 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Globally, an estimated 23 million children missed vaccination in 2020 due to the coronavirus disease 2019 (COVID-19) pandemic. We analyzed vaccination coverage trends and catch-up strategies/recommendations implemented in Latin America during the pandemic. We performed a national administrative database analysis and a systematic literature review to evaluate vaccination coverage data and identify catch-up strategies for missed vaccinations in selected countries in Latin America (Argentina, Brazil, Chile, Colombia, Mexico and Peru). Data were extracted from national health ministry vaccination coverage and supranational databases to identify coverage of rotavirus (RV), pentavalent/hexavalent, measles, Bacillus Calmette-Guérin (BCG) and pneumococcal conjugate vaccines (PCV) at country level before and during the COVID-19 pandemic. A systematic literature review of published papers was conducted to identify vaccination catch-up strategies published in January 2020–June 2021. National administrative database-reported data showed that vaccination coverage trends were declining prior to 2020. The change in vaccination coverage before and during the COVID-19 pandemic ranged from 2.5% to −11.5% (RV), −3.0% to −11.0% (measles), 1.5% to −7.5% (PCV), 9.0% to −14.0% (pentavalent/hexavalent), and 3.0% to −18.5% (BCG). Among 696 identified studies, 14 studies were included in this review. Catch-up vaccination strategies included prioritizing routine vaccinations as per the national immunization schedule. Overall vaccination coverage declined by varying degrees among the countries investigated. This trend was observed prior to 2020, suggesting multifactorial reasons for declining vaccination rates in Latin America.
Plain Language Summary
What is the context?
The coronavirus disease 2019 (COVID-19) led to health immunization disruptions in at least 68 countries, affecting around 80 million children.
Routine childhood vaccination coverage was already suffering a decline in Latin America in the past decade, this situation could deteriorate further due to COVID-19.
Consensus is lacking on the use of current guidelines and recommendations for catch-up vaccinations, as these are difficult to implement.
What is new?
We analyzed national health ministry databases to evaluate vaccination coverage trends in Latin America before and during the COVID-19 pandemic.
We also conducted a systematic literature review to describe catch-up strategies for missed vaccinations during the pandemic.
Vaccination coverage declined for the rotavirus, measles and pneumococcal conjugate vaccines at country level from 2017-2020.
Pentavalent/hexavalent and Bacillus Calmette-Guerin vaccine coverage varied among countries for the same period.
Catch-up vaccination strategies included prioritizing routine vaccinations as per the national immunization schedule.
What is the impact?
Continued efforts from healthcare officials and providers could prevent unvaccinated children from severe disease through catch-up vaccinations.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, which began as a localized outbreak in December 2019, has caused nearly 346 million confirmed cases globally and led to almost 5.5 million deaths as of January, 2022.Citation1 As the outbreak spread rapidly in early 2020, governments around the globe sought to curb transmission through implementing travel bans, social distancing rules, mandated lockdowns, and closure of commercial activities and non-essential services.Citation2 In a recent national pulse survey among 135 participating countries conducted by the World Health Organization (WHO), it was reported that 94% of the responding countries experienced a disruption to essential health services between January and March 2021.Citation3 According to data from the WHO, the United Nations Children’s Fund (UNICEF), the Global Alliance for Vaccines and Immunizations (GAVI), and the Sabin Vaccine Institute, 68 countries have suspended their immunization services and put at least 80 million children <1 year of age at risk.Citation4,Citation5 Furthermore, the WHO reported that an estimated 23 million children missed vaccination in 2020 due to the COVID-19 pandemic.Citation6
For Latin America, there is limited information regarding the barriers to achieving high vaccination coverage in the region.Citation7 However, the COVID-19 pandemic impacted routine immunization programs in Latin America and other regions.Citation8,Citation9 Evidence suggests that this could lead to an increase in mortality from vaccine-preventable diseases (VPDs).Citation5,Citation9–12 Consequently, several supranational organizations and experts have issued guidelines for missed vaccinations during the COVID-19 pandemic.Citation13–15
This study aims to assess and describe vaccination coverage trends in selected countries from Latin America before and during the COVID-19 pandemic. Additionally, we aim to systematically review the implemented catch-up strategies and recommendations for vaccinations missed due to the pandemic.
