ABSTRACT
SARS-CoV-2 vaccine uptake in pregnant women is believed to be low and lags behind the general population contributing to increased hospital admissions, and poor maternal and fetal outcomes. However, there is a paucity of information on the SARS-CoV-2 serostatus of pregnant women to help inform policy planning and assess impact of interventions to improve vaccine uptake in this at-risk group. We analyzed 8,683 residual, anonymized newborn screening dried bloodspot (DBS) specimens during a 15-month period (October 2020 to December 2021) in Wales (UK) for SARS-CoV-2 IgG-antibodies. We compared newborn DBS antibody-positive rates to the percentage number of pregnant women vaccinated and the percentage number of antibody-positive adults. In December 2021, 47.8% of women in Wales had received two doses of the vaccine by their delivery date; however, only 41.1% of DBS specimens had high antibody concentrations. Results indicate that a proportion of pregnant women remain at higher-risk of COVID complications, particularly given the reduction in antibody neutralization of Omicron versus the Delta variant. Our study demonstrates the utility of newborn screening DBS specimens to monitor SARS-CoV-2 serostatus in pregnant women representing maternal vaccination and natural infection in almost real-time, defining the immunity gap and impact of any interventions.
Introduction
Evidence suggests that pregnancy itself is a risk factor for more severe outcomes in COVID-19 and that this risk is further increased in those who have preexisting medical conditions (e.g. diabetes or hypertension), BMI >25 kg/m2, maternal age ≥35 years, living in increased socioeconomic deprivation or are from ethnic minority backgrounds, particularly in the third trimester.Citation1 Increased maternal and fetal risk from viral infection during pregnancy and for a period thereafter are well known for influenza due to the immunological, cardiac and pulmonary physiological adaptions during pregnancy.Citation2 While international evidence demonstrates vaccine efficacy in pregnancy, without safety concerns to the developing fetus,Citation3 there remains concern that vaccine uptake in pregnant women is low and lags behind the general population and that this is contributing to increased hospital admissions impacting on maternal and fetal outcomes. However, there is a paucity of up-to-date information on the SARS-CoV-2 serostatus of pregnant women to help inform policy planning and assess impact of interventions to improve vaccine uptake in this at-risk group. Here, we report on the utility of newborn dried blood spot (DBS) screening specimens to facilitate real-time sero-surveillance of SARS-CoV-2 IgG antibody status in pregnant women.
Materials and methods
We analyzed residual, anonymized newborn-screening DBS specimens received into the Wales Newborn Screening Laboratory, during the first week of every month over a 15-month period (n = 8,683) for SARS-CoV-2 IgG antibodies (S1 domain-SARS-CoV-2 spike-protein), using the EUROIMMUN (PerkinElmer) enzyme-linked immunosorbent assay (ELISA) and performed on an automated platform (DSX, Dynex Technologies, USA) to enable high throughput analysis. This assay will identify individuals who have developed antibodies following both infection and/or vaccination. The antibody results are evaluated using an assay-specific calibrator to report the ratio of the specimen absorbance to the calibrator absorbance to calculate a cutoff index (CI) value. The CI is interpreted as follows: <0.8 negative; ≥0.8 to <1.0 borderline; ≥1.1 positive. Borderline results were considered positive and those ≥6.0 as strongly positive. Validation of the assay demonstrates that plasma and DBS specimens produce equivalent results.Citation4,Citation5 Antibodies detected in newborn DBS specimens reflect maternal antibodies due to neonatal Fc receptor (FcRn) mediated transplacental transfer during pregnancy. The concentrations of SARS-CoV-2 IgG antibodies in screening DBS specimens have previously been shown to reflect the overall population-level trends in case incidence, with a lag that is consistent with the time to the development of detectable antibodies after infection.Citation6
Data on the number of individuals aged 16 years and over who had tested for positive for SARS-CoV-2 antibodies (December 2020 to July 2021) were obtained from the Welsh Government COVID-19 Infection Survey.Citation7 Data on vaccination coverage in pregnant women was sourced from the Welsh Immunization System and linked to maternity datasets using the mother’s NHS number.Citation8 We compared the DBS screening specimen SARS-CoV-2 IgG antibody-positive rates to the percentage number of women vaccinated (dose 1 & 2) by their delivery date and to the overall percentage of adults in Wales (U.K.) testing positive for antibodies to SARS-CoV-2.
