ABSTRACT
Varicella is a contagious disease of children. Qingdao administrated free one-dose and free two-dose universal varicella vaccination schedules in 2013 and 2016 for preschool children. The effectiveness of the vaccination was analyzed in this study. Monthly varicella incidence data of 1–6 years old children during 2007–2020 were obtained from the Qingdao Infectious Disease Reporting Information Management System. We applied Interrupted time series and segmented regression analyses to assess changes in varicella incidence at the beginning of each month and average monthly changes during the vaccination. The vaccination was associated with a reduction of 32.7% in varicella morbidity on average during the 8-year intervention, there is a statistically significant difference between the voluntary period and free vaccination period (χ2 = 290.80,P < 0.001). Immediately after the free one-dose vaccination implementation in 2013 and free two-dose vaccination implementation in 2016, varicella incidence decreased by 0.135 cases per 100 000 population (P < 0.001) and increased by 1.189 cases per 100 000 population (P = 0.039), respectively, the results were statistically significant. There were significant declining trends in varicella incidence after free vaccination: 0.135(P < 0.001) and 0.055 (P = 0.025) per month in 2013.7–2016.6 and 2016.7–2020.12, respectively. This study shows a further decaying trend of varicella incidence based on the impact of free two-dose vaccination. It is necessary to prolong free two-dose universal varicella vaccination to strengthen the immune barrier of preschool children sequentially.
Introduction
Varicella is a highly acute and contagious self-limiting disease caused by varicella zoster virus (VZV), occurring primarily in childhood. In addition, in temperate climates, varicella has the same characteristic seasonal pattern with strong seasonality and peak incidence during early spring and in late winter.
Varicella attenuated live vaccine (VarV) was licensed in the United States in 1995.Citation1 Then, VarV has become widely available worldwide. The World Health Organization’s (WHO) recommends that vaccine coverage should be reached and sustained at ≥80% to further reduce the number of cases and morbidity related to varicella.Citation2
VarV has been introduced into China as a category II vaccine purchased on a private basis in 1998 and acknowledged as an effective measure to prevent and control the spread of varicella. However, the transmission cannot be interdicted thoroughly, and the annual average incidence rate of varicella from 2005 to 2019 has increased by more than 60%.Citation3,Citation4
In Qingdao, one-dose and two-dose schedules of VarV have been offered on 1 July 2013, and 1 July 2016, respectively. This large-scale universal vaccination aims at reducing the incidence of varicella and consolidating a varicella immune barrier among children in Qingdao. The varicella vaccine coverage rate of children aged at 6–11 years is only 69.4%, which is around one-third of the children who did not receive the vaccine.Citation5 However, the schedule has been implemented for 7 years, and the effect of preschool-aged children at the population level remains unknown.
The effect of health intervention policy must be evaluated. Interrupted time series (ITS) is considered strong quasi-experimental research to estimate the causal effect and study the dynamic changes of massive vaccination emphasizing on secular trends of outcome at the population level. Furthermore, ITS can address the lack of traditional studies applying a case-control or screeningCitation6–8 to examine varicella vaccine effectiveness with small sample sizes at the individual level and limited confounding factors caused by group differences.Citation5–Citation9–14
Therefore, in this study, we apply an ITS design and segmented regression analysis based on the trends of varicella incidence among children aged 1–6 years old from 2007 to 2020 in Qingdao, China, to investigate whether this extensive public health intervention has achieved its goals at the population-level.
Methods
Study design
Qingdao is an economically developed coastal city in Shandong Province of China, with a population of about 10,071,722. Universal varicella vaccination aims to reduce the incidence of varicella. Therefore, we conducted a longitudinal study of the reported varicella incidence among preschool children between 2007 and 2020 with a single group of ITS design to evaluate the effect of mass vaccination before and after the intervention.
