could view them as donors for the generation of monoclonal antibody treatments.Citation13
Two inactivated SARS-CoV-2 vaccines (Sinopharm vaccine and Sinovac-CoronaVac) have been approved for mass vaccination in mainland China and listed for WHO Emergency Use Listing (EUL).Citation14 These vaccines have been demonstrated to have good immunogenicity with vaccine-induced neutralizing antibodies against SARS-CoV-2 in previous clinical trials.Citation15,Citation16 However, real-world evidence on the efficacy of vaccines post-marketing is scarce. Emerging variants and recurrent outbreaks pose a great challenge for the various SARS-CoV-2 vaccines. There is an urgent need to evaluate the immune response in real-world settings which could be used to increase public confidence to accept vaccination.
Real-world evidence offers knowledge of the effects of medical care interventions using regular clinical information.Citation17 We aimed to explore the efficacy of an inactivated SARS-CoV-2 vaccine among hospitalized patients in China using a hospital-based case-control study; this study was based on real-world data using patients’ antibody responses and retrospective vaccinations collected from medical records.
Methods
Study design and participants
We designed a hospital-based case-control study in Taizhou, China. During the study period, all the hospitalized patients in our hospital were routinely assayed for anti-SARS-CoV-2 antibodies and nucleic acids prior to admission in accordance with the requirements for prevention and control the epidemic. The patients with negative nucleic acid for SARS-CoV-2 can be admitted to the ward of the hospital. This study included all the inpatients who admitted to the urology ward in our hospital between 1 April 2021 and 30 June 2021. None of the patients had prior COVID-19 infection during the active pandemic. Patients were asked to retrospectively recall whether they had received the SARS-CoV-2 vaccine. We further checked the vaccination records for all participants according to their ID card provided by the China Information Management System for Immunization Programming. The information on SARS-CoV-2 vaccination included the date of vaccine administration, type of vaccine used (Sinopharm vaccine or Sinovac-CoronaVac), injection site and vaccinator. All subjects were not vaccinated during the hospitalization period. In this study, the vaccination status was defined as whether the subjects were vaccinated against COVID-19 before the antibody test and hospitalization. Participants who were vaccinated before antibody testing were considered to have a history of vaccination, and those who were vaccinated on or after antibody testing were considered to have no vaccination. All detailed protocols followed the principles of our institutional research ethics committee and were in accordance with the Declaration of Helsinki. All patient data were anonymized for further analysis. This study was exempted from informed consent as it was a retrospective study, but it was approved by the Medical Ethics Committee of Enze Hospital, Zhejiang Province, China (No: K20210706).
Serological assay
For each participant, 3 mL of peripheral venous blood was drawn upon admission to the hospital, and serum samples were separated from the blood. Serum samples were assayed for qualitative detection of total antibodies (IgM and IgG) against the RBD domain of S1 protein using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Wantai SARS-COV-2 Ab ELISA, Beijing Wantai Biopharmaceutical Co., Ltd., China). Samples with a cutoff ratio higher than 1.0, were considered positive. The sensitivity and specificity of RT-PCR were 86% and 100%, respectively.Citation9 All serological tests were performed at the Clinical Laboratory, Enze Hospital, Taizhou Enze Medical Center (Group).
Statistical analysis
Based on a case-control design, we estimated that an enrollment target of 88 participants would provide the study with greater than 80% statistical power to detect a 30% or more difference in exposure proportion of vaccination between the seropositive group and the seronegative group at a significance level of 0.05 using a two-tailed test.Citation18,Citation19
Continuous data, including age and days after the first vaccination dose, were expressed as mean ± standard deviation and compared between negative and positive serological participants using a two-sample independent t-test. Counts and frequency distributions were displayed for categorical variables, and chi-squared tests or Fisher’s exact tests were used to compare the differences between the negative and positive serological groups. Vaccination status among negative and positive serological participants was compared using the chi-squared test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to assess the association between vaccination doses and seropositivity using the binary logistic regression model with age and sex adjustment. All the data were analyzed using IBM SPSS Statistics software (version 22.0; SPSS Inc., Chicago, IL, USA). All the tests were two-tailed, and a P-value <0.05 or below was considered statistically significant.
