ABSTRACT
The COVID-19 pandemic has had a dramatic impact on society, but little is known about how the pandemic affects the vaccine policy landscape and public perception of vaccines in Thailand. This study aims to describe potential changes in Thailand’s policy landscape post-pandemic. We performed a literature review and in-depth interviews with 12 key informants to understand the policy landscape in Thailand. The findings were shared in a policy forum in December 2021. Several key findings were summarized. Funding and development have been thriving during the pandemic. However, a long-term commitment from all stakeholders is required to maintain policy continuation. A public-private partnership should be considered. The regulatory body needs to be prepared for product authorization. The vaccine introduction decision-making process, and investment in prevention and promotion, should further be discussed. In summary, it is important to reshape the environment and mentality of all stakeholders to create a sustainable and self-sufficient vaccine ecosystem.
KEYWORDS:
Introduction
Vaccination is one of the most cost-effective health interventions.Citation1 It is proven to prevent several communicable diseases at the individual level, control an outbreak on a broader level, and even eradicate certain contagious diseases.Citation2 In addition to the preventive effect, vaccines reduce severity and mortality due to the disease.Citation3 Recently, vaccination has become a promising game changer to control the pandemic of coronavirus disease 2019 (COVID-19) which has caused hundreds of millions of infections and millions of deaths, as well as a tremendous negative impact on local and global socioeconomic systems.Citation4,Citation5
The dramatic impacts that are attributable to the COVID-19 pandemic may alter public perception of vaccines and health care and presumably affect the vaccine policy landscape in Thailand, from research and development (R&D), regulation, vaccine introduction and the decision-making process, to the final step of utilization. However, there is currently limited understanding of how the pandemic may have affected the perception and value of all relevant stakeholders toward vaccines. This study aims to describe potential changes in Thailand’s vaccine policy landscape post-pandemic, and ultimately, stimulate policymakers to consider what is required to be done to improve the vaccine policy landscape.
Data collection approach
We performed a literature review in PubMed and the official sources such as the National Drug Information website, the National Vaccine Institute (NVI) website, and the Thai Food and Drug Administration (FDA) website, to understand the vaccine policy landscape in Thailand. In-depth interviews with 12 key informants, including policy makers (n = 4), research institute (n = 2), researchers (n = 1), vaccine developers and suppliers (n = 2), health professionals (n = 2), and patient representatives (n = 1), were then carried out to verify and explore more information on the current vaccine policy landscape and potential impact of COVID-19 pandemic to all aspects related to the vaccine including R&D, regulation and the decision-making process for the vaccine landscape in Thailand post-COVID-19. Based on information gathered from literature and key informant interviews, we shared our findings with all relevant stakeholders (n = 17) in a policy forum for discussion with the aim to gain insights about the potential changes in the vaccine policy landscape including R&D, vaccine introduction, the decision-making process, procurement system, implementation, and overall future direction. We then summarized the findings obtained from the literature review, key informant interviews, and policy forum and described them in three key parts including 1) the vaccine policy landscape in Thailand before the COVID-19 pandemic, 2) the immediate impact of COVID-19 on the vaccine policy decision-making process, and 3) lessons learned from the pandemic and key challenges for the future of vaccine policy decision-making in Thailand. The study was approved by the Institutional Review Board, University of Utah, and conducted according to the Helsinki Declaration. Informed consent was obtained from all participants.
