ABSTRACT
Introduction
Cancer patients are more vulnerable to coronavirus disease 2019 (COVID-19) owing to their compromised immune status. However, data regarding COVID-19 vaccine safety and immune response in cancer patients are scarce.
Method
This prospective, age- and sex-matched, single-center cohort study included 61 cancer patients and 122 healthy control participants. Seropositivity was defined as anti-S IgG titer >0.8 units/ml. Primary end point was seroconversion rate of immunoglobulin (Ig)G antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein (anti-S IgG) in cancer patients vs. healthy control participants following the second dose of COVID-19 vaccine ChAdOx1 nCoV-19 (AZD1222).
Results
After the second-dose vaccination, there was no difference in seropositivity rate between groups (57 [93.44%] patients with cancer vs. 121 [99.18%] control participants; geometric mean ratio [GMR]: 0.39; 95%CI: 0.01–10.46; p-value = 0.571). In contrast, after the first-dose vaccination, the seropositivity rate was significantly lower in the cancer patients than in the control participants (50/61 [81.97%] vs. 121/122 [99.18%]; GMR: 0.07; 95%CI: 0.01–0.71; p = 0.025). The median anti-S IgG titer after the first-and second dose vaccination were not significantly different between groups. Female sex was significantly associated with a higher anti-S IgG titer. 5FU- and taxane-based chemotherapy regimens were associated with a lower IgG titer. Side effects of vaccination were tolerable.
Conclusions
The anti-S IgG seropositivity rate after completing the second vaccine dose did not differ between the cancer patients and control participants. However, the anti-S IgG seropositivity rate after the first-dose vaccination was lower in cancer patients.
Introduction
Coronavirus disease 2019 (COVID-19) is a new emerging disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the first cluster of COVID-19 cases was reported in China on 31 December 2019, more than 493 million cases and 6 million deaths globally as of 8 April 2022 were reported by World Health Organization (WHO).Citation1 Cancer patients are more vulnerable to infection than are healthy people owing to their compromised immune status and the cancer treatments they undergo.Citation2 In a meta-analysis of 19 retrospective studies involving 63,019 patients (2,682 patients with cancer), the incidence of COVID-19 among cancer patients was 6%, which is much higher than the global incidence (approximately 0.2%), and the COVID-19 mortality rate of cancer patients, especially lung cancer patients, was higher than that of persons without cancer [risk ratio (RR): 1.8].Citation3 Thus, cancer patients should be given priority for receiving COVID-19 vaccination.Citation4,Citation5 However, data supporting the safety and immune response of COVID-19 vaccination in cancer patients are scarce. Most clinical trials exclude cancer patients, and the few clinical trials that have included them had fairly small numbers of cancer patients.
Suboptimal vaccine immune response has been reported in cancer patients.Citation6 Immunogenicity is a crucial point of concern owing to the inherent immunocompromised status of cancer patients as well as the anticancer treatment they receive. The SOAP-02 study was the first study on the safety and immunogenicity of a COVID-19 vaccine in cancer patient populations, including both hematologic and solid malignancy patients, using the BioNTech/Pfizer COVID-19 (mRNA-BNT162b2) vaccine.Citation7 The rates of seroconversion for immunoglobulin (Ig)G antibodies against the SARS-CoV-2 spike (S) protein (anti-S IgG) at approximately 21 days following inoculation with a single vaccine dose were 94%, 38%, and 18% in healthy controls, solid cancer patients, and hematological cancer patients, respectively. Among the patients with available blood samples from 2 weeks after a 21-day-post-vaccine boost, the seroconversion rates were 100%, 95%, and 60% in healthy controls, solid cancer patients, and hematological cancer patients, respectively.
A prospective cohort study in Israel that included 180 participants with solid cancer who received the mRNA-BNT162b2 vaccine also reported the vaccine immunogenicity in cancer patients as compared with that in healthy controls.Citation8 The median anti-S IgG titer in the cancer patients was significantly lower than that in the control participants (1931 arbitrary unit (AU/ml vs 7160 AU/ml; p < 0.001). In a multivariable analysis, the variable that was significantly associated with lower anti-S IgG titers was treatment with chemotherapy plus immunotherapy.