Methods
Six Latin American countries were selected for this study, based on the availability of publicly disclosed vaccine coverage data that are validated by WHO-UNICEF coverage databases. National vaccination coverage data from these countries were extracted from national health ministry databases and the most recent Pan American Health Organization (PAHO) immunization report,Citation16 to investigate and compare vaccination coverage trends for routine childhood vaccines before and during the pandemic. For each vaccine, the pre-pandemic rate was calculated by subtracting the vaccine coverage rate of 2019 from the average vaccine coverage rate of 2018 and 2017. For a better understanding this may be represented by the equation , where and x represents the pre-pandemic coverage rate and a, b and c represent the vaccine coverage rate of 2019, 2018 and 2017 respectively. Similarly, the post-pandemic rate was determined by subtracting the coverage rate of 2020 from the average vaccine coverage rate of 2019 and 2018. This may be represented by the equation
, where y is the post-pandemic coverage rate and d is the vaccine coverage rate of 2020.
We then performed a systematic literature review based on the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) guidelines,Citation17 to identify catch-up strategies/recommendations in Latin America.
Search sources and strategy
A search strategy was developed in Medline and then adapted to mainstream and regional databases. The following online databases were searched for studies published from January 2020 through May 2021: Medline (via PubMed), Scopus, Scientific Electronic Library Online (SciELO), Google Scholar and online articles. A search of gray literature was performed using national administrative and supranational databases, to extract vaccination coverage information for selected vaccines at country-level before and during the pandemic. We also performed a snowball search by manually reviewing relevant references cited within the screened articles. The search strategy was developed using keywords and related Medical Subject Heading (MeSH) terms to capture as many examples as possible (Table S1). Database searches were performed until 16 June 2021 and retrieved articles were first screened by title and abstract following a priori established eligibility criteria (Table S2).
Article selection and data extraction
Identified publications were screened in two phases by three reviewers (AGH, MMC, IL) using the pre-defined inclusion and exclusion criteria (Table S2). The first phase included a screening of titles and abstracts for all publications, while the second phase involved reviewing the full-text of relevant articles. Any discrepancies arising from the search were discussed among all three reviewers; in cases of disagreements about article inclusion, unanimous agreement was sought.
Relevant information about each article was extracted, including title, journal, authorship and year of publication, study setting, study objectives, study design, study period (evaluation period), sample size, study population (age group) and the evaluated vaccine. Lastly, the data extraction was checked and approved by the review team.
Results
Vaccination coverage based on national administrative databases
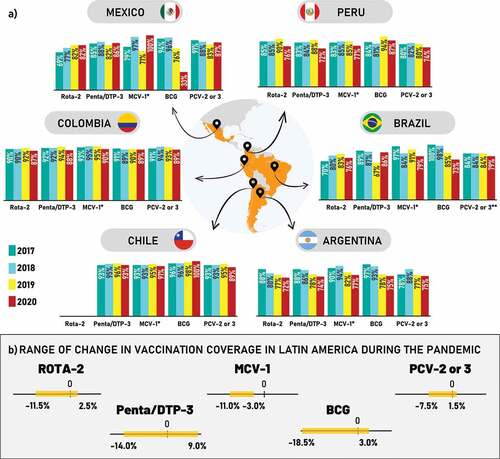
National health ministry vaccine coverage databases were cross-checked with the latest PAHO immunization reports.Citation18 Both sources were utilized to identify the vaccination coverage levels of selected vaccines at country level before and during the pandemic.Citation16,Citation18 The final analysis was performed using the PAHO database due to its standardized methodology and consistency. Selected vaccines included rotavirus vaccine (RV; two doses), pentavalent/hexavalent (diphtheria, tetanus, pertussis, poliomyelitis, Haemophilus influenzae type b and hepatitis B; three doses), measles containing vaccine (MCV; one dose), Bacillus Calmette-Guérin (BCG; one dose) and the pneumococcal conjugate vaccines (PCV; two doses). Data are presented for Argentina, Brazil, Chile, Colombia, Mexico and Peru for the years 2017–2020 (). Overall, the change in vaccination coverage ranged from 2.5% to −11.5% (RV), −11.0% to −3.0% (MCV), 1.5% to −7.5% (PCV), 9.0% to −14.0% (pentavalent/hexavalent), and 3.0% to −18.5% (BCG) ( and Table S3).
Figure 1. (a) Vaccination coverage rates for selected vaccines among six countries in Latin America, 2017–2020. (b) Annual range of the change in vaccination coverage of selected vaccines among six countries in Latin America, 2020a.
