ABSTRACT
A dose-escalation, randomized, double-blind, placebo-controlled phase 1 clinical trial enrolled 145 eligible participants aged 18–55 years in March 2015 in Liuzhou, China. Stratified by age and sex, the participants were randomly assigned to receive either 30, 60, or 90 μg of the HPV-6/11 vaccine (n = 41/40/40) or the parallel placebo vaccine (n = 8/8/8) with a 0/1/6-month dose-escalation schedule. Participants were actively followed-up to record local and systemic AEs occurring within 30 days after each vaccination, and SAEs occurred in 7 months. Blood and urine samples of each participant were collected before and 2 days after the first and third vaccination to determine changes in routine blood, serum biochemical, and urine indexes. Serum HPV-6/11-specific IgG and neutralizing antibody levels at month 7 were analyzed. A total of 79 adverse events were reported, and no SAEs occurred. The incidences of total adverse reactions in the 30 μg, 60 μg, and 90 μg HPV vaccine groups and the control group were 31.7%, 50.0%, 42.5%, and 62.5%, respectively. All but one of the adverse reactions was mild or moderate with grade 1 or 2. No vaccine-related changes with clinical significance were found in paired blood and urine indexes before and after vaccinations. All the participants in the per-protocol set seroconverted at month 7 for both IgG and neutralizing antibodies. The candidate novel Escherichia-coli-produced bivalent HPV-6/11 vaccine has been preliminarily proven to be well tolerated and with robust immunogenicity in a phase 1 clinical study, supporting further trials with larger sample size. The study has been registered at ClinicalTrials.gov (NCT02405520)
Introduction
Genital warts (GWs, also known as anogenital warts or condylomata acuminata) are one of the most common sexually transmitted diseases, and more than 90% of GWs are associated with human papillomavirus (HPV) type 6 and 11 infection.Citation1 Estimating the incidence of GWs is challenging, as GWs are not a mandatory reported condition in many countries.Citation2,Citation3 In China, where sexual activity is relatively conservative, the incidence of GWs reported from the National Notifiable Disease Report System is 0.24–0.29 per 1000 person-yearsCitation4; however, data from a prospective study showed that the incidence was almost ten times higher in Liu Zhou, southern China.Citation2 Based on a systematic review, the estimated global incidence of GWs is approximately 1.6 to 2.9 per 1000 person-years.Citation5 In some high-risk groups, such as 15–26-year-old people, sex workers, men who have sex with men, or HIV-infected individuals, the reported incidences were over 10 per 1000 person-years.Citation6–9 With high recurrence rates and difficulty in healing, GWs cause tremendous psychological pressure and economic burden on patients.Citation10,Citation11
Currently, there are two commercial HPV vaccines available that contain HPV types 6 and 11: a quadrivalent HPV vaccine (Gardasil®, HPV-6/11/16/18) and a nonavalent HPV vaccine (Gardasil®9, HPV-6/11/16/18/31/33/45/52/58). Real-world studies proved the effectiveness of the HPV vaccine, which dramatically reduced the disease burden of GWs in countries with high coverage of the quadrivalent HPV vaccine.Citation12–15 However, the primary indication for HPV vaccines is cervical cancer caused by high-risk HPV infection, such as HPV type 16/18, which has much higher disease burden than other HPV-related cancers and diseases. Thus, relevant immunization strategies and immunization guidelines usually regard women between 9 and 14 years old (usually without sexual debut) as the main target population for vaccination,Citation16,Citation17 leaving some genital wart high-risk groups (such as sex workers and MSM group) with less opportunity to obtain vaccine protection.Citation18,Citation19 The insufficient supply of HPV vaccines further impedes the control of GWs worldwide.
In this study, a randomized and double-blind phase 1 clinical trial was conducted in Liuzhou city, Guangxi Zhuang Autonomous Region of China, to determine the safety and immunogenicity of a new Escherichia coli-produced bivalent HPV-6/11 L1 VLP vaccine candidate. This candidate HPV-6/11 bivalent vaccine is produced by an expression platform similar to that of the licensed Escherichia coli-produced recombinant HPV-16/18 bivalent vaccine (Cecolin®, Xiamen Innovax) in China.Citation20,Citation21
Methods
Study design and participants
The study was a dose-escalation, randomized, double-blind, placebo-controlled phase 1 clinical trial in adult healthy volunteers conducted between March 2015 and April 2016 to assess the safety and immunogenicity of different dosages of HPV-6/11 vaccines. This study was approved by the independent ethics committee of Guangxi Center for Disease Prevention and Control (GXIRB2014–0042), and the study was conducted in accordance with good clinical practice. The study has been registered at ClinicalTrials.gov (NCT02405520). Healthy adults aged 18–55 years living in Liuzhou city, Guangxi Zhuang Autonomous Region, China, were recruited (). Written informed consent was obtained from every volunteer. Participants were screened according to the inclusion and exclusion criteria for eligibility and would be excluded if they had any one of the followings: 1) had received any other study drug or vaccine within 30 d before receiving the study vaccine; 2) had received immune globulin and/or blood preparations within 3 months, inactivated vaccines within 14 d, or live vaccines within 21 d before receiving the study vaccine; 3) had any preexisting severe, acute, or chronic disease; 4) were currently pregnant or lactating; 5) had severe anaphylaxis history for any vaccine ingredient; 6) had an axillary body temperature higher than 37.0°C; or 7) had any other condition that, judged by the investigator, could prevent the participant from complying with this protocol or signing their informed consent.