Qingdao administrated free one-dose universal varicella vaccination regimen for children aged over 1 year old on 1 July 2013. Then the regimen was subsequently revised to vaccinated second-dose VarV for children over 4 years old on 1 July 2016.Citation6 Based on the adjustment of immunization strategy in Qingdao, the period of this study can be divided into three stages based on the amendment on 1 July 2013, and 1 July 2016, which can be regarded as two intervention change points. Stage A (1 January 2007, to 30 June 2013) is the administration of VarV on a voluntary basis. Stage B (1 July 2013, to 30 June 2016) is the operation of the free one-dose VarV schedule operated. Stage C (1 July 2016, to 31 December 2020) is the operation of the free two-dose schedule.
Data source
This study was conducted using the data of monthly reported varicella incidence of children aged 1–6 years old in Qingdao. The reported cases and population from 2007 to 2020 were obtained from the “Infectious Disease Reporting Information Management System,” which is a subsystem of the China Information System for Disease Control and Prevention (CISDCP). One-dose and two-dose VarV coverage rates by year were obtained from the Immunization Information System of Shandong Province.
Statistical analysis
The time series of ITS is a sequence of values of a particular outcome observed continuously and ordered at equally spaced intervals, of which one or more specific change points are divided into two or more segments to differentiate pre-intervention from post-intervention. In addition, the time series of ITS must cover the period before and after the interruption. At the change points, the previously established pattern of events might change because of events, such as interventions or policy changes.Citation12,Citation15
We fitted it through to a generalized least-squares regression line to each segment of the independent variable and time. The level, in which the y-axis-intercept at each segment begins and trends, and the slope inclining by which the observations change over the period in the segment, are two defined parameters to demonstrate the change of outcome. Level change and trend change before and after the change points indicate immediate effectiveness and long-term effect of universal varicella vaccination, respectively.Citation15
We applied the following regression model with two change points:
Yt= β0 + β1×Time + β2 ×Intervention 1+β3×Postslope 1 + β4×Intervention 2 + β5×Postslope 2 +εt
where Yt, the monthly incidence of varicella, is a dependent variable. We used a moving average to pre-process the raw incident data, which was completed to remove the cycles and better capture the trends. Then, we analyzed the data using interrupted time-series analysis. Time is a continuous variable coded 1, 2, 3, … , 168. Intervention 1 is a binary variable, the period before intervention coded 0,and the period after intervention coded 1. Postslope 1 is a continues variable after intervention 1 at time t, which coded 0 before and (time-78) after the intervention 1. Intervention 2 is the same as intervention 1; postslope 2 coded 0 before 2 and (time—114) after the intervention 2. εt is the error term at time t representing random variability, which cannot be explained by the model. β0, the y-axis-intercept, indicates the incidence at the initial moment of the time series. β1, the slope, is the trend of varicella incidence pre-intervention. β2 is the level change reflecting the immediate effect of one-dose varicella vaccination. β3 is the slope change indicating the long-term effect of free one-dose varicella vaccination implementation. β4 is the level change representing the short-term effect of free two-dose VarV universal vaccination.β5 is the slope change reflecting the long-term effect of free two-dose VarV implementation. The Durbin–Watson (DW) method was applied to test the stability of time series data. Varicella incidence = reported cases/population*100,000.
We defined statistical significance as P < 0.05 for a two-tailed test, and all statistical analyses were performed using R software.
Result
A total of 7, 327 varicella cases among preschool-aged (1–6 years old) children without death and outbreak cases were reported in Qingdao, China, from 1 January 2007, to 31 December 2020, with an average annual incidence of 97.08 cases per 100,000 population. The average annual incidence of the voluntary period has decreased from 118.76 cases per 100,000 population to 79.85 cases per 100,000 population during the free vaccination period, and this result shows statistically significant difference (χ2 = 290.80, P < 0.001). The average varicella incidence during the two free vaccination periods is 115.47 cases and 60.74 cases per 100,000 population, showing statistically significant difference (χ2 = 358.75, P < 0.001). The number of reported varicella cases decreased from 859 cases (166.89 cases per 100,000 population) in 2011 to a minimum of 162 cases (25.94 cases per 100,000 population) in 2020. However, the rebound of varicella was observed in 2015. ( and ).