Argentina
The average coverage rate for RV declined by 7.0% pre-pandemic and 6.5% during the pandemic. The average coverage rates for pentavalent/hexavalent vaccine declined by 9.0% pre-pandemic and 8.0% during the pandemic. The average MCV coverage rate declined by 10.0% pre-pandemic, and 11.0% during the pandemic. The average BCG coverage rate declined by 17.0% pre-pandemic and 10.5% during the pandemic. The average coverage rate of PCV declined by 6.0% pre-pandemic and 7.5% during the pandemic ().
Brazil
The coverage rate of the RV vaccine showed an increasing trend while the remaining vaccines indicated a decreasing trend in Brazil from 2017 to 2020. The pre-pandemic average coverage rate for RV showed an increase of 8.0%, which declined by 5.5% during the pandemic. For the pentavalent/hexavalent vaccine, an average decline of 21.0% and an average increase of 9.0% were observed before and during the pandemic, respectively. The average MCV coverage rate increased by 0.5% pre-pandemic and decreased by 8.5% during the pandemic. The average decline for BCG vaccine during the pre-pandemic period was 14.0% and this further declined by 18.5% during the pandemic. For PCV, there was no change in the pre-pandemic vaccination coverage rate but it declined by 5.0% during the pandemic ().
Chile
Vaccination coverage data for the RV vaccine for Chile were unavailable, as this vaccine is not included in the national immunization programme (NIP). Interestingly, from 2017 to 2020, the BCG vaccine coverage rate increased, while pentavalent/hexavalent vaccine coverage remained stable. Meanwhile, MCV and PCV coverage rates decreased slightly during the pandemic. Average pentavalent/hexavalent coverage increased by 2.0% pre-pandemic and decreased by 2.5% during the pandemic. Similarly, average PCV coverage increased by 2.0% pre-pandemic and then decreased by 5.0% during the pandemic. Average MCV coverage increased by 2.0% pre-pandemic and decreased by 3.0% during the pandemic. Average BCG coverage increased by 2.0% pre-pandemic and further increased by 3.0% during the pandemic ().
Colombia
The average RV vaccine coverage rate increased by 2.0% in the pre-pandemic period and decreased by 4.0% during the pandemic. Average coverage for MCV increased by 1.0% pre-pandemic and decreased by 5.0% during the pandemic. Average pentavalent/hexavalent coverage increased by 2.0% pre-pandemic and decreased by 5.0% during the pandemic. Average BCG vaccine coverage rate remained constant at 0% pre-pandemic and decreased by 0.5% during the pandemic, while the average PCV coverage rate increased by 0.5% pre-pandemic and decreased by 4.5% during the pandemic. ().
Mexico
The coverage rate for RV vaccine, pentavalent/hexavalent vaccine and MCV increased, while the coverage for PCV and BCG vaccine decreased from 2017 to 2020. The average coverage rate for RV vaccine increased by 9.0% pre-pandemic and 2.5% during the pandemic. Average pentavalent/hexavalent vaccine coverage declined by 4.5% pre-pandemic and increased by 1.0% during the pandemic. Average MCV coverage declined by 17.0% pre-pandemic and increased by 16.0% during the pandemic. Average coverage for BCG decreased by 19.0% pre-pandemic and further decreased by 53.0% during the pandemic. The average PCV coverage declined by 6.5% pre-pandemic and increased by 1.5% during the pandemic ().
Peru
The coverage rates for the RV, pentavalent/hexavalent, MCV and PCV vaccines decreased, whereas BCG coverage rate increased from 2017 to 2020. Average RV coverage increased by 5.0% pre-pandemic and decreased by 11.5% during the pandemic. Average pentavalent/hexavalent coverage increased by 4.5% pre-pandemic and decreased by 14.0% during the pandemic. Average MCV coverage increased by 1.0% pre-pandemic and decreased by 8.0% during the pandemic, while average BCG coverage increased by 11.5% pre-pandemic and decreased by 0.5% during the pandemic. Average PCV coverage rate declined by 1.0% pre-pandemic and further declined by 7.0% during the pandemic ().
Systematic literature review study characteristics
The systematic literature review included 14 studies out of 696 publications identified through the five databases ().Citation13–15,Citation20–30 A description of the main characteristics of the studies follows; further details are presented in Table S4.
Figure 2. PRISMA diagram.