Table 1. Baseline and demographic characteristics of the participants.
Vaccines
All vaccines used in the clinical trial were produced by Xiamen Innovax, Xiamen, China, satisfying good manufacturing practice (GMP) conditions according to the requirements of the National Medical Products Administration of China (NMPA). The bivalent HPV-6/11 vaccine was a mixture of two aluminum hydroxide adjuvant-absorbed recombinant L1 VLPs of HPV-6 and HPV-11 separately expressed in E. coli as reported previously. The candidate vaccine was formulated to contain either 30 μg, 60 μg, or 90 μg of HPV L1 VLP antigen, in which the amount of HPV-6 L1 VLP was equal to that of HPV-11 L1 VLP, with a total of 0.21 mg of aluminum adjuvant suspended in 0.5 mL of phosphate-buffered saline (PBS). The control placebo vaccine contained 0.21 mg of aluminum adjuvant without HPV antigen and was also suspended in 0.5 mL PBS. The participants allocated to the HPV-6/11 group in stages I to III received dosages of 30 μg, 60 μg, and 90 μg HPV-6/11 bivalent vaccine, respectively.
Procedures
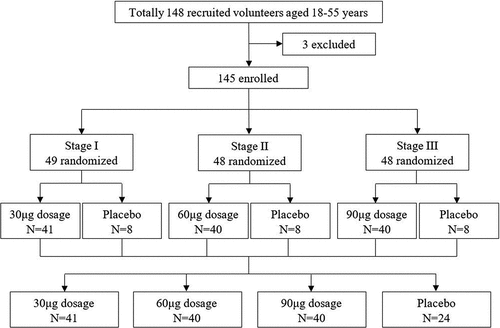
The study contains three stages that were conducted sequentially in a dose-escalating manner. Participants in each stage were stratified by age (18–25 year, 26–35 year, 36–45 year, and 46–55 year) and sex and randomized to receive different dosages of HPV vaccines or the parallel placebo vaccine with a ratio of approximately 5:1 (). Recruitment for the next-stage group did not start unless no vaccine-related serious adverse events occurred within 7 days after the first dose of vaccination in the previous stage. All the eligible participants were vaccinated intramuscularly in the upper arm deltoid muscle at 0, 1 and 6 months.
Figure 1. Trial profile. The dose-escalation phase 1 study was carried out in three stages. Seven days after the first dose of vaccination in each stage, total adverse reactions and events that occurred during the first week were collected and analyzed. If no vaccine-related serious adverse events occurred within the first week, the next stage of study was started. All the participants received three doses of the allocated vaccine according to the protocol.
Safety assessment
All the participants were observed for 30 minutes after each dose for immediate adverse reactions (ARs) and were trained to record all adverse events (AEs) occurring within 30 days after each vaccination in diary cards. Throughout the trial, reporting of all serious adverse events (SAEs) and pregnancy outcomes was requested, and the participants were trained to do so.
Blood and urine samples of each participant were collected before and 2 days after the first and third vaccinations to measure a total of 13 laboratory indexes, including routine blood, serum biochemical, and urine indexes, to assess the possible potential vaccine effects on liver and kidney functions. Among the indexes, there were six routine blood indexes: white blood cell count (WBC), lymphocytes (LY), absolute neutrophil count (ANC), eosinophils (EOS), platelets (PLT), and hemoglobin (HGB); four serum biochemical indexes: total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glucose (GLU); and three routine urine indexes: urinary protein (PRO), urinary glucose (UGLU), and urine occult blood (BLD).
