ABSTRACT
We analyzed serially collected serum samples from healthy adults who underwent BNT162b2 vaccination to elucidate the association between spike (S)-IgG antibody titers determined by ELISA using the WHO international standard (NIBSC code 20/136) and neutralizing antibody titers against three live SARS-CoV-2 variants. This study included 53 health care workers who received two doses of the BNT162b2 vaccine. S-IgG and nucleocapsid (N)-IgG antibody titers were measured by ELISA. Neutralizing (NT) antibody responses against three variants (Wuhan D614 G: KUH003, Alpha, and Delta) were evaluated before and after the first and second vaccination. N-IgG were not detected in any serum samples. S-IgG antibody titers remarkably increased after two BNT162b2 vaccine doses in all participants. S-IgG antibody titers were strongly correlated with NT titers against three variants of live viruses: KUH003 (r = 0.86), Alpha (r = 0.72), and Delta (r = 0.84). Serum samples from participants after one dose of BNT162b2 neutralized Alpha efficiently (median titer, 113.0), but median NT titers against KUH003 and Delta variants were lower, 57.0 and 28.0, respectively (p < .01). Two doses of the BNT162b2 vaccine elicited a strong immune response in this study. The second dose was required for induction of a strong booster effect. Serum collected from BNT162b2 vaccine recipients contained significantly lower neutralizing activity against Delta than that of against KUH003 (p < .0001) and Alpha (p < .0001). If a new variant emerges, live virus-based NT titers should be examined in serum obtained from vaccine recipients to evaluate vaccine efficacy for protection against infection.
Introduction
The new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), which is associated with a devastating disease burden worldwide. SARS-CoV-2 is an enveloped virus with four structural proteins: spike (S) protein, membrane (M) protein, envelope (E) protein, and nucleocapsid (N) protein.Citation1,Citation2 Since S protein is responsible for viral attachment and entry into target cells, this protein plays an important role in the induction of protective humoral and cellular immunity against SARS-CoV-2. COVID-19 mRNA vaccines contain mRNA encoding of the S protein. They can induce strong immune response against it.Citation3–6 The vaccines are highly effective in preventing disease onset as well as viral infection. They are well tolerated despite adverse reaction a few days after vaccination.Citation6–9
Pseudoviruses are useful virological tools because of their safety and versatility, especially for the examination of highly pathogenic emerging and reemerging viruses. Since SARS-CoV-2 is highly pathogenic and infectious, live SARS-CoV-2 has to be handled under biosafety level 3 conditions, which has hindered the development of vaccines and therapeutics. Measurement of humoral immune responses against SARS-CoV-2 is necessary for evaluating the immunogenicity of COVID-19 vaccines. Enzyme-linked immunosorbent assays (ELISAs) for the detection of anti-S IgG antibodies are widely used and reliable methods for evaluating COVID-19 vaccines because they are safe and easy for analyzing a large number of samples.Citation10–12 Neutralizing tests are generally more specific and more accurately reflect the inhibitory effect of viral replication than ELISAs. However, since neutralizing tests with infectious virus are difficult to use widely for serological analysis of SARS-CoV-2, pseudovirus-based neutralization assays, which are much safer than live virus-based neutralizing assays, have been developed as an alternative to serological analysis.Citation13
Pseudovirus-based neutralization assays are convenient for laboratory use. However, a pseudovirus is a recombinant virus particle whose core skeleton and surface proteins derive from a variety of viruses. Therefore, it reflects only the viral attachment and entry steps of viral infection controlled by the S protein. By contrast, live virus-based neutralizing assays can assess the entire inhibition process, from the early stages of viral infection to entry into cells and initiation of replication. In other words, a live virus-based neutralizing assay is thought to reflect the actual inhibition of viral infection more accurately, albeit at the cellular level. Therefore, we sought to elucidate the association between anti-S IgG antibody titers determined by an internationally standardized ELISA and neutralizing antibody titers against three live SARS-CoV-2 variants in healthy adult recipients of the BNT162b2 COVID-19 vaccine.