Results
Basic characteristics of study participants
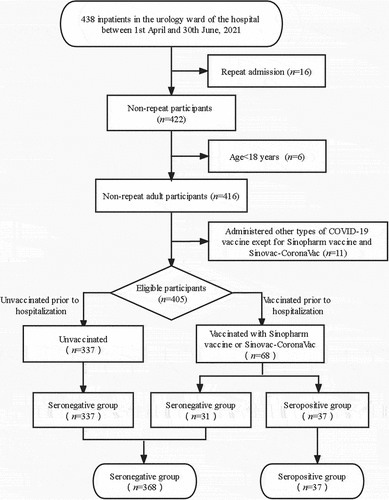
The recruitment for the study subjects are shown in the flow diagram (). The study included 405 hospitalized patients from the Department of Urology with an anti-SARS-CoV-2 antibody assay. Based on serological results, the patients were divided into 37 seropositive (case) and 368 seronegative (control) groups. The average age (63.2 ± 14.8 years vs. 66.4 ± 14.1 years, P = 0.192), proportion of sex (male: 86.5% vs. 77.7%, P = 0.216) and hospitalization days (6.29 ± 5.38 vs. 6.70 ± 5.09, P = 0.648) were not different between seropositive (case) and seronegative (control) groups (). Similarly, there were no differences in sex and age distribution between the vaccinated and unvaccinated participants (P > 0.05).
Table 1. Characteristics of participants between seropositive and seronegative group (n = 405).
SARS-CoV-2 seroconversion and vaccination status
As displayed in , all the 37 positive serologic participants had received at least one dose of COVID-19 vaccine and were assessed for at least 16 days. Of the 368 participants with negative serologic results, 91.6% (337/368) were not vaccinated, and 8.4% (31/368) had received at least one dose of COVID-19 vaccine.
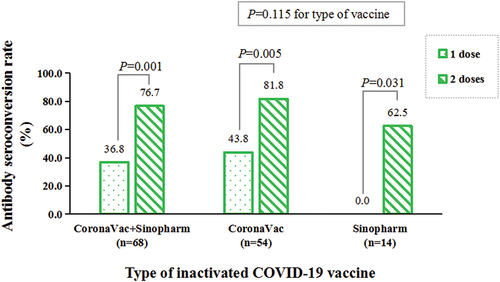
showed that the overall seroconversion rate was 54.4% (37/68) in the participants vaccinated with the inactivated SARS-CoV-2 vaccine. The antibody response to different types of the inactivated SARS-CoV-2 vaccines were similar (59.3% for CoronaVac vs. 35.7% for Sinopharm, P = 0.115). None of the unvaccinated participants had seropositive antibody.
Table 2. The seroconversion rate of the vaccinated participants with the inactivated SARS-CoV-2 vaccines (n = 68).
SARS-CoV-2 serological status and vaccination doses
shows the vaccination data between seropositive and seronegative groups within the vaccinated participants. No differences were observed in the CoronaVac or Sinopharm vaccines for the antibody response to the SARS-CoV-2 vaccine (P > 0.05). Among the 37 seropositive participants, 14 (37.8%) were tested after the first vaccination dose and 23 (62.2%) were tested after the full scheduled vaccination with two doses. Of the 31 seronegative participants who were vaccinated, 24 (77.4%) received only one dose and 7 (22.6%) received two doses of the vaccine before serological tests.
Table 3. Vaccination characteristics between seropositive and seronegative group within the vaccinated participants (n = 68).
The antibody seroconversion rate of participants vaccinated with one or two doses of the inactivated SARS-CoV-2 vaccine are also shown in and . The antibody seroconversion rate was significantly higher in the participants vaccinated two doses than in those vaccinated only one dose, irrespective of type of the inactivated vaccine (P = 0.001).