Immunogenicity assessment
Serum samples were collected at day 0 and month 7 (21–60 days after third vaccination) from all participants to evaluate HPV-6/11specific immunoglobulin G (IgG) and neutralizing antibody (nAb) level by E.-coli-expressed VLP-based enzyme-linked immunosorbent assay (ELISA) and pseudovirion-based neutralization assay (PBNA), both of which had been described in the previous studies.Citation20,Citation22,Citation23 The IgG antibody testing in this study was performed at the China National Institute for Food and Drug Control, and the neutralizing antibody testing was performed at Xiamen Innovax Biotechnology Co., Ltd.
Briefly, for the VLP-based ELISA, samples of serially diluted serum, serially diluted reference serum and negative control, were added to the 96-well microtiter plates coated with E.-coli-expressed HPV-6 or HPV-11 VLPs, incubated (45 min, 37°C), and washed plates before the addition of horseradish peroxidase-conjugated goat anti-human IgG. After another incubation (45 min, 37°C) and washing, tetramethylbenzidine solution was added and further incubated for 15 min at 37°C. Finally, the reactions were stopped with the addition of H2SO4 solution, and the optical density (OD) was read at 450/620 nm. The reference standard curve was generated from a serially diluted reference serum pool from HPV vaccine recipients. The unit of HPV-6/11 IgG antibody concentrations was defined as YU/ml, which was a standard unit of in Xiamen Innovax. Serum samples from 296 healthy children of ages 0–36 months were considered as true negative samples with which to optimize the cutoff value (COV). The ROC curve was used to calculate the best OD value (0.15) with the largest Youden coefficient for HPV-6/11, correspondingly, which were 131.0 YU/mL for HPV-6 antibody and 24.0 YU/mL for HPV-11 antibody. For calculating the geometric mean concentration (GMC), negative samples were artificially set as half of COV.
For the PBNA, human embryonic kidney cells, which are also named 293 FT cells, were seeded in 96-well flat-bottom plates (15,000 cells per well) and then incubated for 4 hours (37°C). Samples of serial diluted serum, negative control, and quality control were added to a U-shaped-bottom plate, and then cultured with HPV-6/11 pseudovirions for 1 hour (room temperature). Subsequently, the mixtures were transferred to 96-well flat-bottom plates and co-cultured with 293 FT cells for 72 h (37°C). The 96-well flat-bottom plates were scanned by an Enzyme-Linked Immunospot (ELISPOT) reader and the neutralizing antibody titers of positive sample were defined as the highest dilution capable of inhibiting 50% of the green fluorescent protein (GFP) compared with the negative control, and the negative samples were artificially set as 1:50, which was half of the initial dilution.
Statistical analysis
Full analysis set (FAS) included participants who received at least one dose. Safety set-1 (SS-1) included participants who received at least one dose and had at least one safety visit. Safety set-2 (SS-2) included participants who received at least one dose and had blood and urine test results before and at 2 days after the vaccination. As no participant withdrew from the study or violated the protocol, FAS, SS-1, and SS-2 are the same. The frequency and incidence of ARs in the four groups were compared to determine whether the vaccine groups had a greater chance of ARs than the control group and the existence of dose-dependent effects. Changes in routine blood, serum biochemical, and urine indexes of each participant before and at 2 days after vaccination were classified into three categories: “maintaining” indicated no grade change; “worsening” indicated a change from normal to abnormal or an increase in grade; and “improving” indicated a change from abnormal to normal or a decrease in grade.
Immunogenicity was analyzed in the per-protocol set for immunogenicity (PPS-I). For IgG or neutralizing antibodies, PPS-I set included all participants who received three doses of the vaccine and had no violation of the protocol, were negative for the corresponding antibody against the relevant types of HPV at day 0, and had antibody results at month 7. Seroconversion was defined as an increase in antibody titers of at least four-fold in an individual’s paired serum.
Chi-square tests and two-sided Fisher’s exact tests were used to compare the frequency and incidence of AEs occurring within 30d among the four different groups. The geometric mean concentration (GMC) of IgG antibody, the geometric mean titer (GMT) of neutralizing antibody, and the seroconversion rate (SCR) were summarized with 95% confidential intervals (CIs) by the Clopper–Pearson method or Student’s t distribution, respectively. Statistical comparisons were made using two-sided tests with an alpha value of 0.05. The statistical analyses were performed by an independent statistician using SAS 9.4.
Results
In total, 148 volunteers were recruited, and 145 participants aged 18–55 years old were enrolled, with 49, 48, and 48 participants, respectively, from stage I to III (). Stratified by age and sex, the participants were randomly assigned to receive the HPV-6/11 vaccine (n = 41/40/40) or the control vaccine (n = 8/8/8) at stages I, II, and III. All enrolled participants received three doses of the assigned vaccines according to the protocol.
After unblinding, data from the placebo group were combined. The mean ages were similar in all four groups, including the three dosages of HPV-6/11 vaccine groups and the placebo group. All the groups contained almost equal numbers of females and males, and no participant withdrew from the study or violated the protocol.