Findings
Vaccine policy landscape in Thailand before the COVID-19 pandemic
In 2002, Thailand enacted the National Health Security Act, together with the establishment of the Universal Health Coverage (UHC) to ensure health security for everyone who lives in Thailand by providing free health services through public medical centers all over the country.Citation6 There are three main public health insurance schemes that cover over 99% of the Thai population, namely the Civil Servant Medical Benefit Scheme (CSMBS), operated by the Comptroller General’s Department of the Ministry of Finance for government employees and their dependents (8%); the Social Security Scheme (SSS), managed by the Social Security Office of Ministry of Labor for employees of the private sector (15%); and the Universal Coverage Scheme (UCS), operated by the National Health Security Office (NHSO) for the remaining Thais who are not covered by the other schemes (76%). These public health insurance schemes cover the healthcare services and health interventions for health promotion, disease prevention, disease diagnosis, treatment, and rehabilitation that are listed as a benefit package. Vaccines are one of the disease prevention interventions that are financially covered and procured by the NHSO for all Thai populations.Citation7
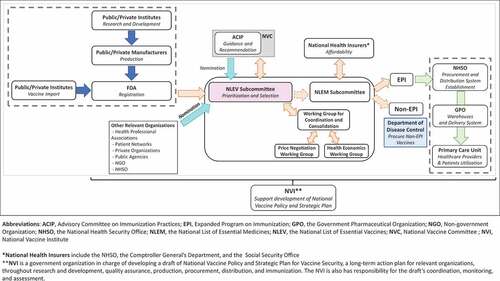
Based on the literature reviews and key informant interviews, we have depicted an overall vaccine landscape in Thailand as shown in . Beginning with the vaccine development and importation, the majority of vaccines used in Thailand are imported. Only certain types of vaccines are locally manufactured while some are being imported as bulk drug products and are finished locally.Citation8 Although local production is not extensive, Thailand has a well-established infrastructure and system developed to support vaccine development. This includes pilot plants of certain technology platforms, the Central Storage for Regional Working Reference Standards of Vaccines, the National Primate Research Center, several Centers of Animal Research that can facilitate vaccine testing, clinical research centers, as well as training programs for related personnel.Citation8 Thailand has legislation called “the National Vaccine Security Act” to support vaccine security policy as well as the National Policy and Strategic Plan for Vaccine Security, developed under the NVI, to facilitate the direction of all development toward vaccine security.Citation9 A bill mandating government providers for domestic purchasing when the vaccine is locally produced has been recently launched to incentivize local production.Citation10 In terms of regulation, a vaccine must be licensed and market-authorized by the Thai FDA before launching onto the market. FDA’s role is to evaluate the quality, safety, and efficacy of vaccines. This involves authorizing their use, distribution, and sales.Citation11 Additionally, following the WHO guideline,Citation12 each lot of vaccine must be assessed by the Institute of Biological Products, Department of Medical Sciences, for the national quality control before marketing.
In order for vaccines to be included in the national benefit package, since 2020, they must be listed in the National List of Essential Vaccines (NLEV) under the National List of Essential Medicines (NLEM). The decision-making process starts with vaccines being prioritized and recommended by the Advisory Committee on Immunization Practices (ACIP) which comprises experts from various professions and is chaired by the director-general of the Department of Disease Control (DDC). Additionally, vaccines of interest can be nominated by experts or relevant organizations, i.e., health professional associations, patient networks, private organizations, public agencies, non-government organizations (NGOs), and NHSO. The vaccines, recommended by ACIP, will be considered further by The NLEV subcommittee which assesses a vaccine from efficacy, safety, quality, and economic impact including cost-effectiveness (value for money) and budget impact (financial affordability), as well as equity and ethical consideration. Once NLEV has decided whether a vaccine should be included in the benefit package, it will be passed on to the public health insurance payers, which are the NHSO (for UCS), the Comptroller General’s Department (for CSMBS), and the SSO (for SSS), to assess the affordability before announcing the vaccine’s inclusion in the Royal Thai Government Gazette.