ChAdOx1 nCoV-19 (AZD1222) is a viral-vectored vaccine with a recombinant structure in which chimpanzee adenovirus encodes the spike (S) glycoprotein of the SARS-CoV-2 virus.Citation9 In a meta-analysis that included 21 studies of COVID-19 vaccination, using either mRNA-1273 (Moderna), mRNA-BNT162b2, AZD1222, or Ad26.COV2.S (Janssen), in 5012 patients with active malignancies, the seroconversion rates of anti-S IgG after complete COVID-19 vaccination, were 85% in cancer patients versus 100% in healthy control participants (p < 0.001).Citation10 Only one paper in this meta-analysis studied the immune response of AZD1222 in cancer patients.Citation11 In that study, only 4% of patients received AZD1222, and the immunogenicity analysis was conducted on just 6% of all patients after their first vaccine dose. To address this gap, we began the present study investigating the safety and seropositivity of AZD1222 in patients with cancer and determining the factors associated with the immunogenicity of this vaccine.
Materials and methods
Study design and participants
This prospective single-centered cohort study was conducted to evaluate the seropositivity of AZD1222 (ChAdOx1 nCoV-19 vaccine) among cancer patients. The study included patients aged ≥18 years who were histologically diagnosed with solid tumors at the Outpatient Department in Chulabhorn Hospital, Thailand. The enrolled patients were required to have an ECOG status of 0–2. We also included a cohort of healthy control participants who were health-care workers from our hospital. Exclusion criteria included: hematologic malignancy, absolute neutrophil count of < 1500 cells/µl and/or platelet count of <100,000 cells/µl, life expectancy of <3 months, pregnancy, and previous COVID-19 infection. The study was approved by the Ethics Committee of Chulabhorn Hospital. All participants signed a written consent form after receiving study information.
Procedure
During the period from 1 June 2021 to 18 July 2021, participants received their second of two doses of AZD1222, 12 weeks after receiving the first dose. To evaluate the immunogenicity of AZD1222 in our cohort, blood samples (6 ml) were collected from each study participant at the Outpatient Department in Chulabhorn Hospital at four timepoints: timepoint 1, before receiving first vaccine dose; timepoint 2, at week 8 after the first vaccine dose; timepoint 3, at week 12 after the first vaccine dose, directly before receiving the second vaccine dose; and timepoint 4, at week 4 after the second vaccine dose. The Roche Elecsys® Anti-SARS-CoV-2 immunoassay was used to detect antibodies against SARS-CoV-2 S protein (anti-S antibody). This assay has been demonstrated to perform reliably, with overall specificity and sensitivity of 99.95% and 97.92%, respectively.Citation12 Samples with serum anti-S IgG antibody levels of >0.8 Units (U)/ml were considered positive. According to the WHO, the international standard for reporting immunogenicity is binding antibody units (BAU)/ml.Citation13 The Elecsys-S U/ml were converted to BAU by using the equation: Elecsys-S U = 0.972 × BAU.Citation14
For the 30 minutes directly following vaccination with a dose of AZD1222, all participants were observed for immediate adverse events (AEs). A questionnaire regarding vaccination side effects was conducted via telephone to evaluate symptoms on days 1, 7, and 30 post-vaccination for both vaccine doses. The grading severity of AEs was defined as follows: mild, AE does not interfere with daily activity; moderate, AE interferes with daily activity; severe, AE limits daily activity; and life-threatening, AE requires emergency department visit or hospitalization.
Outcome
The primary endpoint was the rate of seroconversion for anti-S IgG antibody in cancer patients relative to that in healthy control participants (mostly health-care workers) following second-dose vaccination with AZD1222. The secondary outcomes were the anti-S IgG antibody seroconversion rate after first-dose vaccination, comparisons of anti-S IgG titers between cancer patients and healthy control participants, identification of factors associated with anti-S IgG antibody seropositivity using univariate analyses, and evaluation of AZD1222 vaccination side effects.