A total of 79 adverse events were reported, and no severe adverse events (SAEs) occurred during the 7 months’ follow-up after enrollment. There was no significant difference in the incidences of total adverse events among the three dose groups and the control group. The incidence of total adverse reactions (AR, adverse events possibly related to vaccination) within 30 days in the 30 μg, 60 μg, and 90 μg HPV vaccine groups and the control group was 31.7%, 50.0%, 42.5%, and 62.5%, respectively. Compared with the control group, the three dose groups presented no significant increase in the incidence of local and systemic ARs ().
Table 2. The symptoms of adverse events occurred within 30 days after each vaccination.
The most commonly reported local adverse reaction was pain at the injection site, with 19.5%, 37.5%, 22.5%, and 25.0% of the 30 μg, 60 μg, and 90 μg HPV vaccine group and the placebo group participants, respectively, reported this symptom. Another commonly occurring injection site symptom was itching. All of the reported local adverse reactions were mild (grade 1). For systemic adverse reactions, the most often reported symptom was fever, which was defined as an axillary temperature of 37.3°C or higher and was reported by 0%, 5.0%, 10.0%, and 25.0% of the four vaccine group participants. The incidences of fatigue, cough, and pruritus were also higher than 5%, but no significant difference was found among groups. A total of eight subjects reported systemic adverse reactions of grade 2 or higher, including one symptom of grade 3 in the 30 μg HPV vaccine group, which was judged as “probably related to vaccination” by the investigators. Grade 3 AR was a generalized urticaria occurring on the 13th day after the first vaccination, and the participant recovered on the same day of occurrence after outpatient treatment. The participant did not report similar symptoms during the rest of the study.
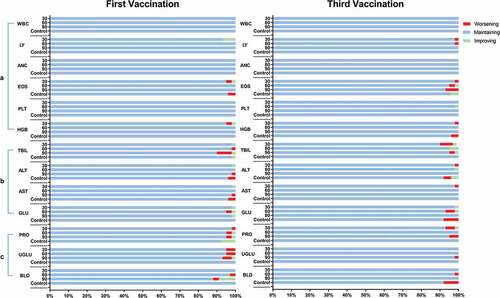
summarizes the changes in paired laboratory parameters, which include routine blood, serum biochemical, and urine indexes before and at 2 days after the first and third vaccinations. Thirteen types of laboratory testing indicators were analyzed, including WBC, LY, ANC, EOS, PLT, HGB, TBIL, ALT, AST, GLU, PRO, UGLU, and BLD, resulting in a total of 3770 pairs of results. Most pairs of parameters (3645, 96.7%) were within the normal range both pre- and post-vaccination, and 50 pairs (1.3%) shifted from abnormal to normal after vaccination. There were also 33 pairs (0.9%) that turned from normal to abnormal after vaccination, and 42 pairs (1.1%) that maintained abnormality. Similar results were observed in categorized analyses, such as routine blood, serum biochemical (commonly reflecting blood coagulation and liver function, respectively), and urine indexes (commonly reflecting kidney function). All these changes were not significant (P > 0.05) by McNemar’s test.
Table 3. The changes of paired blood routine, serum biochemical, and urine routine indexes before and at 2 days after the first and the third vaccination.
The changes in grade in routine blood, serum biochemical, and urine indexes after different vaccination schemes are shown in . Most (3099 out of 3184 pairs, 97.3%) of the parameter grades maintained unchanged before and after vaccination. Fifty-one pairs of parameters improved after vaccination (grade decrease), while 34 pairs worsened. Only one man in the placebo group had an abnormal indicator of grade 3, whose hemoglobin changed from 163 g/L (normal) to 70 g/L (grade 3) after vaccination. As the subject had a history of severe anemia, the change in hemoglobin level was not considered to be related to the vaccine.
Figure 2. Changes in routine paired blood, serum biochemical, and urine indexes before and 2 days after the first and third vaccinations. The fluctuations were classified into three categories: “maintaining” indicated no grade change observed; “worsening” indicated a change from normal to abnormal or an increase in grade; and “improving” indicated a change from abnormal to normal or a decrease in grade. a) Six routine blood indexes were measured: white blood cell count (WBC), lymphocytes (LY), absolute neutrophil count (ANC), eosinophils (EOS), platelets (PLT), and hemoglobin (HGB). b) Four serum biochemical indexes were measured: total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glucose (GLU). c) Three routine urine indexes were measured: urinary protein (PRO), urinary glucose (UGLU) and urine occult blood (BLD).