Thailand is one of the countries that self-procure vaccines because of its upper-middle income status making it ineligible for international aid programs. The NHSO is responsible for vaccine procurement as well as establishing the distribution system of vaccines under the Expanded Program on Immunization (EPI). Simultaneously, the Department of Disease Control (DDC) oversees non-EPI vaccine procurement. The NHSO has assigned Rajavithi Hospital Network, under the Department of Medical Services, to procure vaccines being used under the EPI program. The vaccine supply and logistics system is assigned to the Government Pharmaceutical Organization (GPO), a state enterprise operating under the Ministry of Public Health, as a third-party provider to manage using a vendor managed inventory (VMI).Citation13 The GPO is one of the important local vaccine producers that also assists in the development of vaccine specifications for national procurement. Once the vaccines are delivered from local producers and importers, they will be stored in the GPO warehouse before being distributed to each district hospital warehouse. During the distribution, the vaccines are equipped with an appropriate cold chain delivery system arranged by the GPO, and the vaccines are then distributed to the primary care unit (PCU), or health center where immunizations take place.
The immediate impact of COVID-19 on the vaccine policy decision-making process
The COVID-19 pandemic caused an immediate impact on national healthcare systems worldwide. In Thailand, vaccine R&D and production have thrived since the pandemic. Unprecedented grants have been funded for COVID-19 vaccine R&D through the NVI. The less-conditioned research grants allow the NVI, as the focal point, to plan for more long-term project investments. With the cooperation of R&D institutes and government agencies, a few vaccine candidates discovered in Thailand are currently being investigated in clinical trials. Given that no pharmaceutical or vaccine products have ever been discovered from product development to investigation in a clinical trial in Thailand before, this achievement could be considered an important step for the Thai pharmaceutical R&D area in Thailand. This is good evidence demonstrating that our country is ready to serve society’s need in an emergency with our capacity to develop a vaccine product, aligned with the vaccine security goal of Thailand. Additionally, we believe that the emerging interest in the creation of innovations might serve as a foundation to be built upon and further expanded into other fields. This rapid growth stimulates the regulator to prepare for new product licensing and authorization. Currently, consultation service from the FDA has been provided to synergize expertise from the regulator and sponsors to shape the framework for a vaccine product development plan. Recently, Siam Biosciences, a private pharmaceutical manufacturer, has been selected to be a local manufacturing partner by AstraZeneca to produce the COVID-19 vaccine.Citation14 This partnership demonstrates that Thailand is well equipped with the required facilities and human resource abilities and is in position for vaccine manufacturing. It has attracted big enterprises in Thailand to invest in the industry as well as foreign enterprises to collaborate on technology transfer.
The immediate effect of COVID-19 in Thailand also impacted vaccine procurement. It is obvious that timely procurement of a COVID-19 vaccine would lessen the magnitude of the impact of the pandemic. However, the procurement regulation in Thailand did not allow the government to purchase vaccines or even reserve vaccines for purchasing when evidence of vaccine efficacy and safety is not available. In other words, in order to reserve vaccines, an advance market commitment (AMC) is required. This is viewed as noncompliance with procurement regulations in Thailand. Until recently, a temporary exemption under this emergency was deployed to allow the purchasing process to take place.
Finally, another important impact was the heightened public awareness about the importance of vaccines as an effective disease prevention intervention. The COVID-19 pandemic has large implications globally and in every individual country not only in the healthcare system sector but also in a broader impact on the economy. People are concerned with the inadequate supply of vaccines, helping them to realize the benefits of vaccines and the need for their accessibility. Some key informants of this study believed that this public awareness and concern about vaccine access is not only limited to the COVID-19 vaccine, but it may influence a greater recognition of the importance of other vaccine access as well. In contrast, a lowered EPI vaccination rate was observed during the pandemic. This might be because the public health measures implemented in response to the pandemic such as social distancing measures and lockdown may have prevented them from seeking routine immunization services in addition to difficulties in the provision of health care service delivery.