Figure 2. The antibody seroconversion rate of the vaccinated participants with one or two doses of the inactivated SARS-CoV-2 vaccines (CoronaVac or Sinopharm) (n=68).
The crude OR for seropositivity was 5.63 (95% CI: 1.93–16.46, P = 0.002) in full vaccination with two doses versus partially vaccinated participants with one dose. The magnitude of the estimated association between vaccination doses and seroconversion remained large after adjusting for sex and age (OR = 6.20 , 95% CI: 2.05–18.71, P = 0.001).
SARS-CoV-2 serologic status and vaccination days
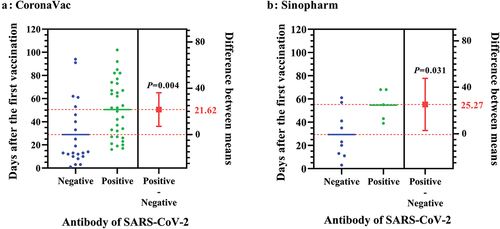
The vaccination days between seropositive and seronegative participants vaccinated with the CoronaVac or Sinopharm vaccine are presented in and . Among the seropositive participants, 14 (37.8%) were assessed after the first vaccination dose for at least 16 days, and 23 (62.2%) were evaluated after the second vaccination dose for 8–76 days and after the first vaccination dose for 37–102 days. Among the 31 seronegative patients after vaccination, 24 (77.4%) were tested after the first vaccination dose for 1–94 days and 7 (22.6%) were tested after the second vaccination dose for 1–60 days and after the first vaccination dose for 23–91 days. The interval between the first vaccination and serologic test was less than 14 days in 45.2% of seronegative individuals (). In the vaccinated participants, the interval days from the first vaccination to serological test were 22.05 days longer in seropositive cases than in seronegative controls (51.08 ± 23.56 vs. 29.03 ± 25.52 days, P < 0.001). The differences in the interval days between the seropositive and seronegative groups were similar for the two types of the inactivated SARS-CoV-2 vaccine ( and ).
Discussion
Clinical implications
In this study, all patients were negative SARS-CoV-2 nucleic acid prior to admission due to the regular prevention and control measures in the hospital. Only 54.4% (37/68) of COVID-19 vaccinated subjects were able to elicited specific antibodies. In contrast, none of the non-vaccinated subjects had antibodies for anti-SARS-CoV-2, suggested no prior history of infection during the active pandemic.
To the best of our knowledge, this study provides the first real-world evidence for the evaluation of immunological reactions to the inactivated SARS-CoV-2 vaccine in China. As of 1 June 2021, at least 13 different vaccines (across four platforms) have been administered, and six different vaccines have been listed for the WHO EUL.Citation14 The two inactivated virus vaccines (Sinopharm vaccine and Sinovac-CoronaVac) were approved for mass vaccination in China. Although there is growing academic evidence on the usefulness and protection of SARS-CoV-2 vaccines and a phase III randomized controlled study in Indonesia showed the seroconversion rate at 14 days after the second injection of the SARS-CoV-2 inactivated vaccine was 97.48% using IgG antibody and 87.15% using neutralization antibody in healthy adults aged 18–59 years,Citation20 limited pragmatic data exist regarding the effectiveness of the inactivated vaccines based on non-control settings in Chinese population.Citation21–23 A comparative study showed that the positive IgG rate was 85.7% at six weeks after fully received Sinopharm vaccine and 99.3% in Pfizer-BioNTech vaccine recipients.Citation24 In the present study, the overall low seroconversion rate (54.4%) was observed, but the antibody response to full-schedule vaccination was much higher than that of no or partial vaccination, which indicated that it was extremely urgent to booster vaccination. This real-world information regarding the effectiveness of vaccination indicates a positive consequence in adults.