HPV-6/11 IgG antibodies and nAbs levels at month 7 were measured for all but two of the participants in the 30 μg group, who had no blood samples obtained at month 7. For both HPV-6/11 IgG antibodies and nAbs, the antibody levels in the three vaccine groups were significantly higher than those of the placebo group. The seroconversion rate (SCR), the geometric mean concentration (GMC) of IgG antibodies, and the geometric mean titer (GMT) of nAbs after vaccination in the PPS-I set are shown in . Compared with the placebo group (none of the subjects was seropositive at month 7), 100% of the participants who received three doses of different dosages of the HPV-6/11 vaccine seroconverted for IgG and neutralizing antibodies against both types. GMC or GMT of antibodies among different dosages of HPV-6/11 vaccine groups was similar, except that GMT of HPV-11 nAbs in the 60 μg group was significantly higher than the 30 μg group ().
Figure 3. HPV-6/11 antibody levels at one month post three doses of vaccine (month 7) in the per-protocol set. Antibody level of each participant is shown (A: HPV-6 IgG; B: HPV-11 IgG; C: HPV-6 nAb; D: HPV-11 nAb). The black lines indicate the GMC/GMT and 95%CI. *: Significant difference between the two dose groups; **: Significant differences between this group and all the other dose groups. nAb: neutralizing antibody. ID50: the highest dilution which blocked 50% of green fluorescent protein (GFP) expression (50% neutralization).
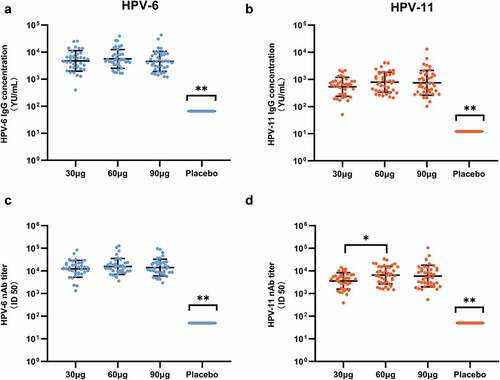
Table 4. Antibody responses at month 7 in participants who received three vaccine doses and were seronegative for antibody against the relevant types of HPV at day 0.
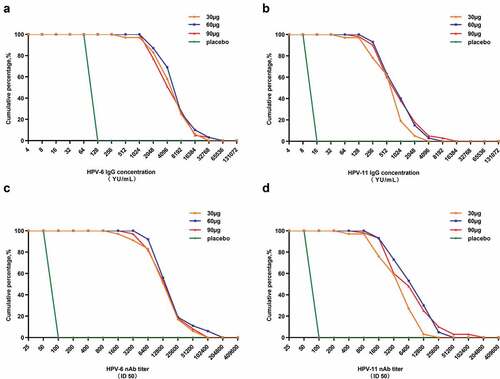
The distribution of the IgG and neutralizing antibody titers is plotted in , which showed that almost all the HPV vaccine receivers produced high level of HPV-6/11 IgG antibodies and nAbs. It is worth noting that the IgG and neutralizing antibodies induced by HPV-6/11 vaccination are highly correlated (r = 0.91 and 0.93 for HPV-6 and HPV-11, respectively, Figure S1).
Figure 4. Reverse cumulative distribution curves of the HPV-6/11 antibodies in the per-protocol set for immunogenicity (PPS-I). A: HPV-6 IgG; B: HPV-11 IgG; C: HPV-6 nAb; D: HPV-11 nAb. nAb: neutralizing antibody. ID50: the highest dilution which blocked 50% of green fluorescent protein (GFP) expression (50% neutralization).
Discussion
This study reports the preliminary safety and immunogenicity data of the first bivalent HPV-6/11 L1 VLP vaccine candidate specifically designed for the prevention of genital warts and other HPV-6/11-related diseases in a dose-escalation, randomized, double-blinded, placebo-controlled phase 1 clinical trial. The results show that in the dose range of 30 μg to 90 μg, the vaccine candidate is well tolerated and has robust immunogenicity in healthy people aged 18–55 years old.
The reported incidence of adverse reactions within 30 days after any of the three doses was similar among the three dosage groups and the control group and ranged from 31.7% to 62.5%. Only one participant reported an adverse reaction of grade 3, and recovered on the same day of occurrence. No SAEs were reported. No clinically significant change in blood or urine parameters was observed. Almost all the HPV-6/11 vaccinees developed high level of specific IgG antibodies and nAbs. These data pave the way for further immunogenicity and efficacy studies of this candidate vaccine in clinical trials with larger sample sizes.