During the COVID-19 pandemic, a special decision procedure has been employed to minimize complexities and streamline all related processes. Policy makers have set up various ad-hoc committees to adopt the use of the COVID-19 vaccine in this emergency situation. Those committees have been working on reviewing the evidence on each aspect of the COVID-19 vaccines (i.e., vaccine quality, safety, and efficacy), making coverage decisions on the vaccines, and utilizing an emergency budget to purchase the COVID-19 vaccine. The attention and resources drawn to the COVID-19 vaccine introduction process have indirectly resulted in delays in the decision-making process for other new vaccine introduction and adoption. However, it is important to note that all key stakeholders involved in this study believed that the COVID-19 pandemic may not directly affect the regular vaccine introduction decision-making process itself.
Lessons learned from the pandemic and key challenges for the future of vaccine policy decision-making in Thailand
Vaccination benefit was underestimated in society since it was perceived as only a disease prevention tool until the COVID-19 pandemic. Recently, it has become a promising health intervention that positively impacts the healthcare system, economic, and political stability, and national security. From the policy forum discussions about the immediate impact of the pandemic on Thailand, we discussed the impacts it has had on the vaccine policy landscape which could provide some key challenges for the future of vaccine policy decision-making in a normal situation. We summarized the key findings in .
Table 1. Summary of findings.
Investment continuity and sustainability in R&D
The significant advance in COVID-19 vaccine R&D observed in Thailand could facilitate advancement in the R&D of other vaccines as well. Domestic vaccine R&D and production is an area to be encouraged and supported as it is a foundation to ensure vaccine security in both emergency and regular situations. In this regard, continuity of funding and policy is crucial to finally reach the state of ‘secured.’ Government, as the major funder and supporter, should view investment in pharmaceutical R&D as a long-term investment. The primary goal should not be to create a successful product. In contrast, the investment should be considered as a steppingstone to escalate the capacity and level of pharmaceutical product discovery and development. Currently, it is also important to note that the direction of the investment for pharmaceutical R&D is likely to be geared toward supporting research based on a technology platform. In other words, instead of providing funding based on the direction of supporting vaccines for specific diseases, investors need to recognize the importance of advancing a technology platform which will eventually support the development of multiple vaccines under a particular platform.
Potential role of public-private partnership in vaccine development
To maintain vaccine policy continuation, long-term commitment from relevant stakeholders is crucial. Private organizations can also be potential partners in various roles, in different models to synergize and create an effective environment. For instance, the support from the private sector could be a fund investment for vaccine development, while the private sector’s expertise can also be a key support for a successful scaleup, from bench to bedside. Finally, a public-private partnership (PPP) is another potential option in the vaccine development area. An example of a PPP that might be applied in Thailand would be the Biomedical Advanced Research and Development Authority (BARDA) model, where the public sector promotes advanced research, innovation and the development of medical devices, tests, vaccines, and therapeutics, by providing grants and other assistance that the private sector proposes to ultimately achieve an objective.Citation15
Importance of strengthening regulatory body authority for vaccine development
The rapid growth in domestic vaccine R&D has brought a challenge to the Thai FDA as the vaccine regulator. There are insufficient internal experts in the organization, especially in biologics. Given that the FDA must comply with the government rules and regulations which might be too rigid, this results in challenges for the FDA to function as a competent public independent authority to achieve its mission.
Process of vaccine introduction to the National List of Essential Vaccines
Even though the pandemic may not have a direct impact on the process of vaccine introduction to the national health benefit package, several key informants shared several important points worth discussing. Because Thailand is a vaccine self-procuring country with limited financial resources, it is necessary to thoroughly consider the cost-effectiveness of a vaccine during the vaccine introduction decision-making process. All stakeholders agreed that the current introduction process is very systematic, evidence-based, and transparent. Yet, the process comprises multiple steps which can cause a significant delay in non-COVID vaccine access during the normal situation. It was also pointed out that since vaccines are developed as a prevention tool, the criteria for decision-making should not be the same as for medicines. To elaborate, those criteria may include cost-effectiveness thresholds and budget limits. Even though these issues were brought up during the policy forum, it remains unclear about what and how these criteria should be changed. All agree that these are important and deserve more attention to improve the efficiency of the decision-making process. Another related issue is that the National Vaccine Security Act, which supports purchasing vaccines that are locally produced, should be included as a key criterion in the decision-making framework of vaccine products in the future.