Methods
Study design
This study was a prospective cohort study of health care professionals working at Fujita Health University. All participants, aged ≥18 years, provided written informed consent before undergoing any study procedures. This study was approved by the Ethics Committee for Clinical Research of the Center for Research Promotion and Support at Fujita Health University (authorization number, HM20–089). All participants had received two doses of the BNT162b2 mRNA COVID-19 vaccine (0.3 mL volume per dose) 21 days apart.
Participants and sample collection
Between 10 March 2021 and 20 May 2021, 53 participants were vaccinated with two doses of the BNT162b2 vaccine. None of the participants had a history of SARS-CoV-2 infection. We did not monitor SARS-CoV-2 infection in these subjects. Blood samples were collected from all participants twice, and three times from 52 participants: (i) before the first vaccination (pre), (ii) before the second vaccination (post 1), and (iii) 1 month after the second vaccination (post 2). Serum was obtained by centrifugation for 15 minutes at 1,500 g at room temperature, aliquoted, and stored at −80°C until use.
ELISA for measuring SARS CoV-2-IgG titers
IgG antibodies against the SARS-CoV-2 S and N proteins were separately measured using a prototype indirect enzyme immunoassay (#DK20-COV4E-S and #DK20-COV4E-N) provided by Denka (Tokyo, Japan). Each well of a 96-well microplate was coated with recombinant SARS-CoV-2 S or N proteins. Serum specimens diluted at a 1:200 ratio with dilution buffer were added to each well. After incubating at room temperature for 1 hour, the wells were washed three times with washing buffer. Horseradish peroxidase-conjugated goat anti-human IgG (H + L) antibodies for N-IgG assay and Horseradish peroxidase-conjugated goat anti-human IgG (Heavy Chain) antibodies for S-IgG assay were added to each well, and the plate was incubated at room temperature for 1 hour. After five washes, the substrate was added to each well, and the plate was incubated at room temperature. Reactions were stopped by the addition of reaction stopper. Finally, optical density (OD)450 and OD630 were measured with a Sunrise™ plate reader (Tecan, Zurich, Switzerland). Antibody titers were calculated in units of binding antibody unit (BAU)/mL with calibrators assigned to the first World Health Organization international standard for anti-SARS-CoV-2 immunoglobulin (National Institute for Biological Standards and Control (NIBSC) code 20/136).Citation14,Citation15 Samples with high titers (>1,000 BAU/mL) were diluted at 1:3,200 and ELISA was performed to calculate the antibody titer. ELISA values were interpreted as follows. For N-IgG, <15 BAU/mL was considered negative, 15–30 BAU/mL was considered indeterminate, and >30 BAU/mL was considered positive. For S-IgG, <45 BAU/mL was considered negative, 45–90 BAU/mL was considered indeterminate, and >90 BAU/mL was considered positive.
SARS-CoV-2 neutralizing test
Heat-inactivated serum was serially diluted with Dulbecco’s Modified Eagle Medium (NACALAI TESQUE, Kyoto, Japan) supplemented with 2% fetal bovine serum (biosera, Nuaillé, France), penicillin (100 units/mL), and streptomycin (100 µg/mL) (Thermo Fisher Scientific, Waltham, MA, USA). Diluted serum samples were mixed with 100 tissue culture infectious dose 50 (TCID50) of a SARS-CoV-2 variant and incubated at 37°C for 1 hour. The mixtures were placed on VeroE6/TMRRSS2 cells (JCRB1819; JCRB Cell Bank, Osaka, Japan) and cultured for 5 days at 37°C with 5% CO2. For the evaluation of cytopathic effect (CPE), plates were fixed with methanol (NACALAI TESQUE, Kyoto, Japan) and stained with methylene blue solution (Polysciences, Warrington, PA, USA). The highest serum dilution factor with 100% CPE inhibition was defined as the authentic virus neutralization titer. The biosafety level (BSL) 3 facility is in the Virus Infection Control of Ōmura Satoshi Memorial Institute, Kitasato University. This BSL3 is an experimental facility approved by the University Biosafety Committee and the Ministry of Education, Culture, Sports, Science, and Technology.
