ABSTRACT
In the global context of the COVID-19 pandemic, the overall benefits of getting any COVID-19 vaccine approved by the World Health Organization for emergency use outweigh the potential risks, even in people with weakened immune systems, including people living with HIV (PLWH). At present, there are no reports of HIV/hepatitis B virus (HBV) co-infected patients receiving a booster dose of the inactivated COVID-19 vaccine. Here, we describe a patient with HIV/HBV co-infection who did not seroconvert to three doses of the inactivated COVID-19 vaccine.
Introduction
PLWH are listed as a high-risk group experiencing more severe outcomes from COVID-19 by the Joint United Nations Program on HIV/AIDS (UNAIDS).Citation1 It is important for this vulnerable population to obtain optimal immunogenicity after vaccination. Due to different degrees of immunodeficiency, the antibody titers and seropositive rates of PLWH are lower than those of the healthy population after vaccination with the inactivated COVID-19 vaccine.Citation2–4 The immune response to the two-dose COVID-19 vaccine is less robust against SARS-CoV-2 variants of concern, and the antibodies induced by the vaccine decline more rapidly over time and provide a shorter duration of protection in immunocompromised populations, such as PLWH.Citation5,Citation6 Therefore, PLWH should be the priority group for vaccination with a booster dose of the inactivated COVID-19 vaccine.Citation3
Shared epidemiological risks result in PLWH having a high prevalence of HBV co-infection. Viral hepatitis caused by HBV infection is one of the most common causes of PLWH’s immune function impairment,Citation7 whereas HIV infection can lead to the depletion of CD4+T cell, which has a negative impact on all stages of the natural history of hepatitis B.Citation8 Compared to patients with HIV-mono-infection, those who are co-infected with HIV/HBV are associated with poorer antiretroviral therapy (ART) outcomes, and their immune function is more difficult to rebuild, especially with lower rate of CD4+T cell recovery.Citation9 Thus, patients with HIV/HBV co-infection generally have lower CD4+T cell counts despite receiving ART.Citation8,Citation10,Citation11 However, the effective response to the vaccine requires CD4+T cell coordination,Citation12 which may results in HIV/HBV co-infected patients with poor immune response to the vaccine.Citation13 Here, we present a patient with HIV/HBV co-infection who did not obtain complete seroconversion after receiving three doses of the BBIBP-CorV vaccine.
Case presentation
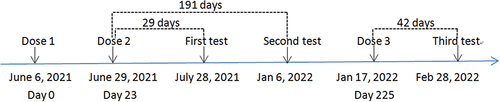
A 39-year-old man was diagnosed as HIV- and HBV-positive (HBsAg+, HBsAb-, HBeAg+, HBeAb-, HBcAb+) on March 6 and 11, 2015, respectively. The patient started ART on 11 March 2015 and received routine follow-up and stable ART at the Third People’s Hospital of Shenzhen. The patient has never been infected with SARS-CoV-2, and the ART drugs taken by the patient during vaccination were TAF (tenofovir alafernade), 3TC (lamivudine) and EFV (efavirenz). The patient received two doses of the BBIBP-CorV vaccine 23 days apart in 2021 and a third dose 202 days after the second dose (). In addition, the time of test is the same as the time of blood collection.
During the vaccination period, we used flow cytometry to analyze CD4+ and CD8+T cell counts and proportions in the peripheral blood of the patient. The patient’s HIV viral load was detected using reverse transcription-polymerase chain reaction with the lowest detection limit of 50 copies/mL. During the vaccination period, the CD4+T cell count of the patient was maintained below 350 cells/µL, and the proportion of CD4+T cells was significantly low. CD8+T cell counts were all within the normal range, although the proportion was slightly high. The CD4+T/CD8+T cell ratio of the patient remained approximately 0.60. In addition, the patient had undetectable VL during vaccination ().
Table 1. The HIV/HBV co-infected patient’s routine laboratory test results during vaccination.