Inadequate investment in prevention and promotion
Another key challenge is the limited investment in the prevention and promotion (P&P) of Thai healthcare system. Even though the annual budget for P&P was proposed to be no less than 14% of the total budget, the actual budget received has always been less than this goal in the last few years. In addition, vaccines, which are part of P&P, are even more under-supported, as only 7% of the health promotion and disease prevention (P&P) budget was allocated for vaccines. This means that the vaccine procurement budget is less than 1% of the total budget.Citation16 The inadequate support of P&P and vaccines is obviously a clear challenge for the healthcare system. Sources of additional funding for vaccines were discussed during the policy forum, but a final solution remains to be explored. Overall, it was clearly agreed that preventable diseases and health promotion should receive more support given the increased awareness of people in our society about the importance of disease prevention, and especially the use of vaccines.
Vaccine procurement regulations
The pandemic clearly demonstrated the necessity for improvement of Thailand’s vaccine procurement regulations. The rigidity of vaccine regulation had resulted in difficulties in decision-making which contributed to the delay of vaccine provision in this emergency. Besides the COVID-19 vaccine, Thailand also had experienced vaccine shortage because of the inability to implement multi-year tender and multi-supplier when required when the producer’s supply was insufficient.Citation16 Hereafter, multi-year tender and multi-supplier have been stated as strategic measures in the National Policy and Strategic Plan for Vaccine Security to ensure vaccine security.Citation8,Citation17 Thus, it is important to review and revise the procurement regulations to be aligned with the oligopoly of the vaccine market to effectively procure vaccines.
Effective supportive system
Apart from the abovementioned key challenges, key informants highlighted the need to enhance the effectiveness of information and surveillance systems. This was based on the challenge encountered during the pandemic. It was clearly recognized that an effective surveillance system could help detect an emergence of infectious diseases in a timely fashion. With a good informatics system, communication and data sharing will also be more effective. Lastly, an effective public communication system is needed to avoid confusion and to gain the trust of citizens.
Application in other low- and middle-income countries
As Thailand is a member of low- and middle-income countries (LMICs), the findings from this study might be applicable to other LMICs in certain aspects. Because the pandemic has highlighted the importance of vaccine security, it is important to encourage local vaccine R&D and production, and technology transfer to create a self-sustainability environment and minimize the risk of vaccine shortage in the long run.Citation18,Citation19 While public awareness about vaccines has been raised, a common issue in LMICs is the limited support and budget for investing in vaccines and other health promotion and prevention interventions.Citation20 This crisis may lead to changes in the decision-making framework and prioritization of those interventions. An effective vaccine advisory group is also required to provide a timely response to upcoming changes. At present, there is support from international organizations, like Gavi, the vaccine alliance, to eligible countries in terms of vaccine recommendations, and product donation. Still, the establishment of the National Immunization Technical Advisory Groups (NITAG) in those countries is a priority to construct an internal vaccine management system such as outlining a framework for vaccine introduction, procurement, and other related processes, to ultimately ensure self-reliability after Gavi graduation.
Conclusion
The COVID-19 pandemic has affected the whole society and has immediate impacts on the current vaccine landscape in Thailand. The lessons learned in coping with the immediate impacts of COVID-19 have led to several key challenges for the future vaccine policy landscape. First, although local vaccine R&D and production were underrated, a thriving in funding and development has been observed during the pandemic. Second, long-term commitment from all relevant stakeholders is important to maintain policy continuation. Private organizations can be potential partners to create an effective environment. Third, the regulatory body needs to be prepared for product authorization. Fourth, though the pandemic may not immediately affect the regular decision-making process, the process should be improved. Fifth, investment in the prevention and promotion of Thailand’s healthcare system is still limited. Sources of additional funding for vaccines have been discussed, but no clear solution was finalized. In summary, it is important that all stakeholders’ mentality should be focused on supporting changes in our environment to create a sustainable and self-sufficient vaccine ecosystem.