Statistical analysis
The sample size was based on historical data from testing the COVID-19 vaccine BNT162b2 in patients with cancer.Citation8 The present study was designed to have 90% power, assuming a two-sided overall alpha of 0.05. We planned to include approximately 60 cancer patients in this study. Every cancer patient was matched to a healthy control participant of the same sex with a maximum age difference of 5 years. Patients were categorized as healthy control participants and cancer patients by timepoint 1 (8 weeks after first-dose vaccination) or timepoint 2 (4 weeks after second-dose vaccination). The significance of differences in the anti-S antibody seroconversion rates between the cancer patient group and the healthy control group at each timepoint were evaluated by logistic regression. Models were adjusted for comorbidities. The significance of differences in the geometric mean ratio (GMR) of anti-S IgG titers between cancer patients and healthy control participants were evaluated by linear regression. We report GMRs with their 95% confidence intervals (95%CIs). Univariate analysis was performed by linear regression. A p-value of <0.05 was considered to indicate a significant difference. All statistical tests were performed using STATA/SE version 16.1 (StataCorp LLC; College Station, TX, USA).
Results
During the period from 1 June 2021 to 18 July 2021, 88 patients with cancer at the Outpatient Department of Chulabhorn Hospital who met the eligibility criteria were included in the study. After age-matching, data from 61 patients in the cancer patient group and 122 control participants were analyzed. The baseline characteristics of the cancer patients and control participants are summarized in .
Table 1. Baseline characteristics of study participants.
Among the study participants in both the cancer patient and control groups, most were women (54.1%). The median age was equal between groups. Among the cancer patient group, the common cancer types were lung cancer (27.87%), colon cancer (27.87%), and breast cancer (26.23%). Half of the patients received chemotherapy (50.82%).
All participants received the AZD1222 vaccine. After the second-dose vaccination, 57 (93.44%) participants in the cancer patient group and 121 (99.18%) participants in the healthy control group were seropositive for anti-S IgG; this difference between groups was not statistically significant (GMR: 0.39; 95%CI: 0.01–10.46; p = 0.571). However, after first-dose vaccination, the rate of anti-S IgG seropositivity in the cancer patient group was significantly lower than that in the healthy control group (50/61 (81.97%) vs 121/122 (99.18%), respectively; GMR: 0.07; 95%CI: 0.01–0.71; p = 0.025) ().
Table 2. Anti-S IgG seroconversion rate following vaccination with AZD1222.
The median anti-S IgG titer after the first-dose vaccination was not significantly different between the cancer patient and the healthy control group (15.42 BAU/ml vs 42.94 BAU/ml; GMR: 0.66; 95%CI: 0.31–1.42; p = 0.294), as well as these titers after the second-dose vaccination (510.12 BAU/mL vs 659.64 BAU/mL; GMR: 1.59; 95%CI: 0.73–3.46; p = 0.242) (). Conducting a subgroup univariate analysis within the cancer patient group by cancer type, systemic treatment, chemotherapy regimen, and stage of cancer revealed that patients who received targeted therapy/immunotherapy and chemotherapy have lower anti-S IgG titers, especially patients who received taxane-based chemotherapy (GMR: 0.06; 95%CI: 0.01–0.63; p = 0.021) or 5-FU-based chemotherapy (GMR: 0.15; 95%CI: 0.03–0.77; p = 0.025). Female participants had significantly higher anti-S IgG levels compared with male participants after first-dose vaccination. There were no statistically significant differences in the anti-S IgG titer among subgroups with different cancer types or stages of cancer ().
Table 3. Anti-S IgG titers following vaccination with AZD1222.
Table 4. Anti-S IgG titer by cancer patient subgroup.
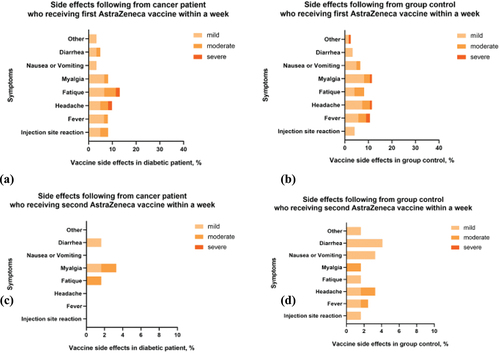
Toxicity data were available for all 183 participants (61 participants in the cancer patient group and 122 participants in the healthy control group). There were 51 of 61 (83.61%) patients with cancer and 101 of 122 (82.69%) healthy control participants who reported no toxicities following the first dose of vaccination with AZD1222. Likewise, after the second dose of AZD1222, 58 of 61 (95.08%) patients with cancer and 114 of 122 (93.44%) healthy control participants reported no toxicity. Compared with the healthy control group, after first-dose vaccination, the cancer patient group had higher incidences of fatigue (13.11% vs 8.20%), diarrhea (4.92% vs 3.28%), and injection site reaction (8.20% vs 4.10%) but less myalgia (1.64% vs 9.84%), headache (8.20% vs 11.4%), and fever (8.20% vs 10.66%). However, after the second dose of vaccination with AZD1222, the cancer patient group had lower incidences of all reported side effects compared with the healthy control group, except for myalgia (3.28% vs 1.64%). There was no death associated with AZD1222 vaccination ().