It is known that developing human-use vaccinations requires a few years and probably millions of dollars, particularly when applying new techniques that have not been fully evaluated for effectiveness, safety, or extended to market manufacturing.Citation1 Many vaccines, including recombinant protein-based subunit vaccines, viral-vector vaccines, mRNA vaccines, live weakened vaccines, and inactivated virus vaccines have both effectiveness and disadvantages, making it difficult to assess which preventive strategy would be safer or more valuable.Citation25 In China, inactivated whole SARS-CoV-2 virus vaccines were developed by the Wuhan Institute of Biological Products and Sinopharm. The whole virus pathogen was cultured in vitro in cell lines, and the infected cells were further inactivated twice by β-propiolactone under specific conditions and further adsorbed to 0.5 mg alum. Phase 1 and Phase 2 trials revealed that 28 days between the first dose and a subsequent booster dose generated higher antibody titers than the shorter interval group (14 days interval).Citation26 The interim analysis of the inactivated vaccine also indicated considerable safety and better immunogenicity, supporting its long-term adverse events evaluation in later studies.Citation27 Our results indicate a satisfactory antibody response to inactivated SARS-CoV-2 virus vaccines. This is consistent with the results of a case-control study, in which the two-dose dosing scheme with the CoronaVac vaccine was effective in protecting against the Delta variant infection in real-world settings.Citation28
Currently, no fully effective drugs are available to treat COVID-19. Although many clinical therapeutic methods are being tried to treat SARS-CoV-2 infections, the only treatments being used worldwide to combat this new infectious disease only help relieve patients’ symptoms.Citation29 These deficiencies of current strategies highlight the essential requirement for vaccines against SARS-CoV-2. The immune system plays a significant role in the pathogenesis of SARS-CoV-2 infection, and an understanding of the immune response and of the underlying mechanism is required to manufacture a cost-effective vaccine. The inactivated SARS-CoV-2 vaccines with empirical evidence of safety and effectiveness are worth promoting in order to prevent infection and contain the COVID-19 pandemic.
Strengths and limitations
The main strengths of our study include the real-world design to better reflect real life, data collection using an active surveillance method, and very limited missing data. We not only asked patients to recall whether they had received the COVID-19 vaccine, but also checked the vaccination records according to each patient’s ID. Accordingly, recall bias need not be considered in this study.
However, there are still several limitations that should be noted when interpreting the findings of this case-control study. First, in the collection of the study samples, hospitalized patients from one clinical department likely presented a selection bias. More older male than female patients were included due to the urological department. A portraits bias may be introduced, because of differences in immune responses between the genders. Some patients with cancer were included in this study, whose antibody responses to vaccine were reported to be relatively poor.Citation30,Citation31 Second, it is difficult to adjust for other potentially important confounding variables because of the lack of communication in this database. The explanation of the findings is, thus, restricted to some aspects. Third, the time required for antibody testing was uncertain. The interval days between the first vaccination and serological testing at admission ranged from 16 to 102 in the seropositive group, and from 1 to 94 in the seronegative group. The partial vaccination and short interval from vaccination to serological testing may be responsible for the lower seroconversion rate (54.4%) compared to the reported rate in literature.Citation24 Furthermore, total antibody, rather than a neutralizing antibody was detected. Fourth, the rate of vaccination in the study population was relatively low (16.8%, 68/405). The study period was the initial stage of mass vaccination campaign in general adult population over 18 years of age in mainland China. The cumulative doses administered nationwide were 124.4675 million by June 30, which was only 45.5% of those (273.2749 million) have been administered as of 23 December 2021. Moreover, we did not include vaccination information after antibody testing. Fifth, this study was a case-control design. The serological assay was only done once before admission, so we did not observed how the SARS-CoV-2 antibody changes over time. Finally, our study only included patients from one medical center hospital in China as the study sample. Therefore, the results may not be generalized or widely applicable to hospitals in other regions of China.