Research Ethics Committee approval (REC 20/SW/0104) for the COVID-19 Dried Bloodspot Antibody Measurement (DREAM) study was obtained (20/NE/0176). However, this study was deemed exempt as residual anonymized specimens were used.
Results
Despite the high background rates for SARS-CoV-2 infection in the general population at the end of 2020 and during 2021, the overall number of newborn DBS specimens with positive antibody-titers remained relatively low throughout this period (October 2020 (1.5%) and March 2021 (12.5%)) compared to 58% of individuals aged ≥16 years in the Wales population for March 2021 (). In July 2021, over 9 in 10 people (93.2%) aged ≥16 years tested positive for antibodies (95% credible interval: 91.8% to 94.5%),Citation7 whereas only 26.2% of newborn DBS specimens were positive.
Figure 1. Percentage number of DBS screening specimens with positive SARS-CoV-2 antibody titres, the Wales population estimates for positive antibody status and the percentage number of pregnant women vaccinated by their delivery date (dose 1 and dose 2). The antibody cut-off index (CI) value is interpreted as follows: <0.8 negative; ≥0.8 to <1.0 borderline; ≥1.1 positive. Borderline results were considered positive and those ≥ 6.0 as strongly positive.
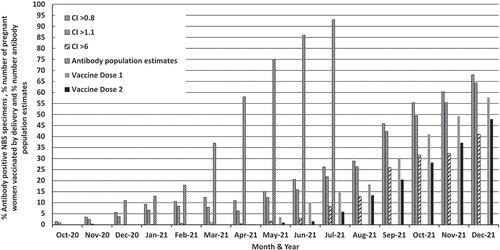
In December 2021, it was reported that 47.8% of pregnant women had received two doses of the vaccine by their delivery date,Citation8 whilst 68.0% of DBS specimens tested positive for antibodies during December 2021; however, only 41.1% had very high antibody concentrations.
Discussion
The results of our study demonstrate the utility of newborn DBS screening specimens to monitor SARS-CoV-2 serostatus in pregnant women representing maternal vaccination and natural infection in almost real-time.
Vaccination against COVID-19 was available for some pregnant women from January 2021 during phase 1 of the roll-out, where there was a high risk of exposure to SARS-CoV-2 for occupational reasons; however, the vaccine was not recommended for routine use in pregnant women at this time.Citation9 The recommendation to routinely include pregnant women in the COVID-19 vaccination program was made in April 2021.Citation10 In December 2021, following recognition of pregnancy as a risk factor for severe outcomes from infection with the Delta variant of SARS-CoV-2, pregnant women were added to list of clinical risk groups for whom vaccination should be prioritized.Citation11 Our data indicate that a significant proportion of pregnant women could still be considered to be at higher risk of COVID complications, particularly given the reduction in antibody neutralization of Omicron versus the Delta variant.Citation12 Furthermore, these findings could also be indicative of waning immunity following primary vaccination. In contrast, uptake of the influenza vaccination in Wales in pregnant women is 82% indicating the significant delay and challenges in ‘catch-up’ efforts for SARS-CoV-2 vaccination specifically.Citation13
Vaccine coverage in pregnant women has remained lower than that in the general population. The reasons for this and the much lower seropositivity in pregnant women compared to the population as a whole may be due to several factors; (1) vaccine hesitancy following early messaging around initial limited evidence of vaccine safety in pregnancy (2) non-evidence-based concerns about effects on fertility or the wellbeing of the baby (and the changing narrative as more evidence emerged about the risks of infection) and (3) reflective of other protective behaviors within the non-vaccinated group to reduce their risk of natural infection, e.g. limiting social contacts. Vaccination is of benefit to both mother and baby during and after pregnancy as maternal antibodies passively transferred to the fetus across the placenta (IgG) particularly during the third trimester and in breast milk (IgA) offer protection.Citation14 A further benefit of vaccination in this group is that seropositive protected individuals would be less likely to require additional interventions such as monoclonal antibodies or antiviral drugs. These therapies are aimed primarily at immunosuppressed individuals unable to mount an immune response preserving this expensive and limited resource. Pressure on Extracorporeal membrane oxygenation (ECMO) capacity would also be reduced as pregnant women accounted for 32% of ECMO facility use.Citation15
There are several limitations to our study in that the type and timing of the vaccination were not recorded. In addition, we were unable to distinguish between vaccine-induced antibodies and infection-induced antibodies and that the lag time in development of antibodies may result in antibodies not being detected in the newborn screening DBS specimen. However, used in combination with available vaccination surveillance data and case surveillance data, the DBS antibody data can still provide useful information in determining the need for additional population interventions to improve protection from severe outcomes of COVID-19 in this group. To ensure maximum utility of the newborn screening DBS antibody testing approach, the SARSCoV-2 antibody titers should be linked to the mother’s health records (using demographic data that is recorded on the newborn screening card) to identify additional risk factors (e.g. age, BMI, ethnicity and social deprivation) and to ensure that seronegative mothers are offered vaccination given that the risk period extends beyond delivery.