Three SARS-CoV-2 variants were used in neutralizing assays. The KUH003 strain (DNA Data Bank of Japan (DDBJ) accession number: LC630936) was used as the Wuhan type standard strain with having D614 G amino acid change in spike protein.Citation16 The QK002 strain (Pango type, B.1.1.7; hCoV-19/Japan/QK002/2020; Global Initiative on Sharing Avian Influenza Data (GISAID) accession ID: EPI_ISL_768526) was used as the Alpha variant. The TY11–927 strain (Pango type, B.1.617.2; hCoV-19/Japan/TY11-927/2021; GISAID accession ID: EPI_ISL_2158617) was used as the Delta variant. The QK002 and TY11–927 strains were kindly provided by the National Institute of Infectious Diseases. A neutralizing antibody titer <5 was defined as titer 5. A titer >160 was defined as titer 320.
Data analysis
All statistical analyses were conducted with the use of JMP software, version 15.2.0 (SAS Institute, Cary, NC, USA) and GraphPad Prism 9 for Windows (GraphPad Software, San Diego, CA, USA). Statistical differences were evaluated using a Wilcoxon paired signed-rank test. Box-and-whisker plots were drawn with GraphPad Prism 9 for Windows. The whiskers extended to 1.5 times the interquartile range (IQR). Correlations between S-IgG and neutralizing antibody titers against three variants of live viruses were evaluated using Spearman’s correlation analysis. The Kruskal–Wallis tests and the Dunn’s multiple comparison test were used to determine the differences in S-IgG titers by age group with GraphPad Prism 9 for Windows (GraphPad Software, San Diego, CA, USA); p < .05 was considered to be statistically significant. Wilcoxon signed-rank and Mann–Whitney tests (based on the non-normal distribution of the data of several groups) were applied to analyze changes in paired and unpaired data, respectively.
Results
Demographic characteristics of the participants
Among the 53 participants, serum was not collected at post 2 from 1 participant. The study participants were health care workers with a mean age of 38.9 years (range, 22–63 years); 43 (81.1%) were female. Post 1 sampling occurred 15.1 ± 1.7 days after the first vaccination and post 2 sampling occurred 14.3 ± 1.6 days after the second vaccination.
Measuring IgG antibodies with ELISA
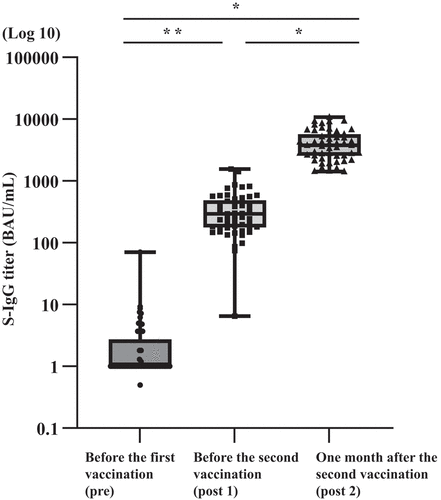
N-IgG and S-IgG antibody titers were measured by ELISA. Before the first vaccination, no participants had detectable N-IgG antibodies. N-IgG antibody titers were below 30 BAU/mL at post 1 and post 2. Serum S-IgG antibody titers in all participants ranged 0–70.1 BAU/mL, with a median of 3.7 BAU/mL before the first vaccination (pre). Post 1 S-IgG antibody titers ranged 6.5–1,566.4 BAU/mL, with a median of 294.9 BAU/mL. Post 2 S-IgG antibody titers ranged 1,412.8–1,0728.0 BAU/mL, with a median of 3,792.0 BAU/mL (). There was a statistically significant increase in S-IgG antibody titers in post 1 (p = .0145), and post 2 (p < .0001) serum samples. In addition, a significant booster effect was observed after the second vaccine dose (p < .0001).
Figure 1. Results of SARS-CoV-2 S-IgG antibody titers in BNT162b2 vaccine recipients.