Although the statistical power achieve 80%, the sample size of the seropositive group is still small. Future prospective clinical studies with larger sample sizes in hospitals over a wider range of regions would validate these findings. The immunopathological basis of COVID-19 still needs to be further evaluated so that its immune evasion mechanism can be better understood, in order to provide more effective vaccine planning schemes.
Conclusions
Our study demonstrates that the inactivated COVID-19 vaccine has a considerable antibody response in adults. The evidence may help boost confidence in the effectiveness of the vaccine Further longitudinal studies that provide more data for different groups are warranted.
Author contributions
F.P.L., T.H.T., and M.X.Z. conceived the study and participated in its design and coordination. T.H.T. and M.X.Z. conducted the study and drafted the manuscript. G.F.S. and Z.Z.L. collected and checked the data, X.L.Z., and L.J.W. participated in the coordination of the study and data collection. All of the authors read and approved the final manuscript.
Data sharing statement
All data underlying the findings are within the paper.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Enze Hospital, Taizhou Enze Medical Center (Group) of Zhejiang Province in China (Reference No. K20210706, approved on 27 July 2021). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. All data, including demographic, serological and vaccination information, are collected on the basis of measures to block hospital and community transmission. Personal information is not involved in this article.
Acknowledgments
We are grateful to our colleague Zhe-Han Zhou in the information centre for his assistance in providing us with relevant information on inpatients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Awadasseid A, Wu Y, Tanaka Y, Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int J Biol Sci. 2021;17(1):1–8. doi:10.7150/ijbs.52569.
- MacDonald NE, et al.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi:10.1016/j.vaccine.2015.04.036.
- Dubé È, Ward JK, Verger P, MacDonald NE. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev Public Health. 2021;42(1):175–191. doi:10.1146/annurev-publhealth-090419-102240.
- Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, Cornaby C, Bartelt L, Weiss S, Park Y, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48):eabc8413. doi:10.1126/sciimmunol.abc8413.
- Tan Y, Liu F, Xu X, Ling Y, Huang W, Zhu Z, Guo M, Lin Y, Fu Z, Liang D, et al. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front Med. 2020;14(6):746–751. doi:10.1007/s11684-020-0822-5.
- Yao YF, Wang ZJ, Jiang RD, Hu X, Zhang HJ, Zhou YW, et al. The multifaceted roles of TAM receptors during viral infection. Virol Sin. 2021;36(1):1–11. doi:10.1007/s12250-020-00264-9.
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang Y-Y, Xiao G-F, Yan B, Shi Z-L, et al. Molecular and serological investigation of 2019-nCov infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. doi:10.1080/22221751.2020.1729071.
- Sule WF, Oluwayelu DO. Real-Time RT-PCR for COVID-19 diagnosis: challenges and prospects. Pan Afr Med J. 2020;35(Suppl 2):121. doi:10.11604/pamj.supp.2020.35.2.24258.
- Ejazi SA, Ghosh S, Ali N. Antibody detection assays for COVID-19 diagnosis: an early overview. Immunol Cell Biol. 2021;99(1):21–33. doi:10.1111/imcb.12397.
- La Marca A, Capuzzo M, Paglia T, Roli L, Trenti T, Nelson SM. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online. 2020;41(3):483–499. doi:10.1016/j.rbmo.2020.06.001.
- Jing R, Kudinha T, Zhou ML, Xiao M, Wang H, Yang WH, Xu Y-C, Hsueh P-R. Laboratory diagnosis of COVID-19 in China: a review of challenging cases and analysis. J Microbiol Immunol Infect. 2021;54(1):17–26. doi:10.1016/j.jmii.2020.10.004.
- Peterhoff D, Glück V, Vogel M, Schuster P, Schütz A, Neubert P, Albert V, Frisch S, Kiessling M, Pervan P, et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49(1):75–82. doi:10.1007/s15010-020-01503-7.
- Xiao SY, Wu Y, Liu H. Evolving status of the 2019 novel coronavirus infection: proposal of conventional serologic assays for disease diagnosis and infection monitoring. J Med Virol. 2020;92(5):464–467. doi:10.1002/jmv.25702.