Discussion
To the best of our knowledge, this is the first report of the immunogenicity and safety of the AZD1222 vaccine in solid cancer patients relative to that in healthy control participants. The anti-S IgG seroconversion rate after the second-dose vaccination with AZD1222 did not differ between cancer patients and healthy control participants. The median anti-S IgG titer after the first-and second dose vaccination were not significantly different between groups. However, after first-dose vaccination, the anti-S IgG seroconversion rate was significantly lower in the cancer patient group than in the healthy control group. The vaccine was generally well-tolerated in cancer patients.
In the large (N = 32,451 participants) phase III landmark clinical trial of ChAdOx1 nCoV-19, study participants were randomized in a 2:1 ratio to receive two doses of AZD1222 vaccine or placebo (administered 4 weeks apart on days 1 and 29). The primary endpoint was prevention of the onset of symptomatic or severe COVID-19 from ≥15 days after the second dose; no severe or critical symptomatic COVID-19 cases were observed among the participants in the AZD1222 group, whereas 8 cases were noted among the 8,550 participants in the placebo group (<0.1%). The rate of nucleocapsid-specific antibody seroconversion, as measured by the validated Roche Elecsys® Anti-SARS-CoV-2 nucleocapsid serology test at ≥15 days after the second dose of AZD1222 was 64.3%. However, cancer patients comprise approximately 6.5% of the total population, and there were no provided efficacy data for the AZD1222 vaccine in cancer patients owing to an insufficient number of cancer patients in the study.Citation15 Notably, our study had a different vaccine dosing schedule, with a longer interval between the first and second dose to achieve greater vaccine efficacy. A pooled analysis of four randomized trials demonstrated that AZD1222 vaccine efficacy was higher in individuals vaccinated with a longer prime-boost interval (vaccine efficacy: 81.3% [95%CI: 60.3%–91.2%] at ≥12 weeks) than in those vaccinated with a short prime-boost interval (vaccine efficacy: 55.1% [33.0%–69.9%] at <6 weeks).Citation16
There is concern regarding the immune response to the AZD1222 vaccine in cancer patients because of their impaired immune function such that antibodies may be produced insufficiently.Citation4 This study demonstrated that although cancer patients had a poor antibody response, in terms of anti-S IgG seroconversion and titers, after one dose of AZD1222, they had a sufficient antibody response after receiving a second dose of vaccine. Many studies evaluating the immune response of COVID-19 vaccines in cancer patients found that the tested vaccine worked sufficiently in this immunocompromised group. A prospective observational study in Italy conducted on 293 solid cancer patients evaluated the anti-S IgG antibody seropositivity rate and safety of a two-dose regimen of mRNA-BNT162b2 or mRNA-1273 vaccine relative to those in healthy volunteers. SARS-CoV-2-specific antibody was analyzed by the LIAISON XL automated platform. Most of the enrolled patients were undergoing active anticancer treatment (85%). The study found a seropositivity rate after complete course of vaccination of 75.88% for the cancer patient group, as compared with one of 100% for the control group. Moreover, the median anti-S IgG titer was significantly lower in the cancer patient group than in the control group. The seroconversion rates and antibody titers after first-dose vaccination were significantly lower than those after second-dose vaccination.Citation17 Our data proves the humoral immune response in patients with cancer. The immune system is partially suppressed from cancer and their treatments, however humoral immune response is still function, thus the immune response can still be elicited especially after the second dose of vaccination.In our study, the anti-S IgG antibody titers after first-dose vaccination were significantly higher in the female participants compared with those in the male participants, which is consistent with the previous reports.Citation17–19 There are well-established explanations for female individuals having a significant immunological advantage over male individuals. One interesting hypothesis suggests that the X chromosome contains a high density of immune-related genes and regulatory elements that are extensively involved in both the innate and adaptive immune responses.Citation20 Another study proposed that possible mechanisms for this difference include differences in human ACE2 (hACE2) receptor expression and behavioral differences, such as smoking or the prevalence of comorbidities that consequently influence immunity.Citation21