Neutralizing antibody titers against three variants of live viruses
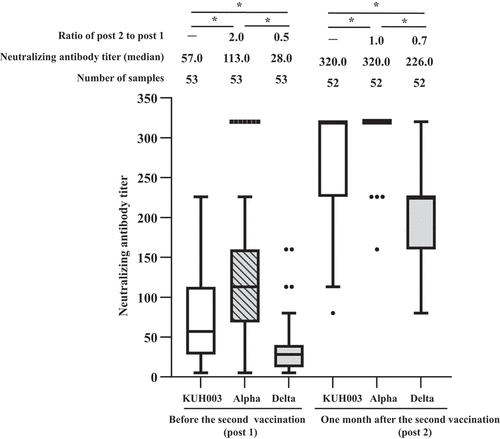
Next, we measured neutralizing antibody titers against KHU003, Alpha, and Delta. As shown in , S-IgG titers were strongly correlated with neutralizing antibody titers against KUH003 (Spearman’s correlation coefficient, r = 0.86; p < .0001), Alpha (r = 0.72; p < .0001), and Delta (r = 0.84, p < .0001). The dynamics of neutralizing antibody titers in serially collected serum samples before and after two doses of the BNT162b2 vaccine are shown in . Neutralizing antibody titer against KUH003 in females (n = 43, median = 80) was significantly higher than in males (n = 10, median = 28) (p < .05). Meanwhile, there were no significant differences between males and females for neutralizing antibodies against Alpha, and Delta, S-IgG, and N-IgG antibody. Neutralizing antibody titers against Delta was significantly lower than titers against KUH003 (p < .0001) and Alpha (p < .0001) before the second vaccination (post 1). After the second vaccine dose (post 2), neutralizing antibody titers against Alpha for post 2, many samples (48/52) scale out at 320, the upper limit of measurement, and the median value, upper quartile, and upper whisker are 320 in the box-and-whisker diagram. The N501Y mutation, common to the alpha, beta, gamma, and Omicron BA.1 variants, but absent in delta, increases angiotensin-converting enzyme 2 (ACE2) affinity. The K417N/T mutation, which is present in beta, gamma, and Omicron BA.1, decreases ACE2 affinity.Citation17 Thus, neutralizing antibody titers against Alpha were the highest. Neutralizing antibody titers against Delta were significantly lower than titers against KUH003 (p < .0001) and Alpha (p < .0001), respectively.
Figure 2. Correlation between spike glycoprotein IgG titers and neutralizing antibody titers against KUH003 (a), Alpha (b), and Delta (c).
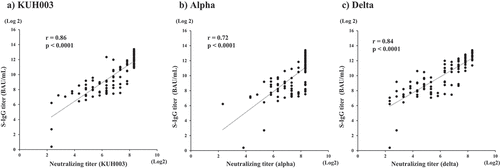
Figure 3. Antibody responses elicited by the BNT162b2 vaccine. Neutralizing antibodies titers against KUH003, Alpha, and Delta were examined in participants vaccinated with one or two doses of BNT162b2.
Relationship between SARS-CoV-2 antibody titers and age in vaccinated individuals
Correlations between S-IgG antibody titers or neutralizing antibody titers and age are shown in . Negative correlations were demonstrated between age and S-IgG titers (r = −0.311) and neutralizing antibody titers against KUH003 (r = −0.332) () in post 1 serum samples. By contrast, neutralizing antibody titers against Alpha and Delta in post 1 serum samples were not negatively correlated with age. In post 2 serum samples, the percentage of participants with neutralizing antibody titer 320, including those above the highest serum dilution, was 92.3% (48/52) for Alpha, 69.2% (36/52) for KUH003, and 23.1% (12/52) for Delta. Therefore, no statistical correlations between age and antibody titers were observed in post 2 serum samples. In post 1 serum samples, the median S-IgG antibody titer in participants aged 20–29 years (n = 14), 30–39 years (n = 13), 40–49 years (n = 16), and ≥50 years (n = 10) was 547.3 BAU/mL, 252.7 BAU/mL, 234.7 BAU/mL, and 232.0 BAU/mL, respectively (). We assessed differences in antibody levels between time periods using the Kruskal‐Wallis tests (p < .05). S-IgG antibody titers were lower in participants aged ≥50 years versus participants aged 20–29 years using Dunn’s multiple comparison test (p < .05).