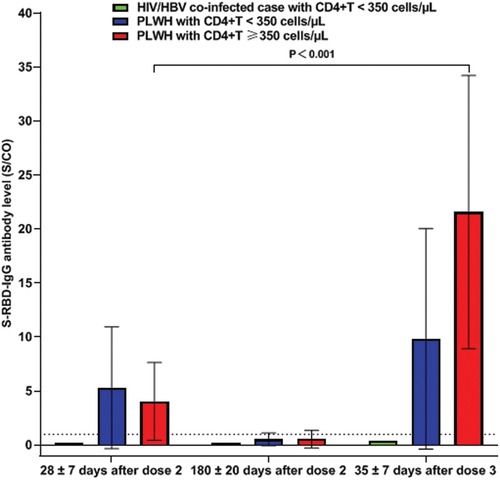
The above case, along with two PLWH with CD4+T <350 cells/µL and 11 PLWH with CD4+T ≥350 cells/µL were recruited into our cohort study, all of whom had received three doses of the BBIBP-CorV vaccine (Sinopharm, 4 μg/0.5 mL) successively. Peripheral venous blood was collected from the participants at 28 ± 7 days and 180 ± 20 days after the second dose and at 35 ± 7 days after the third dose. Magnetic particle chemiluminescence kits (Shengxiang Biotechnology, Changsha, China) were used to detect specific S-RBD-IgG antibodies in blood samples. The participants’ antibody levels were tested once at each time point, whereas we tested the case’s antibody level twice at 42 days after the third dose. The test result was expressed as the ratio of the sample luminescence value (S) to the cutoff (CO) (S/CO value), the S/CO value indirectly reflected the antibody level, and an S/CO value ≥1 was defined as S-RBD-IgG antibody seropositivity. Twenty-nine days after two doses of the BBIBP-CorV vaccine, the S-RBD-IgG antibody S/CO value of the case was 0.13; 42 days after the third dose, the case’s S-RBD-IgG antibody S/CO values of two tests were 0.39 and 0.44 (mean 0.42), respectively, suggesting that the patient failed to seroconvert after vaccination ().
Two PLWH with CD4+T <350 cells/µL had successful seroconversion not only 28 days after receiving two doses of the BBIBP-CorV vaccine but also 35 days after receiving the third dose, although one PLWH had low antibody levels after receiving the third dose of the BBIBP-CorV vaccine. Among the 11 PLWH with CD4+T ≥350 cells/µL, 2 of them failed to seroconvert after receiving two doses of BBIBP-CorV vaccine; however, all of them succeeded to seroconvert after receiving the third dose of the BBIBP-CorV vaccine, and the S/CO value of the S-RBD-IgG antibody increased significantly from 4.05 ± 3.60 at 28 days after the second dose to 21.57 ± 12.65 at 35 days after the third dose (p < .001) ().
Discussion
The immunogenicity of inactivated COVID-19 vaccines in immunocompromised populations, such as PLWH, has received extensive attention. To date, there are very few preliminary reports on the early efficacy of PLWH after receiving the third dose of inactivated COVID-19 vaccine,Citation14 while the effectiveness of PLWH vaccination with the third dose of inactivated COVID-19 vaccine still lacks research.
The level of immunogenicity induced by the vaccine in PLWH is related to their CD4+T cell counts and VL.Citation15 The CD4+T cell count, which was considered as an indicator of the PLWH immune status,Citation16 was significantly associated with reduced humoral responses of PLWH to a variety of vaccines, including inactivated COVID-19,Citation2,Citation3 hepatitis A virus,Citation17 and pneumococcal vaccine.Citation18 ART can permanently suppress HIV to an undetectable level in plasma, usually rebuilds the immune function of PLWH and restores its CD4+T cell count, improving the immune responses of PLWH after vaccination.Citation19 This patient received stable ART and his VL was well suppressed. However, the recovery of his CD4+T cell counts was poor due to co-infection with HBV, and the CD4+T cell count remained below 350 cells/µL constantly. In addition, the CD4+T/CD8+T ratio of this patient remained approximately 0.60, and the CD4+T/CD8+T ratio was also significantly associated with reduced humoral response of PLWH to the vaccine.Citation17 A previous study noted that compared with PLWH with a CD4+T/CD8+T ratio of 0.6–1.0 or a ratio >1.0, the BBIBP-CorV vaccine induced lower antibody level in PLWH with a CD4+T/CD8+T ratio of <0.6.Citation20 These may be related to the fact that the patient failed to seroconvert not only after receiving two doses of the BBIBP-CorV vaccine but also after receiving the third dose of the vaccine.