The subgroup of patients treated with chemotherapy had considerably lower anti-S IgG antibody titers than did the subgroup of patients receiving hormonal treatment. These data are consistent with previous reports.Citation18,Citation19 Because chemotherapy interferes with DNA synthesis and cell proliferation, the activation of lymphocytes by vaccination is suppressed by this therapy.Citation22 However, such suppression must be incomplete because an immune response to vaccination can still be generated. Another finding of our study is that taxane- and 5FU-based chemotherapy influenced the immune response to vaccination more than did other chemotherapy regimens. Taxane inhibits T-cell and natural killer-cell proliferation and activation, mainly by stabilizing GDP-bound tubulin in microtubules, thereby stopping cell division and migration.Citation23 5FU is an antimetabolite that inhibits RNA and DNA synthesis. Possible explanations would be that 5FU-containing regimen was generally for first and also subsequent line of treatment, e.g., FOLFOX and FOLFIRI in patients with colon cancer. However, carboplatin containing regimen was less likely used as subsequent or rechallenging treatment. Previous chemotherapy regimens may influence more immune suppression in 5FU-based chemotherapy. Moreover, data from breast cancer patients showed taxane-based chemotherapy lower levels of all the measured lymphocytes especially memory B-cell. Surprisingly, lymphocyte recovery in taxane-based chemotherapy was significantly slower than taxane-free chemotherapy.Citation24
Moreover, the anti-S IgG antibody titer was lower in the subgroup of patients who received targeted therapy than in the subgroup of those who underwent hormonal treatment.Citation25 There are also data demonstrating less durable responses for patients receiving targeted therapy or chemotherapy.Citation25,Citation26 In 2021, Chumsri et al. studying humoral responses after SARS-CoV-2 mRNA vaccination in cancer patients also found that the CDK4/6 inhibitor may cause an impaired antibody-mediated response to SARS-CoV-2 mRNA vaccines.Citation26
Regarding side effects of vaccination with AZD1222, cancer patients had relatively higher incidences of fatigue, diarrhea, and injection site reaction after first-dose vaccination. The reason for these side effects may be the cancer itself or the systemic anticancer treatment that the patients received concurrently with vaccination.Citation19,Citation27 However, after the second-dose vaccination, the cancer patient group had a lower incidence of all reported side effects, except for myalgia, compared with the healthy control group. The safety profile of the vaccine was consistent with previous studies.Citation19,Citation27
The biggest strength of our study is its age-matching and prospective design, which minimizes the interference of age and sex confounders on the results. However, our study has some limitations. First, it has insufficient power to differentiate vaccine immunogenicity within specific patient subgroups (e.g., those with different cancer types and distinct treatment modalities, which could have differential affects on host immune responsiveness). Second, at the time the study was conducted, the predominant SARS-CoV-2 strain was Delta, but the Omicron strain is now predominant. Third, we assessed only anti-S IgG antibody levels and did not evaluate neutralizing antibody levels.Citation28 However, there is evidence that anti-S antibody levels correlate with neutralizing antibody levels.Citation28
Conclusion
Our study shows the seropositivity and safety of AZD1222 in solid cancer patients. The anti-S IgG seropositivity rate after completing the second dose of AZD1222 was not different between the cancer patient group and the control group. In contrast, after the first-dose vaccination, the anti-S IgG seropositivity rate was lower in cancer patients, suggesting that antibody responses to AZD1222 in cancer patients might be delayed such that they strongly benefit from completing the second dose of COVID-19 vaccination. The side effects of AZD1222 are tolerable. Our findings suggest that the benefits of vaccination with AZD1222 outweigh the risks of side effects in cancer patients. Further studies with larger sample sizes are needed to determine the efficacy of COVID-19 vaccines in cancer patients.