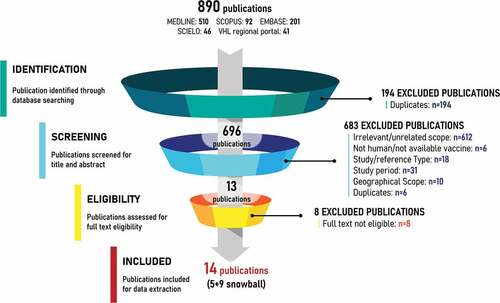
Geographically, the included studies presented nationwide (n = 6),Citation20–21,Citation26–29 regional (n = 4)Citation13,Citation15,Citation24,Citation25 or international (n = 4)Citation14,Citation22,Citation23,Citation30 data (Figure S1). Among the studies reporting nationwide data, the highest number reported data from Brazil (n = 3).Citation20,Citation27,Citation28 One study each reported nationwide data for Argentina,Citation29 Peru,Citation26 and Chile.Citation21 Regional studies that reported data for the Latin American region were also identified (n = 4).Citation13,Citation15,Citation24,Citation25 Studies reporting international level data were produced by WHO, Centers for Disease Control and Prevention (CDC), and PAHO (n = 4).Citation14,Citation22,Citation23,Citation30
Most of the included studies were recommendations/guidelines (n = 5)Citation15,Citation22−Citation24,Citation30 followed by a narrative or retrospective administrative database analyses (n = 4).Citation26–29 The remaining studies were qualitative analyses (n = 2),Citation21,Citation25 an interrupted time series analysis (n = 1),Citation20 expert opinion (n = 1)Citation13 and a descriptive review (n = 1).Citation14
Study populations in the studies were broken down by age groups (Figure S1). Study objectives were reported for all studies, based on either vaccination coverage or catch-up strategies/recommendations (Figure S1). Vaccines evaluated varied based on the study population, with 11 studies reporting the vaccines being evaluated (Figure S1).Citation14,Citation20,Citation22–30
Catch-up strategies/recommendations
One study from Chile reported national level catch-up strategies/recommendations.Citation21 The remaining vaccination guidelines were published by supranational and regional organizations.
The recommendations from Chile were provided by the Advisory Committee on Vaccines and Vaccination strategies and aligned with those provided by PAHO and WHO.Citation21 It was recommended to maintain the vaccination of newborns in hospital birth-centers as per the vaccination schedule. Primary vaccination series should be prioritized, especially vaccines protective against measles, rubella, poliomyelitis and pneumococcal disease. For influenza, it was recommended to prioritize the most vulnerable groups for vaccination.
For individuals with COVID-19, it was recommended that all vaccinations be deferred until the patient had completely recovered. Similarly, vaccinating a person who was a contact of a COVID-19 case should be deferred for the quarantine period (14 days since their last exposure).Citation21
CDC provided similar recommendations regarding COVID-19 patients and those exposed to COVID-19 cases.Citation22 Additional recommendations included the use of reminder and recall systems, immunization information systems and health records to promote vaccine adherence and ensure timely catch-up vaccination.
WHO’s Strategic Advisory Group of Experts on Immunization recommended that a clearly defined catch-up vaccination policy and schedule should be in place for all NIPs.Citation15 All preventive mass vaccination campaigns were to be halted where there are no active VPD outbreaks while simultaneously assessing the risk-benefit of continuing vaccination programs during outbreaks/endemic situations.Citation13 Catch-up vaccination strategies should be based on the epidemiology of VPDs in the region and include outreach programs, mobile immunization sessions or intensified routine immunization services for a given period.
Guidance from PAHO, in the context of the COVID-19 pandemic,Citation24 recommended similar strategies to those from Chile, i.e., vaccination against influenza and measles should be prioritized and vaccination of newborns must remain a priority in all settings, especially against hepatitis B. It was also recommended that countries with pneumococcal vaccination programs for older adults and individuals in risk groups should maintain such programs whenever possible. Maintaining a periodic and systematic registry of the population including newborns pending vaccination was also recommended. Additionally, it was recommended to temporarily suspend mass vaccination campaigns due to the risk of increasing COVID-19 transmission in the community and in health establishments. A risk-benefit analysis of conducting outbreak response vaccination was recommended in the case of a VPD outbreak, to safely implement this decision in the context of the COVID-19 pandemic. Guidance also recommended to prioritize vaccination of newborns according to the national immunization calendar.Citation23 School-based vaccination programs should be maintained only if they can be implemented safely. Individuals infected with COVID-19 or those exposed to COVID-19 cases should be vaccinated following recovery or after completion of quarantine, respectively.