The two currently commercially available HPV vaccines that contain HPV-6/11 antigens are quadrivalent (HPV-6/11/16/18) and nonavalent HPV vaccines (HPV-6/11/16/18/31/33/45/52/58). With higher antigen content, both vaccines have higher incidences of adverse events post vaccination.Citation24,Citation25 Based on the combined analysis of several Phase 3 studies, the incidences of total AEs in female subjects aged 16–26 years within 14 days after receiving any dose of the quadrivalent and nonavalent HPV vaccine were 90.7% and 93.9%, respectively, and those of injection-site AEs in 4 days after vaccination were 84.9% and 90.7%, which were significantly higher than that of the candidate HPV-6/11 vaccine in our study, although not head-to-head compared. Additionally, the incidences of severe injection-site adverse reactions are much higher for quadrivalent and nonavalent HPV vaccines. However, both vaccines have been proven to be safe and efficacious against GWs after wide administration worldwide.Citation12,Citation14,Citation26–28 These data indicate that the application of HPV-6/11 vaccine can reduce the burden of genital warts in the real world.
The test recombinant HPV-6/11 vaccine was produced by an E. coli expression system, which has the characteristics of high yield, short turnaround time, and easy scale-up.Citation29 Two recombinant vaccines produced by the E. coli system have been successfully developed and licensed, namely a recombinant hepatitis E vaccine (Hecolin®) and the recombinant HPV 16/18 vaccine (Cecolin®), respectively. Both vaccines have shown good safety, robust immunogenicity, and excellent efficacy in the Phase III trials.Citation20,Citation30
Both PBNA and VLP-ELISA are commonly used methods for measuring specific antibody responses against HPV, and PBNA has been considered the gold standard because of unbiased assessment. However, the use of the PBNA in large clinical trials is challenging because it is a complex and labor-intensive assay. Therefore, the ELISA method is usually used as an alternative to PBNA, especially for the detection of vaccine-induced antibodies. This study also showed a high correlation for measuring vaccine-induced anti-HPV-6/11 responses between the ELISA and the PBNA, which is consistent with previous studies.Citation23,Citation31
All the three tested dosages of HPV-6/11 vaccine are highly immunogenic; however, the antibody level in this study cannot be directly compared to the antibody levels induced by other HPV vaccines that also contain HPV-6/11 VLPs, which use a proprietary multiplex competitive Luminex immunoassay (cLIA), due to the different tests used and the lack of international standard for HPV-6/11 antibodies. Dose-dependent effects were almost not observed, except that the GMT of HPV-11 specific nAbs in 60 μg group was significantly higher than that of the 30 μg group. Due to the small sample size of this Phase I study, it is necessary to further analyze the safety and immunogenicity of different dosages of HPV-6/11 vaccine with different HPV-6 and HPV-11 antigen ratios in a larger cohort and determine the optimal preparation for further efficacy trials.
Some prospective cohort studies have indicated that males might have a higher incidence of HPV-6/11 infection than females, and there are no significant differences in the clearance of HPV-6/11 infection by sex.Citation32,Citation33 Therefore, the burden of genital warts among male, especially homosexual male populations, should also be taken seriously. Although 107 countries have introduced HPV vaccines into the national immunization program, which is limited by the production of HPV vaccines, most countries, especially developing countries with severe disease burdens of cervical cancer, still regard women as the only target population. The opportunities for men to be educated and get vaccinated of these HPV-6/11-containing vaccine are relatively low. In 2018, the World Health Organization called for a global alliance to put all countries on the path to the elimination of cervical cancer within the century. This public health problem might be solved mainly by the high coverage of HPV vaccines around the world.Citation34 Furthermore, the WHO emphasized the importance of the HPV vaccine for elimination of genital warts in the global health sector strategy on sexually transmitted infectionsCitation35 and encouraged countries to determine national genital wart elimination goals. In this context, a new HPV-6/11 bivalent vaccine based on an E. coli expression system will be of significance to expand the supply, reduce the cost, and increase the accessibility of vaccines especially in males, which might accelerate the achievement of the goal of elimination of GWs. Therefore, the vaccine is believed to have practical value.
The study has some limitations. The first is that we did not analyze all the routine blood, serum biochemical, and urine indexes tested due to the lack of clear grading criteria in available guidelines or the unstable characteristics of some of the indexes themselves. Nevertheless, all the test indicators were reviewed by clinicians, and no clinically significant changes were found in the analyzed indicators in this study. The second limitation is that since the lack of an international standard for quantifying antibodies against HPV-6/11, the GMC of nAbs cannot be calculated as IgG antibodies. The third limitation is that memory B cell responses and persistence of antibody immunity were not investigated in this study. However, this study is the first-in-human study with very small sample size, and the primary objective is the safety and tolerability of this vaccine candidate, immunogenicity-related issues remain to be addressed in future studies.