Figure 4. Correlation between age and SARS-CoV-2 S-IgG antibody titers (a), neutralizing antibody titers against KHU003 (b), Alpha (c), and Delta (d).
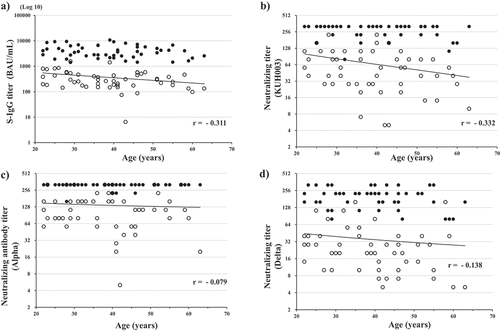
Table 1. The S-IgG titer and the neutralizing antibody titer of KUH003, alpha variant, and delta variant after BNT162b2 vaccination.
Discussion
Measuring antibody titers provides important information on vaccine immunogenicity and SARS-CoV-2 seroepidemiology. ELISA-based S-IgG antibody assays are useful for testing large numbers of serum samples. In fact, various types of assays are widely used in the clinical setting. However, standardization of many ELISA-based S-IgG antibody assays is crucial for an accurate understanding of vaccine immunogenicity and seroepidemiology. Therefore, the World Health Organization created an international standard for measuring SARS-CoV-2 antibody titers in December 2020,Citation14,Citation15 which facilitates the comparison and harmonization of datasets across laboratories. The SARS-CoV-2 S-IgG and N-IgG ELISA kits used in this study meet the international standard. They are extremely reliable for clinical use and can be compared with overseas data.
Since N-IgG antibodies were not detected in any participants before vaccination, they were considered naïve to SARS-CoV-2. A marked increase in SARS-CoV-2 S-IgG antibody titers after two doses of the BNT162b2 vaccine was demonstrated in all 53 healthy adults (). As shown in , regarding antibody titers in all serum samples, S-IgG antibody and neutralizing antibody titers against three variants of live viruses were all strongly correlated. However, the correlation between S-IgG and live virus-based neutralizing antibody titers was slightly different in the neutralization tests for KUH003 (r = 0.86), Delta (r = 0.84), and Alpha (r = 0.72). Since neutralizing antibody titers against Alpha exceeded the maximum dilution in many post 2 serum samples, the strength of the correlation between S-IgG and neutralizing antibody titers might be slightly lower for Alpha. Most patients in the present study were females (43/53, 81.1%). The previous study Citation18 using a cohort of 248 health care workers reported that gender was statistically associated with difference in antibody response after vaccination. It is consistent with our results, the neutralization antibody titer against KUH003 before the second vaccination (post 1) of females is significantly higher than of males (p < .05). Assays for measuring IgG antibodies against the S protein based on ELISA are suitable for testing a large number of samples, but these methods only reflect the binding capacity of IgG antibodies to an epitope on the S protein, which does not reflect the true level of protection against viral infection. In addition, pseudovirus-based neutralizing tests are widely used due to their high biosafety levels,Citation13–Citation19–23 but they also do not entirely reflect total protection against viral infection. Thus, although live virus-based neutralizing tests are troublesome for clinical use due to biohazard limitations and the time involved, they are important because they offer a more accurate assessment of protection against infection. In addition, as shown in this study, it is important to evaluate the reliability of ELISA-based S-IgG antibody assays based on comparisons with neutralization tests.
As already demonstrated in a previous phase 2/3 study,Citation4,Citation24 two doses of the BNT162b2 vaccine elicited a strong immune response in this study (). The second vaccine dose was required for induction of a strong booster effect. Live virus-based neutralizing antibody titers after two vaccine doses were statistically different among the three variants (), with Delta having the lowest antibody titers. This finding is consistent with previous studies showing that serum collected from BNT162b2 vaccine recipients has lower neutralizing activity against Delta.Citation25,Citation26 New variants that have mutations mostly in the gene encoding the S protein will continue to emerge. These new variants are highly infectious and can escape vaccine-induced host immunity.Citation27,Citation28 In fact, neutralizing antibody titers induced by the BNT162b2 vaccine have been demonstrated to be significantly lower against the latest SARS-CoV-2 Omicron variant.Citation29 Therefore, if a new variant emerges, live virus-based neutralizing antibody titers should be examined in serum obtained from vaccine recipients to evaluate vaccine efficacy for protection against infection with the new variant.