A study that provided recommendations based on expert opinionCitation13 stated that VPD surveillance should be continued, and health services should be adapted to sustain vaccinations during the pandemic. Experts also recommended the prioritization of catch-up vaccinations if the situation was safe to do so. One study recommended the vaccination schedule be updated as soon as the pandemic situation allowed the immunization program to be implemented safely.Citation14 A summary of these recommendations is shown in .
Table 1. Summary of strategies and recommendations.Citation13–15,Citation21–25,Citation30..
PAHO provided guidance regarding vaccination of newbornsCitation23 and recommended that the vaccination of newborns with hepatitis B (HepB) and BCG vaccines (as per the national immunization schedule) should be prioritized. For mothers who tested positive for COVID-19, but whose newborn may or may not be COVID-19 positive, it was recommended to postpone the BCG vaccine until both mother and child were discharged from hospital or until 14 days after symptoms had subsided in the newborn. The HepB vaccine should be prioritized for all births within 24 hours of life or as soon as possible, regardless of COVID-19 symptoms.
Discussion
All six studies that reported data on vaccination coverage in Latin America reported an overall decrease in coverage from March 2020.Citation20–21,Citation26–29 Several studies in this review also reported regional variations in the decline of vaccination coverage.Citation13,Citation15,Citation24,Citation25 These variations could be due to regional/cultural differences affecting compliance with health guidelines, level of concern regarding infection, and differences in socioeconomic status.Citation31,Citation32 Additionally, our results suggest that vaccination coverage for some vaccines in various countries was declining even prior to the pandemic. Hence, some reasons for the declining vaccination coverage post-pandemic could be assumed to be outside the direct effects of the pandemic. These reasons could include declining birth rates, inaccessibility to health centers due to shortage of healthcare resources or parents canceling scheduled vaccination appointments due to fear of COVID-19 infection. However, diphtheria – tetanus–pertussis (DTP) vaccine coverage rates increased slightly in Brazil during the pandemic. Mexico also reported a higher vaccine coverage rate for most vaccines, except for the BCG vaccine. This may have been influenced by the stringent vaccine policy introduced following the 2019 measles outbreak in Mexico.Citation33 Further evidence on this subject may help to improve vaccination coverage in the region.
The decline in vaccination coverage in Latin America in 2020 was similar to declines reported for the South-East Asia and Western Pacific regions, where 18 of the 19 (95%) countries included in the study reported disruptions to vaccination, especially infancy and school-entry age vaccinations.Citation34 The study reported that DTP vaccine coverage decreased by 42.0%, oral polio vaccine coverage decreased by 79.0%, and measles vaccine coverage decreased by 9.0% among the school-entry age group.Citation34 Similar findings were reported in Pakistan, where a 52.5% decrease was observed in the daily average total number of vaccinations administered from September 2019 to March 2020.34 The largest decline was 40.6%, for the BCG vaccine.Citation35 The Spanish Association of Pediatrics also recorded a decline in vaccination coverage (range: 5.0%–60.0%) in March 2020 compared with the monthly average from January 2019 to February 2020.Citation36 However, while the above-mentioned studies reported declining vaccination coverage, not all countries reported such declines in 2020. Brazil recorded a 5.79-fold increase in MCV coverage, from 0.86% to 4.98%, following a vaccination campaign in 2020.Citation37 Australia reported a 17.0% absolute increase in coverage for influenza vaccination (adults/elderly), and South Korea reported a 1.0% increase in coverage for HepB, BCG (early infancy), measles, and pneumococcus (infancy) vaccines.Citation34 Best practices developed by these countries could be utilized to improve vaccination coverage within Latin America.
Studies describing vaccination catch-up strategies/recommendations were aligned in their recommendations for prioritizing primary vaccination series, vaccination of COVID-19 cases and individuals suspected of COVID-19 infection, continued surveillance of VPD outbreaks, and catch-up strategies for missed vaccinations. We found that while vaccination strategies exist during times of service disruptions, a lack of standardization remains that hinders the successful implementation of these strategies within the Latin American region.