In our cohort, two PLWH with CD4+T count ≥350 cells/µL did not seroconvert to two doses of the BBIBP-CorV vaccine. However, they not only succeeded in seroconversion after receiving the third dose of the vaccine but also showed a significant increase in the S-RBD-IgG antibody level. This suggests that they are not really “non responders” and their immune memory has been established, therefore the repeated antigen stimulation may be required. It is also worth noting that one PLWH with CD4+T <350 cells/µL had low antibody levels after receiving the third dose of the BBIBP-CorV vaccine, which may not have been enough to provide him with immunity against SARS-CoV-2. Strategies such as higher antigen titer per dose of vaccine or increased doses for repeated stimulation, should be studied to improve the immune response of the PLWH population after vaccination, especially in PLWH with low CD4+T cell counts due to HBV infection or other reasons.
Conclusion
In this report, we present the case of a patient with HIV/HBV co-infection who did not respond to three doses of the BBIBP-CorV vaccine. We suggest that local authorities should strengthen health education and regular HBV screening for early detection and appropriate management of PLWH. Drug therapy that can simultaneously suppress both HIV and HBV should be initiated as early as possible to improve the immune response of PLWH to conventional vaccines. For PLWH with low CD4+T cell counts who failed to seroconvert after receiving three doses of inactivated COVID-19 vaccine, it is necessary to consider appropriately higher antigen titers per dose of vaccine or increase the number of doses to stimulate repeatedly on the premise of safety; to improve the immune response of PLWH after vaccination.
Authors’ contribution
GZ and JT did the ideation, conceptualization, data collection, literature collection, writing original draft, reviewing, and editing. SF and LX did the data collection, reviewing, and editing. WX did the reviewing and editing. ZY did the ideation, reviewing and editing. GZ and JT contributed equally to this work as co-first authors. All authors critically reviewed and approved the final version of the manuscript.
Ethical approval
This study was approved by the Ethics Committee of Shenzhen Center for Disease Control and Prevention (No.QS2021070043, 10 August 2021). The patient consent to the publication of this case report.
Acknowledgments
We thank the patient for allowing us to share his details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this case report are available on request from the corresponding author. The data are not publicly available according to the ethical committee decision on the conduct of this study.
Additional information
Funding
References
- UNAIDS. [accessed 2022 May 20]. https://www.unaids.org/en/resources/documents/2021/HIV_COVID-19_brochure.
- Netto LC, Ibrahim KY, Picone CM, Alves AP, Aniceto EV, Santiago MR, Parmejani PS, Aikawa NE, Medeiros-Ribeiro AC, Pasoto SG, et al. Safety and immunogenicity of CoronaVac in people living with HIV: a prospective cohort study. Lancet HIV. 2022;9(5):1–4. doi:10.1016/S2352-3018(22)00033-9.
- Ao L, Lu T, Cao Y, Chen Z, Wang Y, Li Z, Ren X, Xu P, Peng M, Chen M, et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg Microbes Infect. 2022;11(1):1126–1134. doi:10.1080/22221751.2022.2059401.
- Huang X, Yan Y, Su B, Xiao D, Yu M, Jin X, Duan J, Zhang X, Zheng S, Fang Y, et al. Comparing immune responses to inactivated vaccines against SARS-CoV-2 between people living with HIV and HIV-negative individuals: a cross-sectional study in China. Viruses. 2022;14(2):277. doi:10.3390/v14020277.
- Kerneis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boëlle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(8):1130–1139. doi:10.1093/cid/cit937.