Figure 1. Varicella monthly incidence in Qingdao, China from 2007 to 2020. Solid line depicts smoothing spline in backward moving average with twelve periods in twice. Dashed line depicts the trend of incidence in Qingdao, China from 2007 to 2020. Int1: July 1st, 2013, the date of free one-dose VarV vaccination. Int2: July 1, 2016, the date of free two-dose VarV vaccination.
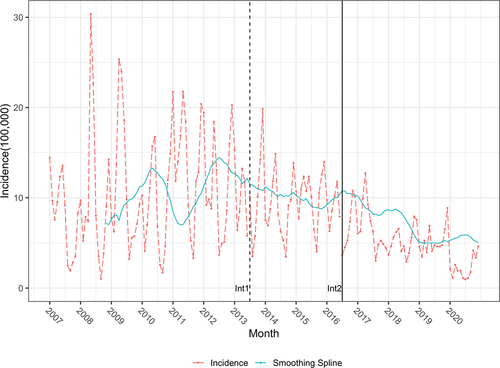
Table 1. The incidences and immunization strategies of varicella in Qingdao, China, 2007–2020.
Based on the results of the segmented regression analysis, the trend of the voluntary self-paying period indicates that the predicted monthly incidence rate increased by 0.077 per 100,000 population, and this result is statistically significant (P < 0.001).
At the first intervention change point, the level change after the date of free one-dose VarV vaccination demonstrates that the monthly varicella incidence decreased by 1.637 per 100,000 population compared with that during the voluntary self-paying period. This result is statistically significant (P = 0.005).
The stage following the voluntary self-paying period and preceding free two-dose VarV vaccination shows a descending trend. The trend change of this segment demonstrates that the predicted monthly incidence of varicella decreased by 0.135 per 100,000 population compared with the previous segment. This result is also statistically significant (P < 0.001).
At the second intervention change point, a level change shows that the monthly incidence of varicella transitorily increased by 1.189 per 100 000 population after two-dose free VarV. The finding is statistically significant (P = 0.0387).
The trend change of free two-dose VarV indicates that the trend of this predicted monthly incidence of varicella in this stage decreased by 0.055 cases per 100,000 population compared with the previous segment. This finding is statistically significant (P = 0.0245).
All these findings show that each segment has a different slope, which indicates a descending trend of varicella morbidity. Moreover, the levels of the predicted mean rates seem to decrease at the first change point and rebound at the second change point ( and ).
Figure 2. ITS scatter plot of the monthly incidence rate of varicella after adjusted from 2007 to 2020 in Qingdao.Int1: July 1st, 2013, the date of free one-dose VarV vaccination. Int2: July 1st, 2016, the date of free two-dose VarV vaccination.
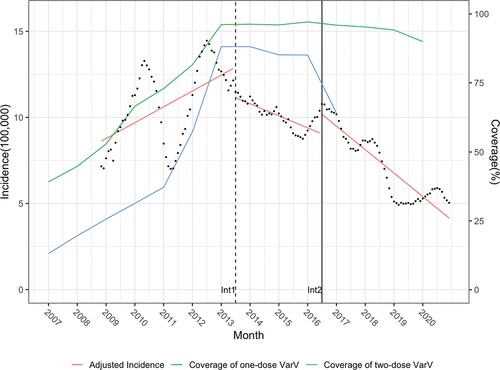
Table 2. Estimated level and trend changes of reported varicella incidence before and after VarV vaccination.