A negative correlation between antibody responses and age of the vaccine recipient was demonstrated for anti-S antibody titers and neutralizing antibody titers against KUH003 after one vaccine dose. This finding is consistent with previous studies showing that age is a major determinant of the immune response elicited by COVID-19 vaccines.Citation30 However, no age-dependent reduction in immune response was observed in serum samples obtained after two vaccine doses because antibody titers in many samples exceeded the maximum dilution.
In conclusion, S-IgG antibody titers based on ELSA were strongly correlated with neutralizing antibody titers against three different variants. However, the correlations between S-IgG antibody titers and neutralizing antibody titers against three different variants were slightly different. Neutralization of each variant was demonstrated by different S-IgG antibody titers determined by an internationally standardized ELISA. As previously reported,Citation25,Citation29,Citation31 the humoral immune responses elicited by the BNT162b2 vaccine were weaker against the Delta than against two other variants. This ELISA for measuring S-IgG antibody titers is reliable for clinical use, but it should be reassessed when a new variant emerges. The present study was not designed for evaluation of immune response induced by the third dose of vaccination. We would like to try it in future.
Author contribution
YH contributed to study conceptualization, data curation, formal analysis, methodology, investigation, writing the original draft, and editing of the manuscript. KK, HM, and YK contributed resources and investigation. HH and RS contributed to data curation. KH, RT, AS, and KK contributed to data curation, formal analysis, investigation, methodology, and visualization. TY contributed to study conceptualization, funding acquisition, project administration, providing resources, supervision, visualization, writing, and manuscript editing. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Acknowledgements
SARS-CoV-2 N-IgG and S-IgG antibody titers were measured by Denka at Gosen site (Niigata, Japan) using an originally developed ELISA kit.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siu YL, Teoh KT, Lo J, Chan CM, Kien F, Escriou N, Tsao SW, Nicholls JM, Altmeyer R, Peiris JSM, et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol. 2008;82(22):1–7. doi:10.1128/JVI.01052-08.
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen K-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi:10.1080/22221751.2020.1719902.
- Walsh EE, Frenck RWsJr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Frenck RWsJr., Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi:10.1056/NEJMoa2107456.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi:10.1038/s41586-020-2639-4.
- Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi:10.1016/S0140-6736(21)00790-X.
- Chen G, Li X, Sun M, Zhou Y, Yin M, Zhao B, Li X. COVID-19 mRNA vaccines are generally safe in the short term: a vaccine vigilance real-world study says. Front Immunol. 2021;12:669010. doi:10.3389/fimmu.2021.669010.
- Blumenthal KG, Robinson LB, Camargo CAsJr., Shenoy ES, Banerji A, Landman AB, Wickner P. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi:10.1001/jama.2021.3976.
- Krüttgen A, Lauen M, Klingel H, Imöhl M, Kleines M. Two novel SARS-CoV-2 surrogate virus neutralization assays are suitable for assessing successful immunization with mRNA-1273. J Virol Methods. 2022;299:114297. doi:10.1016/j.jviromet.2021.114297.
- Azak E, Karadenizli A, Uzuner H, Karakaya N, Canturk NZ, Hulagu S. Comparison of an inactivated Covid19 vaccine-induced antibody response with concurrent natural Covid19 infection. Int J Infect Dis. 2021;113:58–64. doi:10.1016/j.ijid.2021.09.060.
- Van Elslande J, Decru B, Jonckheere S, Van Wijngaerden E, Houben E, Vandecandelaere P, Indevuyst C, Depypere M, Desmet S, André E, et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect. 2020;26(11):1557.e1–1557.e7. doi:10.1016/j.cmi.2020.07.038.