The disruption of essential services due to COVID-19 also impacted the supply chains of healthcare systems. In France, during the lockdown, routine vaccination saw the greatest decrease during the week of May 30 to 5 April 2020. Penta/hexavalent vaccination coverage fell by 28.9%, and deliveries of measles, mumps and rubella (MMR) and human papillomavirus (HPV) vaccines decreased by more than half (50.8% and 78.1%, respectively).Citation38 In the United States, two studies highlighted a notable decline (approximately 75.0%) in vaccines ordered by doctors since January 2020.Citation8,Citation39 This decline in vaccine orders and deliveries could lead to an increased risk of VPD outbreaks in communities with low vaccine coverage.
Study limitations included inconsistencies in vaccination coverage registration and estimation methods in each country, which makes cross-country comparisons difficult. Therefore, we utilized the WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) database, which simplifies comparisons between countries due to standardized coverage estimates. Additionally, this study does not analyze specific risk factors that affected the change in vaccination coverage, considering these are multi-factorial and some related or unrelated to our main effect variable (the COVID-19 pandemic). These unrelated risk factors affecting vaccination coverage could be a subject of future research.
The disruption of essential health services due to the COVID-19 pandemic greatly impacted routine childhood vaccination in the Latin American region. Studies also reported declining coverage prior to the pandemic, suggesting multifactorial reasons for lagging vaccine coverage in the region. Recommendations to prioritize routine vaccination, continue surveillance of VPDs within communities, and develop vaccination campaigns with strategic intervention plans and catch-up strategies based on outreach programs could reverse this declining coverage.Citation37
A plain language summary of this article’s context and findings can be found in .
Contributorship
Maria Mercedes Castrejon: Conceptualization (lead); data collection and analysis (supporting); interpretations (supporting); manuscript – review and editing (equal).
Ingrid Leal: Conceptualization (supporting); data collection and analysis (supporting); interpretations (supporting); manuscript – review and editing (equal).
Thatiana Pinto: Data collection and analysis (supporting); interpretations (supporting); manuscript – review and editing (equal).
Adriana Guzmán-Holst: Conceptualization (supporting); methodology (lead); data collection and analysis (lead); interpretations (supporting); manuscript – review and editing (equal).
All authors had full access to the data and gave final approval before submission.
Previous congress activities
The results of this manuscript were previously presented at the SLIPE 2021 congress held in Buenos Aires, Argentina and virtually, from 13–15 October 2021.
Supplemental Material
Download MS Word (175.4 KB)Acknowledgements
The authors would like to thank Diana Caceres for her support as a reviewer and for accessing data for this study. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Amrita Ostawal (Arete Communication UG, on behalf of GSK) and Kavin Kailash provided writing assistance.
Disclosure statement
MMC, IL, TP and AGH are employed by the GSK group of companies. MMC, IL and AGH hold shares in the GSK group of companies. All authors declare no other financial and non-financial relationships and activities.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2102353
Additional information
Funding
References
- World Health Organization. Weekly epidemiological update on COVID-19 - 25 January 2022. World Health Orgnization; 2022 [ accessed Feb 3]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022.
- Oraby T, Tyshenko MG, Maldonado JC, Vatcheva K, Elsaadany S, Alali WQ, Longenecker JC, Al-Zoughool M. Modeling the effect of lockdown timing as a COVID-19 control measure in countries with differing social contacts. Sci Rep. 2021;11(1):1. doi:10.1038/s41598-021-82873-2.
- World Health Organization. Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic. World Health Organization; 2021 [ accessed Nov 11]. https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS-continuity-survey-2021.1.
- Nelson R. COVID-19 disrupts vaccine delivery. Lancet Infect Dis. 2020;20(5):546. doi:10.1016/s1473-3099(20)30304-2.
- World Health Organizaion. At least 80 million children under one at risk of diseases such as diphtheria, measles and polio as COVID-19 disrupts routine vaccination efforts, warn Gavi, WHO and UNICEF. World Health Organization; 2020 [ accessed Nov 11]. https://www.who.int/news/item/22-05-2020-at-least-80-million-children-under-one-at-risk-of-diseases-such-as-diphtheria-measles-and-polio-as-covid-19-disrupts-routine-vaccination-efforts-warn-gavi-who-and-unicef.
- World Health Organization. Immunization coverage. World Health Organizaion; 2021 [ accessed Nov 11]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- Guzman-Holst A, DeAntonio R, Prado-Cohrs D, Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38(3):470–9. doi:10.1016/j.vaccine.2019.10.088.
- Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Gee J, Glover M, Herring B, Kang Y. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):591–593. doi:10.15585/mmwr.mm6919e2.