Discussion
ITS is characterized as one of the most efficient quasi-experimental approaches to assess the longitudinal effects of interventions. Retrospective data and the outcomes were collected compared before and after implementation of intervention. As described in the theory of ITS analyses, level changes indicate an immediate effect of an intervention, whereas changes in trend indicate long-term effects.Citation12,Citation15
Qingdao, as one of the earliest cities administrated free two-dose VarV for preschool children in China, is the first try to apply ITS to explore the impact of the implementation of universal varicella vaccination. The decrease of targeted diseases caused by direct and indirect vaccine effects is progressive. Previous published literature mostly applied traditional epidemiological study designs such as cohort and case-control studies by descriptive epidemiology or simply comparison which can provide important evidence about disease etiology rather than conduct the comprehensive assessment of universal vaccination intervention on population level.Citation8,Citation9,Citation16 Our study would provide a useful analytical tool for assessing the intervention effect of public health policies. In the model of varicella vaccine intervention, after the free one-dose VarV massive implementation, both level change and trend change show significant immediate reduction of 1.637 cases per 100,000 population and long-term reduction of 0.135 cases per 100 000 population. Therefore, the one-dose VarV massive intervention can be considered as effective.
Although the reverse phenomenon has little impact with regard to burden on society, analyzing the possible causes of this phenomenon remains instructive for vaccination and policy improvement. Evidence suggested that one-dose VarV was around 81% to 84.5% effective at preventing all varicella and it only provided 80.2% efficacy to children aged 1–4 years.Citation17–20 Epidemiologic studies observed a waning of effectiveness after one-dose vaccination,Citation11,Citation19,Citation21 and vaccine effectiveness decreased to 62.6% after 4 years later and further to 49.9% after 6 years.Citation22 Serologic evidence also showed that VarV produces shorter-lived seropositivity, and the positive titer decreased for each successive year.Citation23 Considering the limited immune effectiveness and the short duration for one-dose VarV vaccination, it’s generally believed that an increase in small breakthrough casesCitation11,Citation24,Citation25 and in new cases new cases among vaccine-eligible but unvaccinated susceptible children contributed to the transient increase in varicella incidence in 2016.
Evidence in different countries suggests that high-level coverage is a critical success factor affecting universal vaccination.Citation26 One-dose varicella vaccine coverage of Costa Rica,Citation27 Germany,Citation28 and AustraliaCitation29 has increased over 20% after vaccination program, and more than 60% reductions in varicella incidence have been observed. Meanwhile, multiple studies have elucidated that sufficient and extensive two-dose varicella vaccination coverage is an important factor in controlling the outbreak and further reducing the incidence of varicella.Citation30–33 As outlined, 4 years after the implementation of the free two-dose varicella vaccination in Qingdao, first-dose and second-dose vaccination coverage of age-eligible children have increased from 86.86% to 97.78% and from 42.13% to 85.17%, respectively.Citation34 On the contrary, the average annual incidence of children aged 1–6 years has decreased from 122.58 to 92.96 cases per 100 000 population and further decreased to 26.74 cases per 100 000 population in 2020. Qingdao has maintained 90% and 80% coverage for the one-dose and two-dose VarV during the free immunization period, which results in the significant reduction in the incidence of varicella in the long term. Meanwhile, no case of pre-school varicella outbreak was presented during these years.Citation34 This result may be related not only to the high-level coverage of VarV but also to active vaccination by parents during the self-paying vaccination period.
Considering the quality of data, Qingdao’s active surveillance system for varicella infection is based on cases confirmed by experienced physicians. The absence of reported cases collected from sentinel hospitals rather than from preschool children across the city is an important factor that may underestimate the incidence of the disease. In addition, this study did not control for potential variables, including social and economic factors and migration. Further information on vaccine coverage and multifactorial analysis should be followed up.
Conclusion
The universal varicella vaccination in Qingdao produces satisfactory effects. It confers a further decaying trend of varicella incidence and a stronger herd immunity of preschool children after the free two-dose VarV vaccination. Therefore, extending the implementation of free universal vaccination of two-dose VarV is important to establish and strengthen the immune barrier of children sequentially.
Ethical approval
Ethical approval for this type of study is not required by our institute
Authors’ contributions
Conception and design: ZG, SPL, FY, FQ
Acquisition of data: ZG, XFL
Collectingdata: ZG, XFL, FQ
Analysisand interpretation of data: ZG, FY, SPL, XFL
Writing, review, and/or revision of the manuscript: ZG, FY, XFL, FQ
Revising: SPL, FY, FQ, XFL
All authors read and approved the final manuscript.