- Bayart JL, Douxfils J, Gillot C, David C, Mullier F, Elsen M, et al. Waning of IgG, total and neutralizing antibodies 6 months post-vaccination with BNT162b2 in healthcare workers. Vaccines (Basel). 2021;9.
- Mattiuzzo G, Bentley EM, Hassall M, Routley S, Richardson S, Bernasconi V, et al. Establishment of the WHO international standard and reference panel for anti-SARS-CoV-2antibody. WHO/BS.2020.2403: World Health Organization; 2020.
- Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, Plotkin S, Knezevic I. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi:10.1016/S0140-6736(21)00527-4.
- Ebisudani T, Sugimoto S, Haga K, Mitsuishi A, Takai-Todaka R, Fujii M, Toshimitsu K, Hamamoto J, Sugihara K, Hishida T, et al. Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening. Cell Rep. 2021;35(10):109218. doi:10.1016/j.celrep.2021.109218.
- Cai Y, Zhang J, Xiao T, Lavine CL, Rawson S, Peng H, Zhu H, Anand K, Tong P, Gautam A, et al. Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants. Science. 2021;373(6555):642–648. doi:10.1126/science.abi9745.
- Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, Conti L, De Virgilio A, De Marco F, Di Domenico EG, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. E Clin Med. 2021;36:100928. doi:10.1016/j.eclinm.2021.100928.
- Muik A, Wallisch AK, Sänger B, Swanson KA, Mühl J, Chen W, Cai H, Maurus D, Sarkar R, Türeci Ö, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371(6534):1152–1153. doi:10.1126/science.abg6105.
- Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, Kemp SA, Bassi J, Pinto D, Silacci-Fregni C, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857):136–141. doi:10.1038/s41586-021-03412-7.
- Kuzmina A, Khalaila Y, Voloshin O, Keren-Naus A, Boehm-Cohen L, Raviv Y, Shemer-Avni Y, Rosenberg E, Taube R. SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe. 2021;29(4):522–8.e2. doi:10.1016/j.chom.2021.03.008.
- Lucas C, Vogels CBF, Yildirim I, Rothman JE, Lu P, Monteiro V, Gehlhausen JR, Campbell M, Silva J, Tabachnikova A, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021;600(7889):523–529. doi:10.1038/s41586-021-04085-y.
- McDade TW, Demonbreun AR, Sancilio A, Mustanski B, D’Aquila RT, McNally EM. Durability of antibody response to vaccination and surrogate neutralization of emerging variants based on SARS-CoV-2 exposure history. Sci Rep. 2021;11(1):17325. doi:10.1038/s41598-021-96879-3.
- Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, Barnett ED, Muñoz FM, Maldonado Y, Pahud BA, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386(1):35–46. doi:10.1056/NEJMoa2116298.
- Davis C, Logan N, Tyson G, Orton R, Harvey WT, Perkins JS, Mollett G, Blacow RM, Peacock TP, Barclay WS, et al. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021;17(12):e1010022. doi:10.1371/journal.ppat.1010022.
- Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, Nunna N, Huang W, Oestreicher J, Colpitts T, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi:10.1038/s41591-021-01527-y.
- Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, Zhao C, Zhang Q, Liu H, Nie L, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182(5):1284–94.e9. doi:10.1016/j.cell.2020.07.012.
- Liu Z, VanBlargan LA, Bloyet LM, Rothlauf PW, Chen RE, Stumpf S, Zhao H, Errico JM, Theel ES, Liebeskind MJ, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29(3):477–88.e4. doi:10.1016/j.chom.2021.01.014.
- Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CBF, Malik AA, De la Cruz E, Jorge A, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481–485. doi:10.1038/s41591-022-01705-6.
- Collier DA, Ferreira I, Kotagiri P, Datir RP, Lim EY, Touizer E, Meng B, Abdullahi A, Baker S, Dougan G, et al. Age-Related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi:10.1038/s41586-021-03739-1.
- Kato H, Miyakawa K, Ohtake N, Go H, Yamaoka Y, Yajima S, Shimada T, Goto A, Nakajima H, Ryo A, et al. Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. J Infect Chemother. 2022;28(2):273–278. doi:10.1016/j.jiac.2021.11.021.