- Ota MOC, Badur S, Romano-Mazzotti L, Friedland LR. Impact of COVID-19 pandemic on routine immunization. Ann Med. 2021;53(1):2286–2297. doi:10.1080/07853890.2021.2009128.
- Gaythorpe K, Abbas K, Huber J, Karachaliou A, Thakkar N, Woodruff K, Li X, Echeverria-Londono S, Disease VWGoC-IoVP, Ferrari M, et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. medRxiv. 2021: 2021.2001.2025.21250489. doi:10.1101/2021.01.25.21250489
- Lassi ZS, Naseem R, Salam RA, Siddiqui F, Das JK. The impact of the COVID-19 pandemic on immunization campaigns and programs: a systematic review. Int J Environ Res Public Health. 2021;18(3):988. doi:10.3390/ijerph18030988.
- Chiappini E, Parigi S, Galli L, Licari A, Brambilla I, Angela Tosca M, Ciprandi G, Marseglia G. Impact that the COVID-19 pandemic on routine childhood vaccinations and challenges ahead: a narrative review. Acta Paediatr. 2021;110(9):2529–2535. doi:10.1111/apa.15949.
- Ávila-Agüero ML, Ospina-Henao S, Pirez MC, Gentile Á, Araya S, Brea J, Mendoza L, Falleiros-Arlant LH. Latin American forum on immunization services during the COVID-19 pandemic. Expert Rev Vaccines. 2021;20(3):231–234. doi:10.1080/14760584.2021.1886930.
- Dinleyici EC, Borrow R, Safadi MAP, van Damme P, Munoz FM. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccines Immunother. 2021;17(2):400–407. doi:10.1080/21645515.2020.1804776.
- World Health Organization. Immunization as an essential health service: Guiding principles for immunization activities during the COVID-19 pandemic and other times of severe disruption. World Health Organization; 2020 [ accessed Nov 11]. https://www.who.int/publications/i/item/immunization-as-an-essential-health-service-guiding-principles-for-immunization-activities-during-the-covid-19-pandemic-and-other-times-of-severe-disruption.
- Pan American Health Organization. Immunization brochure. Pan American Health Organization; 2021 [ accessed Nov 11]. https://www.paho.org/en/tag/immunization-brochure.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100.
- Pan American Health Organization. Immunization data and statistics. Pan American Health Organization; [ accessed Nov 11]. https://www.paho.org/en/topics/immunization/immunization-data-and-statistics.
- World Health Organization. WHO/UNICEF estimates of national immunization coverage. WUENIC; 2021 [ accessed Nov 11]. https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/immunization-coverage/who-unicef-estimates-of-national-immunization-coverage.
- Alves JG, Figueiroa JN, Urquia ML. Impact of COVID-19 on immunization of Brazilian infants. Int J Infect Dis. 2021;107:252–253. doi:10.1016/j.ijid.2021.04.089.
- CAVEI. Considerations of the Advisory Committee on Vaccines and Vaccination Strategies (CAVEI) in relation to vaccination services during the health emergency COVID-19. Rev Chil Pediatr. 2020;91(4):620–622. doi:10.32641/rchped.vi91i4.2475.
- Centers for Disease Control and Prevention. Interim guidance for routine and influenza immunization services during the COVID-19 pandemic. Centers for Disease Control and Prevention; 2021 [ accessed Nov 11]. https://www.cdc.gov/vaccines/pandemic-guidance/index.html.
- Pan American Health Organization. Vaccination of newborns in the context of the COVID-19 pandemic, 19 May 2020. Pan American Health Organization; 2020 [ accessed Nov 11]. https://covid-19pharmacovigilance.paho.org/img/recursos/60a8355b7aab338b7d6574ee3.pdf.
- Pan American Health Organization. The immunization program in the context of the COVID-19 pandemic. Version 2 (24 April 2020). Pan American Health Organization; 2020 [ accessed Nov 11]. https://iris.paho.org/handle/10665.2/52056.
- Pan American Health Organization. Summary of the status of national immunization programs during the COVID-19 pandemic, July 2020. Pan American Health Organization; 2020 [ accessed Nov 11]. https://iris.paho.org/handle/10665.2/52544.
- Pereira-Victorio C-T, Libertad T, Valladares-Garrido MJ. Vaccination coverage in times of COVID-19: an analysis from social epidemiology in the region of Cusco/Coberturas de vacunación en tiempos de COVID-19: un análisis desde la epidemiología social en la región del Cusco. Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo. 2020;13:167–174.