- World Health Organization (WHO). What vaccines are there against COVID-19? [accessed 2021 Oct 7]. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey=%7badgroupsurvey%7d&gclid=CjwKCAjwuvmHBhAxEiwAWAYj-JdhqOJLAI512AEUA168OCO7onh05UxiEbFdFvkq_s2XdNWfz2YWqRoCICkQAvD_BwE.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi:10.1126/science.abc1932.
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. doi:10.1016/j.cell.2020.06.008.
- Gokhale M, Stürmer T, Buse JB. Real-World evidence: the devil is in the detail. Diabetologia. 2020;63(9):1694–1705. doi:10.1007/s00125-020-05217-1.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New Jersey (NJ): Routledge; 1988.
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149.
- Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, Khrisna CV, Sari RM, Setyaningsih L, Surachman F, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520–6528. doi:10.1016/j.vaccine.2021.09.052.
- Benjamanukul S, Traiyan S, Yorsaeng R, Vichaiwattana P, Sudhinaraset N, Wanlapakorn N, Poovorawan Y. Safety and immunogenicity of inactivated COVID-19 vaccine in health care workers. J Med Virol. 2021;94(4):1442–1449. ( Epub ahead of print). doi:10.1002/jmv.27458.
- Jantarabenjakul W, Chantasrisawad N, Puthanakit T, Wacharapluesadee S, Hirankarn N, Ruenjaiman V, Paitoonpong L, Suwanpimolkul G, Torvorapanit P, Pradit R, et al. Short-Term immune response after inactivated SARS-CoV-2 (CoronaVac®, Sinovac) and ChAdox1 nCov-19 (Vaxzevria®, Oxford-AstraZeneca) vaccinations in health care workers. Asian Pac J Allergy Immunol. [accessed 2021 Oct 31]. ( Epub ahead of print). doi:10.12932/AP-250721-1197.
- Ma Y, Liu N, Wang Y, Zeng J, Hu YY, Hao W, Shi H, Zhu P, Lv J, Fan W, et al. Immune checkpoint blocking impact and nomogram prediction of COVID-19 inactivated vaccine seroconversion in patients with cancer: a propensity-score matched analysis. J Immunother Cancer. 2021;9(11):e003712. doi:10.1136/jitc-2021-003712.
- Alqassieh R, Suleiman A, Abu-Halaweh S, Santarisi A, Shatnawi O, Shdaifat L, Tarifi A, Al-Tamimi M, Al-Shudifat A-E, Alsmadi H, et al. Pfizer-BioNtech and Sinopharm: a comparative study on post-vaccination antibody titers. Vaccines (Basel). 2021 Oct 21;9(11):1223. doi:10.3390/vaccines9111223.
- Rawat K, Kumari P, Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. doi:10.1016/j.ejphar.2020.173751.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi:10.1016/S1473-3099(20)30843-4.
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al.; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi:10.1016/S0140-6736(21)01429-X.
- Li XN, Huang Y, Wang W, Jing QL, Zhang CH, Qin PZ, Guan W-J, Gan L, Li Y-L, Liu W-H, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case–control real-world study. Emerg Microbes Infect. 2021;10(1):1751–1759. doi:10.1080/22221751.2021.1969291.
- Biswas P, Hasan MM, Dey D, Dos Santos Costa AC, Polash SA, Bibi S, Ferdous N, Kaium MA, Rahman MH, Jeet FK, et al. Candidate antiviral drugs for COVID-19 and their environmental implications: a comprehensive analysis. Environ Sci Pollut Res Int. 2021;28(42):59570–59593. doi:10.1007/s11356-021-16096-3.
- Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, Mesa-Chavez F, Barrientos-Gutiérrez T, Tagliamento M, Lambertini M, Villarreal-Garza C. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. doi:10.1016/j.ejca.2021.10.014.
- Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi:10.1001/jamaoncol.2021.2675.