Acknowledgment
We would like to express special gratitude to all the personnel who supported or helped with this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Choo PW, Donahue JG, Platt MR. The epidemiology of varicella and its complications. J Infect Dis. 1995;172(3):1–6. doi:10.1093/infdis/172.3.706.
- Varicella and herpes zoster vaccines: WHO position paper, June 2014–recommendations. Vaccine. 2016;34(2):198–199. doi:10.1016/j.vaccine.2014.07.068.
- Siu HT, Wang LJ, M LY, Yin DP. Varicella epidemiology in China, 2005-2015. Chin J Vaccines Immun. 2019;25:155–159.
- Dong Pumei WM, Yanmin L. Epidemiological characteristics of varicella in China, 2016-2019. Chin J Vaccines Immun. 2020;26:403–406.
- Wang Y, et al. Effectiveness and failure rate of the varicella vaccine in an outbreak in Jiangsu, China: a 1:2 matched case-control study. Hum Vaccin Immunother. 2019;16(3):506–512.
- Hu P, Yang F, Li X, Wang Y, Xiao T, Li H, Wang W, Guan J, Li S. Effectiveness of one-dose versus two-dose varicella vaccine in children in Qingdao, China: a matched case-control study. Hum Vaccin Immunother. 2021;17(12):5311–5315. doi:10.1080/21645515.2021.1982281.
- Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, LaRussa PS, Gershon AA. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203(3):312–315. doi:10.1093/infdis/jiq052.
- Suo L, Lu L, Zhao D, Pang X. Impact of a 2-dose voluntary vaccination strategy on varicella epidemiology in Beijing, 2011–2017. Vaccine. 2020;38(20):3690–3696. doi:10.1016/j.vaccine.2020.01.087.
- Xu Y, et al. Epidemiology of varicella and effectiveness of varicella vaccine in Hangzhou, China, 2019. Hum Vaccin Immunother. 2020;17(1):211–216.
- Wu QS, Liu J-Y, Wang X, Chen Y-F, Zhou Q, Wu A-Q, Wang L. Effectiveness of varicella vaccine as post-exposure prophylaxis during a varicella outbreak in Shanghai, China. Int J Infect Dis. 2018;66:51–55. doi:10.1016/j.ijid.2017.10.016.
- Chan YD, Edmunds WJ, Chan H-L, Wong M-L, Au KWA, Chuang S-K, van Hoek AJ, Flasche S. Varicella vaccine dose depended effectiveness and waning among preschool children in Hong Kong. Hum Vaccin Immunother. 2020;16(3):499–505. doi:10.1080/21645515.2019.1663121.
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi:10.1046/j.1365-2710.2002.00430.x.
- Kawamura Y, Hattori F, Higashimoto Y, Kozawa K, Yoshikawa T. Evaluation of varicella vaccine effectiveness during outbreaks in schools or nurseries by cross-sectional study. Vaccine. 2021;39(21):2901–2905. doi:10.1016/j.vaccine.2021.04.009.
- Soumerai SB, Starr D, Majumdar SR. How do you know which health care effectiveness research you can trust? a guide to study design for the perplexed. Prev Chronic Dis. 2015;12:E101. doi:10.5888/pcd12.150187.
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38–44. doi:10.1016/j.acap.2013.08.002.
- Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. Bmj. 2015;350:h2750. doi:10.1136/bmj.h2750.
- Marin M, et al. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137(3):e20153741. doi:10.1542/peds.2015-3741.
- Henry O, et al. One or two doses of live varicella virus-containing vaccines: efficacy, persistence of immune responses, and safety six years after administration in healthy children during their second year of life. Vaccine. 2018;36(3):381–387. doi:10.1016/j.vaccine.2017.11.081.
- Seward JF, Marin M, Vázquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis. 2008;197(2):S82–9. doi:10.1086/522145.