- Sato APS. Pandemic and vaccine coverage: challenges of returning to schools. Rev Saude Publica. 2020;54:115. doi:10.11606/s1518-8787.2020054003142.
- Silveira MF, Tonial CT, Goretti KMA, Teixeira AMS, Hallal PC, Maria BMA, Horta BL, Hartwig FP, Barros AJD, Victora CG. Missed childhood immunizations during the COVID-19 pandemic in Brazil: analyses of routine statistics and of a national household survey. Vaccine. 2021;39(25):3404–3409. doi:10.1016/j.vaccine.2021.04.046.
- Torres F, Domínguez P, Aruanno ME, Macherett MJ, Nocent ES, Risoli L, Sasso M, Cabello C, Seoane MN. Impact of the SARS-CoV-2 pandemic on the administration of vaccines as per the national immunization schedule in children younger than 2 years. Arch Argent Pediatr. 2021;119(3):198–201. doi:10.5546/aap.2021.eng.198.
- World Health Organization. Immunization in the context of COVID-19 pandemic. World Health Organization; 2020 [ accessed Nov 11]. https://www.who.int/publications/i/item/immunization-in-the-context-of-covid-19-pandemic.
- Chandir S, Siddiqi DA, Setayesh H, Khan AJ. Impact of COVID-19 lockdown on routine immunisation in Karachi, Pakistan. Lancet Glob Health. 2020;8(9):e1118–e1120. doi:10.1016/s2214-109x(20)30290-4.
- McDonald HI, Tessier E, White JM, Woodruff M, Knowles C, Bates C, Parry J, Walker JL, Scott JA, Smeeth L, et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Eurosurveillance. 2020;25(19):2000848. doi:10.2807/1560-7917.ES.2020.25.19.2000848.
- Rincón-León HA, Navarro-Fuentes KR. Measles: a millennial itch in the COVID-19 era. Rev Med Inst Mex Seguro Soc. 2020;58(6):644–647. doi:10.24875/RMIMSS.M20000095.
- Harris RC, Chen Y, Côte P, Ardillon A, Nievera MC, Ong-Lim A, Aiyamperumal S, Chong CP, Kandasamy KV, Mahenthiran K, et al. Impact of COVID-19 on routine immunisation in South-East Asia and Western Pacific: disruptions and solutions. Lancet Reg Health West Pac. 2021;10:100140. doi:10.1016/j.lanwpc.2021.100140.
- Chandir S, Siddiqi DA, Mehmood M, Setayesh H, Siddique M, Mirza A, Soundardjee R, Dharma VK, Shah MT, Abdullah S, et al. Impact of COVID-19 pandemic response on uptake of routine immunizations in Sindh, Pakistan: an analysis of provincial electronic immunization registry data. Vaccine. 2020;38(45):7146–7155. doi:10.1016/j.vaccine.2020.08.019.
- Moraga-Llop FA, Fernández-Prada M, Grande-Tejada AM, Martínez-Alcorta LI, Moreno-Pérez D, Pérez-Martín JJ. Recovering lost vaccine coverage due to COVID-19 pandemic. Vacunas. 2020;21(2):129–135. doi:10.1016/j.vacun.2020.07.001.
- Lopes-Júnior LC, de Souza TM, Sobreira LB, Daleprane CLV, Denadai IR, Martins NB, Dall’Orto TLC, Rabelo LC, Martins EA, Silva VR, et al. Analysis of vaccination coverage during the COVID-19 pandemic in Vitória, Brazil. J Human Growth Dev. 2021;31(3):387–397. doi:10.36311/jhgd.v31.12122.
- Alain Weill JD, Desplas D, Cuenot F, Dray-Spira R, Zureik M. Usage des médicaments de ville en France durant l’épidémie de la Covid-19 – point de situation après les 8 semaines de confinement et une semaine de post-confinement. EPIPHARE - Groupement d’intérêt scientifique (GIS) ANSM-CNAM; 2020 [ accessed Nov 11]. https://ansm.sante.fr/uploads/2020/10/13/20201013-epi-phare-rapport-covid-3-1usage-medic.pdf.
- Chanchlani N, Buchanan F, Gill PJ. Addressing the indirect effects of COVID-19 on the health of children and young people. CMAJ. 2020;192(32):E921–e927. doi:10.1503/cmaj.201008.