In conclusion, the new candidate E. coli-produced bivalent HPV-6/11 L1 VLP vaccine has been preliminarily proven to be well tolerated and highly immunogenic in a phase I clinical study, which encourages further immunogenicity and efficacy trials of this vaccine and represents a crucial step toward a more accessible HPV-6/11 vaccine.
Author contributions
J Z, T W, Z-J M, S-J H, H-R P, and N-S X designed the study. Z-F B, W S, T H, X-L C, Y-H WJ, Y-F L, Y Z, S-J Z, and Y-Y S collected the data, conducted data analysis; J-H N, Q C, B-Z L, F-Z Z performed laboratory tests. Z-F B and T W drafted the manuscript. Z-J M, M-Q L, G S, W-J H, S-J H, T W, J Z, and N-S X were responsible for supervision of the study. All authors critically reviewed the manuscript and approved the final version.
Supplemental Material
Download MS Word (34.3 KB)Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: Zhao-Jun Mo, Zhao-Feng Bi, Wei Sheng, Qi Chen, Teng Huang, Ming-Qiang Li, Xue-Lian Cui, Ya-Hui Wangjiang, Ya-Fei Li, Ya Zheng, Si-Jie Zhuang, Ying-Ying Su, Shou-Jie Huang, Ting Wu, Jun Zhang, and Ning-Shao Xia reported no potential conflicts of interest relevant to this article. The authors declare the following financial interests/personal relationships that may be considered as potential competing interests: The study funder, Xiamen Innovax, prepared the vaccines in the phase I trial. Bi-Zhen Lin, Feng-Zhu Zheng, Guang Sun, and Hui-Rong Pan are current employees of Xiamen Innovax.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2092363
Additional information
Funding
References
- Lacey CJN, Guimera N, Garland SM. Chapter 10 - low-risk human papillomavirus: genital warts, cancer and respiratory papillomatosis. In: Jenkins D, Bosch F, editors. Human papillomavirus. Academic Press; 2020. p. 1–11.
- Wei F, Sheng W, Wu X, Yin K, Lan J, Huang Y, Ma X, Zheng Y, Zhuang S, Huang S, et al. Incidence of anogenital warts in Liuzhou, South China: a comparison of data from a prospective study and from the national surveillance system. Emerg Microbes Infect. 2017;6(1):e113. doi:10.1038/emi.2017.100.
- Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2015;61(Suppl 8):S849–55. doi:10.1093/cid/civ813.
- Yue X, Gong X, Li J, Teng F., Jiang N, Men P, Wang J, Wang Y, Chen X, Gu H. Epidemiological features of condyloma acuminatum in national sexually transmitted disease surveillance sites in China from 2008 to 2016. Chinese Journal of Dermatology. 2017;50:321–325.
- Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13(1):39. doi:10.1186/1471-2334-13-39.
- Moreira ED Jr., Giuliano AR, Palefsky J, Flores CA, Goldstone S, Ferris D, Hillman RJ, Moi H, Stoler MH, Marshall B, et al. Incidence, clearance, and disease progression of genital human papillomavirus infection in heterosexual men. J Infect Dis. 2014;210(2):192–199. doi:10.1093/infdis/jiu077.
- Dareng EO, Adebamowo SN, Famooto A, Olawande O, Odutola MK, Olaniyan Y, et al. Prevalence and incidence of genital warts and cervical Human Papillomavirus infections in Nigerian women. BMC Infect Dis. 2019;19:27. doi:10.1186/s12879-018-3582-y.
- Su S, Chow EP, Muessig KE, Yuan L, Tucker JD, Zhang X, Ren J, Fairley CK, Jing J, Zhang L, et al. Sustained high prevalence of viral hepatitis and sexually transmissible infections among female sex workers in China: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):2. doi:10.1186/s12879-015-1322-0.
- Lukacs A, Mate Z, Farkas N, Miko A, Tenk J, Hegyi P, Németh B, Czumbel LM, Wuttapon S, Kiss I, et al. The quadrivalent HPV vaccine is protective against genital warts: a meta-analysis. BMC Public Health. 2020;20(1):691. doi:10.1186/s12889-020-08753-y.
- Qi SZ, Wang SM, Shi JF, Wang QQ, Chen XS, Sun LJ, Liu A, Zhang N, Jiang N, Siva P, et al. Human papillomavirus-related psychosocial impact of patients with genital warts in China: a hospital-based cross-sectional study. BMC Public Health. 2014;14(1):739. doi:10.1186/1471-2458-14-739.
- Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10(1):113. doi:10.1186/1471-2458-10-113.
- Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, Wand H, Fairley CK. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011;11(1):39–44. doi:10.1016/S1473-3099(10)70225-5.
- Drolet M, Benard E, Perez N, Brisson M, Ali H, Boily M-C, Baldo V, Brassard P, Brotherton JML, Callander D; Group HPVVIS. Population-Level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. doi:10.1016/S0140-6736(19)30298-3.
- Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health. 2018;108(1):112–119. doi:10.2105/AJPH.2017.304119.
- Herweijer E, Ploner A, Sparen P. Substantially reduced incidence of genital warts in women and men six years after HPV vaccine availability in Sweden. Vaccine. 2018;36:1917–1920. doi:10.1016/j.vaccine.2018.02.097.
- Paul P, Fabio A. Literature review of HPV vaccine delivery strategies: considerations for school- and non-school based immunization program. Vaccine. 2014;32:320–326. doi:10.1016/j.vaccine.2013.11.070.
- Ng SS, Hutubessy R, Chaiyakunapruk N. Systematic review of cost-effectiveness studies of human papillomavirus (HPV) vaccination: 9-valent vaccine, gender-neutral and multiple age cohort vaccination. Vaccine. 2018;36(19):2529–2544. doi:10.1016/j.vaccine.2018.03.024.
- van der Loeff SMF, Vorsters A, Marra E, Van Damme P, Hogewoning A. Should female sex workers be offered HPV vaccination? Hum Vaccin Immunother. 2019;15:1544–1548. doi:10.1080/21645515.2019.1602432.
- Lin A, Ong KJ, Hobbelen P, King E, Mesher D, Edmunds WJ, Sonnenberg P, Gilson R, Bains I, Choi YH, et al. Impact and cost-effectiveness of selective human papillomavirus vaccination of men who have sex with men. Clin Infect Dis. 2017;64(5):580–588. doi:10.1093/cid/ciw845.
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang S-J, Zhao F-H, Li M-Q, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020;112(2):145–153. doi:10.1093/jnci/djz074.
- Pan H, Li Z, Wang J, Song S, Wang D, Wei M, Gu Y, Zhang J, Li S, Xia N, et al. Bacterially expressed human papillomavirus type 6 and 11 bivalent vaccine: characterization, antigenicity and immunogenicity. Vaccine. 2017;35(24):3222–3231. doi:10.1016/j.vaccine.2017.04.064.
- Hu YM, Guo M, Li CG, Chu K, He WG, Zhang J, Gu J-X, Li J, Zhao H, Wu X-H, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. 2020;63(4):582–591. doi:10.1007/s11427-019-9547-7.
- Zhao H, Lin ZJ, Huang SJ, Li J, Liu XH, Guo M, Zhang J, Xia N-S, Pan H-R, Wu T, et al. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum Vaccin Immunother. 2014;10(3):740–746. doi:10.4161/hv.27619.
- Moreira ED Jr., Block SL, Ferris D, Giuliano AR, Iversen OE, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 phase III clinical trials. Pediatrics. 2016;138(2): 2015–4387. 10.1542/peds.2015-4387.
- Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-Of-Study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105(1):28–37. doi:10.1038/bjc.2011.185.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi:10.1056/NEJMoa1405044.
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GWK, Ferris DG, Steben M, Bryan J, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi:10.1056/NEJMoa061760.
- Goldstone SE, Giuliano AR, Palefsky JM, Lazcano-Ponce E, Penny ME, Cabello RE, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2021:10. doi: 10.1016/S1473-3099(21)00327-3.
- Huang X, Wang X, Zhang J, Xia N, Zhao Q. Escherichia coli-derived virus-like particles in vaccine development. NPJ Vaccines. 2017;2(1):3. doi:10.1038/s41541-017-0006-8.
- Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang C-L, Jiang H-M, Cai J-P, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376(9744):895–902. doi:10.1016/S0140-6736(10)61030-6.
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–434. doi:10.4161/hv.4.6.6912.
- Wei F, Guo M, Huang S, Li M, Cui X, Su Y, Wu X, Ma X, Zheng Y, Huang Y, et al. Sex differences in the incidence and clearance of anogenital human papillomavirus infection in Liuzhou, China: an observational cohort study. Clin Infect Dis. 2020;70(1):82–89. doi:10.1093/cid/ciz168.
- Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, Monsonego J, Franceschi S. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer. 2015;136(12):2752–2760. doi:10.1002/ijc.29082.
- WHO. Global health sector strategy on sexually transmitted infections 2016–2021-towards ending STIs. Geneva, Switzerland: World Health Organization; 2016.
- WHO. Draft global health sector strategies-sexually transmitted infections, 2016–2021. Geneva, Switzerland: World Health Organization; 2016.