- Hao B, Chen Z, Zeng G, Huang L, Luan C, Xie Z, Chen J, Bao M, Tian X, Xu B, et al. Efficacy, safety and immunogenicity of live attenuated varicella vaccine in healthy children in China: double-blind, randomized, placebo-controlled clinical trial. Clin Microbiol Infect. 2019;25(8):1026–1031. doi:10.1016/j.cmi.2018.12.033.
- Vázquez M, et al. Effectiveness over time of varicella vaccine. Jama. 2004;291(7):851–855. doi:10.1001/jama.291.7.851.
- Hong K, Sohn S, Choe YJ, Rhie K, Lee JK, Han MS, Chun BC, Choi EH. Waning effectiveness of one-dose universal varicella vaccination in Korea, 2011–2018: a propensity score matched national population cohort. J Korean Med Sci. 2021;36(36):e222. doi:10.3346/jkms.2021.36.e222.
- Duncan JR, et al. Varicella seroepidemiology in United States air force recruits: a retrospective cohort study comparing immunogenicity of varicella vaccination and natural infection. Vaccine. 2017;35(18):2351–2357. doi:10.1016/j.vaccine.2017.03.054.
- Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert Rev Vaccines. 2017;16(4):391–400. doi:10.1080/14760584.2017.1294069.
- Kurugöl Z, Gökçe Ş. Outbreak of varicella in preschool children despite one-dose vaccination. Turk J Pediatr. 2018;60(1):56–62. doi:10.24953/turkjped.2018.01.008.
- Holl K, et al. Coverage, efficacy or dosing interval: which factor predominantly influences the impact of routine childhood vaccination for the prevention of varicella? a model-based study for Italy. BMC Public Health. 2016;16(1):1103. doi:10.1186/s12889-016-3738-x.
- Avila-Aguero ML, Ulloa-Gutierrez R, Camacho-Badilla K, Soriano-Fallas A, Arroba-Tijerino R, Morice-Trejos A. Varicella prevention in Costa Rica: impact of a one-dose schedule universal vaccination. Expert Rev Vaccines. 2017;16(3):229–234. doi:10.1080/14760584.2017.1247700.
- Hecht J, Siedler A. [The epidemiology of varicella disease in Germany after introduction of a vaccination recommendation : analysis of mandatory and sentinel data between 2002 and 2014]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60(1):118–126. doi:10.1007/s00103-016-2475-8.
- Khandaker G, Marshall H, Peadon E, Zurynski Y, Burgner D, Buttery J, Gold M, Nissen M, Elliott EJ, Burgess M, et al. Congenital and neonatal varicella: impact of the national varicella vaccination programme in Australia. Arch Dis Child. 2011;96(5):453–456. doi:10.1136/adc.2010.206037.
- Doll MK, Rosen JB, Bialek SR, Szeto H, Zimmerman CM. An evaluation of voluntary 2-dose varicella vaccination coverage in New York City public schools. Am J Public Health. 2015;105(5):972–979. doi:10.2105/AJPH.2014.302229.
- Zeng WB, Zhang F, Cheng S, Sun J-Y, Shen H, Luo M-H. Concerns on vaccine against varicella caused by varicella-zoster virus infection. Virol Sin. 2021;36(1):159–162. doi:10.1007/s12250-020-00231-4.
- Beleni AI, Borgmann S. Mumps in the vaccination age: global epidemiology and the situation in Germany. Int J Environ Res Public Health. 2018;15(8):1618. doi:10.3390/ijerph15081618.
- Li Z, Yao Y, Lu X, Liu J, Huang Z, Sun X, Lu Y. Impact of a two-dose varicella immunization program on the incidence of varicella: a multi-year observational study in Shanghai, China. Expert Rev Vaccines. 2021;20(9):1177–1183. doi:10.1080/14760584.2021.1963236.
- Li Xiaofan XX, Wencheng W, Tingting X, Feng Y. Epidemiological characteristics of varicella public health emergency events in Qingdao city, 2006-2019. Chin J Vaccines Immun. 2020;26:283–286.