A strength of our study is that we estimated the incidence of infection and vaccine response during a period of high community prevalence of SARS-CoV-2 that was associated with the rapid emergence of both the Delta and subsequent Omicron variants and during vaccine roll out in the UK. Furthermore, the reported coverage for the Wales newborn blood spot screening program is >99% which enables results to be both representative and generalizable to the pregnant population (and one of the most relevant indicators of immunity at a point in time reflective of post-partum risks of infection).
Although surveillance data are available on vaccination coverage in pregnant women, it is challenging to obtain robust information systematically across Wales in a timely manner. Many women currently pregnant will have been vaccinated months before their pregnancy began and with issues of waning immunity, historical vaccination status may not be a reliable marker of protection during the current period of risk. Use of serological testing of residual DBS samples provides a reliable and relatively low-cost method of systematically monitoring levels of protection in pregnant women during their period of highest risk.
Serology for SARS-CoV-2 is essential for population-level surveillance; to better understand immunity conferred by infection and vaccination(s) against variants as they emerge, to assess dose combinations and boosters that are required for long-term protection and to assess waning humoral immunity in the population. Serology is also important for individual-level risk assessment in susceptible patient groups to improve immunity by providing additional vaccine doses and to improve targeted therapeutic interventions. Using the newborn DBS antibody testing approach data can be generated using specimens routinely collected that are close to real-time and enable assessment of any changes in vaccination policy and messaging. The results also underpin the need to enhance efforts to improve vaccine uptake in this group supported both by the accumulation of safety data of vaccination for mother and child and by the growing evidence that pregnancy is a risk for adverse outcomes in COVID, as has been shown for influenza. Furthermore, this work demonstrates that maternal antibodies pass to their baby and can be measured in their first days of life. Vaccination protecting children is now supported by the data that vaccination of children prevents pediatric multisystem inflammatory syndrome (PIMSTS) in over 90% of children.Citation16
High throughput analysis of DBS specimens facilitates large-scale sero-surveillance which does not require phlebotomy staff, blood collection tubes, patient travel or risk nosocomial infection exposure. At present, we are not routinely testing the newborn DBS specimens for SARS-CoV-2 antibodies. However, we are seeking funding to continue testing and to assess various approaches for linking antibody titers to the mother’s health records not only for SARS-CoV-2, but to monitor immunity from other infections for which there are maternal vaccination programs, such as influenza and pertussis, especially as the primary aim for the pertussis vaccination program is to provide immunity to neonates and infants too young for their routine primary vaccinations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Centers for Disease Control and Prevention. COVID-19 vaccination for pregnant people to prevent serious illness, deaths, and adverse pregnancy outcomes from COVID-19. 2021 Sept 29 [accessed 2022 Apr 4]. https://emergency.cdc.gov/han/2021/han00453.asp.
- Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):1–4. doi:10.3201/eid1401.070667.
- Medicines & Healthcare products Regulatory Agency. Coronavirus vaccine - weekly summary of yellow card reporting. gov.uk. 2021 [accessed 2022 Apr 4]. www.gov.uk/government/publications/coronavirus-covid-19-vaccineadverse-reactions/coronavirus-vaccine-summary-of-yellow-cardreporting
- Zava T, Zava D. Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays. Bioanalysis. 2020;13(1):13–28. doi:10.4155/bio-2020-0289.