Author’s contributions
Conceptualization, P.Y. (Piyarat Limpawittayakul); investigation, P.L., P.S., W.C., A.S., B.W., J.S., C.P., C.S., W.L., T.U., W.T.; methodology, K.T.; supervision, K.T. All authors have read and agreed to the published version of the manuscript.
Ethics
The study was conducted in accordance with the principles laid out in the Declaration of Helsinki guidelines for research involving human subjects. The study protocol was reviewed and approved by the Ethics Committee for Human Research, Chulabhorn Research Institute (Certificate No. 056/2564).
Acknowledgments
We thank the clinical research management unit, especially Kamonwan Soonklang and Kerati Jirawattanapalin, for managing this project. We also thank Katie Oakley, PhD, from Edanz (www.edanz.com/ac) for editing a draft of this manuscript. Importantly, we thank all patients and health-care workers for being participants in this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO Coronavirus (COVID-19) Dashboard: 263,563,622 confirmed cases and 5,232,562 deaths from COVID-19 pandemic [Internet]. [accessed 2022 Apr 8]. https://covid19.who.int/.
- Pathania AS, Prathipati P, Abdul BA, Chava S, Katta SS, Gupta SC, Gangula PR, Pandey MK, Durden DL, Byrareddy SN et al. COVID-19 and cancer comorbidity: therapeutic opportunities and challenges. Theranostics. 2021;11(2):1–8. PMID: 33391502; PMCID: PMC7738845. doi:10.7150/thno.51471.
- Yang L, Chai P, Yu J, Fan X. Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants. Cancer Biol Med. 2021;18(1):298–307. PMID: 33628602; PMCID: PMC7877167. doi:10.20892/j.issn.2095-3941.2020.0559.
- He Y, Ding Y, Cao B, Huang Y, Wang X. COVID-19 vaccine development from the perspective of cancer patients. Hum Vaccin Immunother. 2021;17(10):3281–3287. Epub 2021 Jun 25. PMID: 34170788; PMCID: PMC8437497. doi:10.1080/21645515.2021.1943988.
- Rajan S, Akhtar N, Sharma S, Chakrabarti D, Kumar V. COVID-19 vaccination for cancer patients: evidence, priority, and practice. Vaccine. 2021;39(36):5075–5077. Epub 2021 Jul 24. PMID: 34340859; PMCID: PMC8302852. doi:10.1016/j.vaccine.2021.07.063.
- Nordøy T, Aaberge IS, Husebekk A, Samdal HH, Steinert S, Melby H, Kolstad A. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol. 2002;19:71–78. PMID: 12180483. doi:10.1385/MO:19:2:71.
- Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. Epub 2021 Apr 27. PMID: 33930323; PMCID: PMC8078907. doi:10.1016/S1470-2045(21)00213-8.
- Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, Benouaich-Amiel A, Ben-Zvi H, Moskovits N, Brenner B, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–1140. PMID: 34047765; PMCID: PMC8164144. doi:10.1001/jamaoncol.2021.2155.
- Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. Epub 2020 Dec 8. PMID: 33306990; PMCID: PMC7832220. doi:10.1016/S0140-6736(20)32623-4.
- Tran S, Truong TH, Narendran A. Evaluation of COVID-19 vaccine response in patients with cancer: an interim analysis. Eur J Cancer. 2021;159:259–274. Epub 2021 Oct 25. PMID: 34798454; PMCID: PMC8542448. doi:10.1016/j.ejca.2021.10.013.
- Heudel P, Favier B, Assaad S, Zrounba P, Blay J-Y. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann Oncol. 2021:1e2. Epub 2021 Jul 30. PMID: 34333128; PMCID: PMC8321960. doi:10.1016/j.annonc.2021.07.012.
- Riester E, Findeisen P, Hegel JK, Kabesch M, Ambrosch A, Rank CM, Pessl F, Laengin T, Niederhauser C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J Virol Methods. 2021;297:114271. Epub 2021 Jul 30. PMID: 34333128; PMCID: PMC8321960. doi:10.1016/j.annonc.2021.07.012.
- Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, Plotkin S, Knezevic I. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. Epub 2021 Mar 23. PMID: 33770519; PMCID: PMC7987302. doi:10.1016/S0140-6736(21)00527-4.