Author contributions
Conceptualization, N.C.; Methodology, S.Y., N.C.; Data Collections, W.K., S.Y., N.C.; Resources, S. Taychakhoonavudh, S. Techathawat; Writing – Original Draft Preparation, W.K., S.Y., N.C.; All authors contributed to the critical revision of the manuscript.
Acknowledgment
We would like to thank our 12 key informants for sharing their perspectives in the in-depth interview, Dr.Nakorn Premsri for the participation as panelists, Dr.Somsak Chunharas for being a chair, all participants for sharing their opinions during in the policy forum, and Dr.Vijj Kasemsup for help review and comment on the manuscript. We would also like to extend our gratitude to Dr.Lynda Oderda and Dr.Gary Oderda for their assistance in the editing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rémy V, Zöllner Y, and Heckmann U. Vaccination: the cornerstone of an efficient healthcare system. Journal of Market Access & Health Policy. 2015;3(1):1 doi:10.3402/jmahp.v3.27041.
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645). doi:10.1098/rstb.2013.0433.
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86(2):140–7. doi:10.2471/BLT.07.040089.
- UNICEF. Socio-economic impact assessment of COVID-19 in Thailand 2020.
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. Geneva; 2021.
- Office NHS. About NHSO. Bangkok; 2021.
- National Health Security Office. National health security act B.E.2545 (A.D.2002). Bangkok; 2021.
- National Vaccine Committee. Draft of the national policy and strategic plan for vaccine security 2023-2027. Institute TNV, editor. Bangkok; 2021.
- National vaccine security act. 2019 Nov 21. p. 50–71.
- National vaccine committee regulations: funding encouragement and cooporation for vaccine research & development, and production. Committee NV, editor. Bangkok; 2019.
- Thai Food and Drug Administration. Guideline on quality, non-clinical and clinical assessment regarding market authorizations of vaccines in Thailand. 2008.
- World Health Organization. Guidelines for independent lot release of vaccines by regulatory authorities, Annex 2, TRS No 978. Geneva; 2010.
- Riewpaiboon A, Sooksriwong C, Chaiyakunapruk N, Tharmaphornpilas P, Techathawat S, Rookkapan K, Rasdjarmrearnsook A, Suraratdecha C. Optimizing national immuniation program supply chain management in Thailand: an economic analysis. Public Health. 2015;129(7):899–906. doi:10.1016/j.puhe.2015.04.016.
- AstraZeneca. AstraZeneca’s COVID-19 vaccine manufactured in Thailand authorised for World Health Organization emergency use. 2021.
- Office of the Assistant Secretary for Preparedness & Response. Biomedical advanced research and development authority. Washington (DC); 2022.
- Health Intervention and Technology Assessment Program. A review and recommendations for fiscal and monetary policy management of immunization systems in Thailand. 2020.
- National Vaccine Committee. Draft of the national policy and strategic plan for vaccine security 2019-2022. Institute TNV, editor. Bangkok; 2017.
- World Health O. Increasing access to vaccines through technology transfer and local production. Geneva: World Health Organization; 2011.
- Peacocke EF, Heupink LF, Frønsdal K, Dahl EH, Chola L. Global access to COVID-19 vaccines: a scoping review of factors that may influence equitable access for low and middle-income countries. BMJ Open. 2021;11(9):e049505. doi:10.1136/bmjopen-2021-049505.
- Onishchenko K, Hill S, Wasserman M, Jones C, Moffatt M, Ruff L, Pugh SJ. Trends in vaccine investment in middle income countries. Hum Vaccin Immunother. 2019;15(10):2378–2385. doi:10.1080/21645515.2019.1589287.