- Moat S, Zelek W, Carne E, Ponsford MJ, Bramhall K, Jones S, El-Shanawany T, Wise MP, Thomas A, George C, et al. Development of a high-throughput SARS-CoV-2 antibody testing pathway using dried blood spot specimens. Ann Clin Biochem. 2021;58(2):123–131. doi:10.1177/0004563220981106.
- Liu F, Nguyen M, Vijayakumar P, Kaplan A, Meir A, Dai Y, Wang E, Walsh H, Ring AM, Omer SB, et al. Newborn dried blood spots for serologic surveys of COVID-19. Pediatr Infect Dis J. 2020;39(12):e454–e456. doi:10.1097/INF.0000000000002918.
- Welsh Government -coronavirus (COVID-19) infection survey (antibodies data): 26 to 29 July 2021 analysis of the proportion of people in Wales testing positive for COVID-19 antibodies for 26 to 29 July 2021. 2021 [ accessed 2022 Apr 4]. Coronavirus (COVID-19) infection survey (antibodies data): 26 to 29 July 2021 | GOV.WALES.
- Public Health Wales COVID-19 vaccination enhanced surveillance equality report 13: February 2022. [accessed 2022 Apr 4]. https://www2.nphs.wales.nhs.uk/CommunitySurveillanceDocs.nsf/3dc04669c9e1eaa880257062003b246b/e61c928e715ece3180258680003449c3/$FILE/Wales%20COVID-19%20vaccination%20enhanced%20surveillance%20-%20equality%20report.pdf.
- UK Government - Department of Health & Social Care. Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination. 2020 [accessed 2022 April 4].
- UK Government - Department of Health & Social Care. Joint Committee on Vaccination and Immunisation: interim statement on phase 2 of the COVID-19 vaccination programme. 2021 Feb 26 [accessed 2022 Apr 4]. https://www.gov.uk/government/publications/priority-groups-for-phase-2-of-the-coronavirus-covid-19-vaccination-programme-advice-from-the-jcvi/jcvi-interim-statement-on-phase-2-of-the-covid-19-vaccination-programme.
- UK Health Security Agency. COVID-19: the green book, chapter 14a. Coronavirus (COVID-19) vaccination information for public health professionals. 2022 Feb 28 [accessed 2022 Apr 4]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1057798/Greenbook-chapter-14a-28Feb22.pdf.
- Sheward DJ, Kim C, Ehling RA, Pankow A, Castro Dopico X, Dyrdak R, Martin DP, Reddy ST, Dillner J, Karlsson Hedestam GB, et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: a cross-sectional study. Lancet Infect Dis. 22(6):813–820. [2022 Mar 17]. doi:10.1016/S1473-3099(22)00129-3.
- Public Health Wales – Vaccine Preventable Disease Programme. Coverage of pertussis and influenza vaccination in pregnant women in Wales 2020/21. [accessed 2022 Apr 4]. https://www2.nphs.wales.nhs.uk/CommunitySurveillanceDocs.nsf/3dc04669c9e1eaa880257062003b246b/452c3aab0c3e7d70802586f6002f2b68/$FILE/Coverage%20of%20pertussis%20and%20influenza%20vaccination%20in%20pregnant%20women%20in%20Wales%202020-21_v1.pdf.
- Post AL, Li SH, Berry M, Itell H, Martinez DR, Xie G, Permar SR, Swamy GK, Fouda GG. Efficiency of placental transfer of vaccine-elicited antibodies relative to prenatal Tdap vaccination status. Vaccine. 2020;38(31):4869–4876. doi:10.1016/j.vaccine.2020.05.036.
- The Royal College of Obstetricians and Gynaecologists (RCOG). Maternity colleges urge pregnant women to have flu vaccine and COVID-19 vaccine this winter | RCOG.
- Zambrano LD, Newhams MM, Olson SM, Halasa NB, Price AM, Boom JA, Sahni LC, Kamidani S, Tarquinio KM, Maddux AB, et al. Effectiveness of BNT162b2 (Pfizer-BioNtech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years — United States, July–December 2021. MMWR Morb Mortal Wkly Rep. 71(2):52–58. [2022 Jan 14]. doi:10.15585/mmwr.mm7102e1.