- Resman Rus K, Korva M, Knap N, Avšič Županc T, Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. Epub 2021 Apr 10. PMID: 33865031; PMCID: PMC8035809. doi:10.1016/j.jcv.2021.104820.
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. AstraZeneca AZD1222 clinical study group. Phase 3 safety and efficacy of AZD1222 (ChAdox1 nCov-19) COVID-19 vaccine. N Engl J Med. 2021;385(25):2348–2360. Epub 2021 Sep 29. PMID: 34587382; PMCID: PMC8522798. doi:10.1056/NEJMoa2105290.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-Dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdox1 nCov-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. Epub 2021 Feb 19. Erratum in: Lancet. 2021 Mar 6;397(10277):880. PMID: 33617777; PMCID: PMC7894131. doi:10.1016/S0140-6736(21)00432-3.
- Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, Cremona G, Muroni M, Bernuzzi P, Lo Cascio G, et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: seropositivity and safety. A prospective observational study in Italy. Eur J Cancer. 2021;157:441–449. Epub 2021 Sep 2. PMID: 34601285; PMCID: PMC8410513. doi:10.1016/j.ejca.2021.08.035.
- Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, Kaklamani V, Dietrich P-Y, Taylor BS, Simand P-F, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091–1098.e2. Epub 2021 Jun 18. PMID: 34214473; PMCID: PMC8218532. doi:10.1016/j.ccell.2021.06.009.
- Linardou H, Spanakis N, Koliou GA, Christopoulou A, Karageorgopoulou S, Alevra N, Vagionas A, Tsoukalas N, Sgourou S, Fountzilas E, et al. Responses to SARS-CoV-2 vaccination in patients with cancer (ReCover Study): a prospective cohort study of the Hellenic Cooperative Oncology Group. Cancers (Basel). 2021;13(18):4621. PMID: 34572848; PMCID: PMC8466969. doi:10.3390/cancers13184621.
- Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13(1):2. PMID: 30621780; PMCID: PMC6325731. doi:10.1186/s40246-018-0185-z.
- Ahmed SB, Dumanski SM. Sex, gender and COVID-19: a call to action. Can J Public Health. 2020;111(6):980–983. Epub 2020 Sep 29. PMID: 32990927; PMCID: PMC7523484. doi:10.17269/s41997-020-00417-z.
- Hwang JK, Zhang T, Wang AZ, Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14(1):38. PMID: 33640005; PMCID: PMC7910769. doi:10.1186/s13045-021-01046-w.
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. PMID: 18097448. doi:10.1038/nri2216.
- Verma R, Foster RE, Horgan K, Mounsey K, Nixon H, Smalle N, Hughes TA, Carter CR. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016 Jan 26;18(1):10. PMID: 26810608; PMCID: PMC4727393. doi:10.1186/s13058-015-0669-x.
- Jackson-Spence F, Toms C, Yang Y-H, Walshaw L, Riddell A, Cutino-Moguel M-T. The effect of anti-cancer therapy on immunologic response to COVID-19 vaccination. Presented at ASCO GU 2022. 2022 Feb 17–19. Abstract 319.
- Chumsri S, Advani PP, Pai TS, Li Z, Mummareddy A, Acampora M, Reynolds GA, Wylie N, Boyle AW, Lou Y, et al. Humoral responses after SARS-CoV-2 mRNA vaccination and breakthrough infection in cancer patients. Mayo Clin Proc Innov Qual Outcomes. 2022;6(2):120–125. Epub 2021 Dec 13. PMID: 34926993; PMCID: PMC8666324. doi:10.1016/j.mayocpiqo.2021.12.004.
- Yasin AI, Aydin SG, Sümbül B, Koral L, Şimşek M, Geredeli Ç, Öztürk A, Perkin P, Demirtaş D, Erdemoglu E, et al. Efficacy and safety profile of COVID-19 vaccine in cancer patients: a prospective, multicenter cohort study. Future Oncol. 2022;18(10):1235–1244. Epub 2022 Jan 27. PMID: 35081732; PMCID: PMC8793921. doi:10.2217/fon-2021-1248.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. Epub 2021 May 17. PMID: 34002089. doi:10.1038/s41591-021-01377-8.