- Costa C, Migliore E, Galassi C, Scozzari G, Ciccone G, Coggiola M, Pira E, Scarmozzino A, La Valle G, Cassoni P, et al. Factors influencing level and persistence of anti SARS-CoV-2 IgG after BNT162b2 vaccine: evidence from a large cohort of healthcare workers. Vaccines. 2022;10(3):474. doi:10.3390/vaccines10030474.
- Wondimeneh Y, Alem M, Asfaw F, Belyhun Y. HBV and HCV seroprevalence and their correlation with CD4 cells and liver enzymes among HIV positive individuals at University of Gondar Teaching Hospital, Northwest Ethiopia. Virol J. 2013;10(1):171. doi:10.1186/1743-422X-10-171.
- Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatol (Baltimore, Md). 2009;49(5 Suppl):S138–145. doi:10.1002/hep.22883.
- Kwofie TB, Adigbli D, Osei-Yeboah J, Ativi E, Lokpo SY. Hepatitis B and C infections in HIV-1 patients on combination antiretroviral therapy (cART) in Ghana: implications for immunologic recovery, clinical response to treatment, and hepatotoxicity. Heliyon. 2021;7(6):e07172. doi:10.1016/j.heliyon.2021.e07172.
- Maitha GM, Kikuvi G, Wanzala P, Kirui F. Influence of hepatitis B virus co-infection on virological and immunological response to antiretroviral treatment among HIV patients attending comprehensive care clinics in Makueni County, Kenya. Pan Afr Med J. 2021;38:103. doi: 10.11604/pamj.2021.38.103.25793.
- Malagnino V, Cerva C, Teti E, Campogiani L, Compagno M, ForoghiBil L, Saderi L, Armenia D, Salpini R, Svicher V, et al. Poor CD4/CD8 ratio recovery in HBcAb-positive HIV patients with worse immune status is associated with significantly higher CD8 cell numbers. Sci Rep. 2021;11(1):3965. doi:10.1038/s41598-021-83616-z.
- Peng X, Ouyang J, Isnard S, Lin J, Fombuena B, Zhu B, Routy JP. Sharing CD4+T cell loss: when COVID-19 and HIV collide on immune system. Front Immunol. 2020;11:596631. doi:10.3389/fimmu.2020.596631.
- El Chaer F, El Sahly HM. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am J Med. 2019;132(4):437–446. doi:10.1016/j.amjmed.2018.12.011.
- Tan Y, Zou S, Ming F, Zhang Z, Xing Z, Wu S, Guo W, Tang W, Liang K. Early efficacy and safety of the third dose inactivated COVID-19 vaccine among people living with HIV. J J Acquir Immune Defic Syndr. 2022;90(3):e1–e3. doi:10.1097/QAI.0000000000002953.
- Menson EN, Mellado MJ, Bamford A, Castelli G, Duiculescu D, Marczyńska M, Navarro LM, Scherpbier HJ, Heath PT. Guidance on vaccination of HIV-infected children in Europe. HIV Med. 2012;13(6):333–336. doi:10.1111/j.1468-1293.2011.00982.x.
- Duro R, Rocha-Pereira N, Figueiredo C, Piñeiro C, Caldas C, Serrão R, Sarmento A. RoutineCD4 monitoring in HIV patients with viral suppression: is it really necessary? a Portuguese cohort. J Microbiol Immunol Infect. 2018;51(5):593–597. doi:10.1016/j.jmii.2016.09.003.
- Fritzsche C, Bergmann L, Loebermann M, Glass A, Reisinger EC. Immune response to hepatitis a vaccine in patients with HIV. Vaccine. 2019;37(16):2278–2283. doi:10.1016/j.vaccine.2019.018.064.
- Song JY, Cheong HJ, Noh JY, Choi MJ, Yoon JG, Kim WJ. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in HIV-infected adults in the era of highly active antiretroviral therapy: analysis stratified by CD4 T-cell count. Hum Vaccin Immunother. 2020;16(1):169–175. doi:10.1080/21645515.2019.1643677.
- Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi:10.1056/NEJMoa1506816.
- Feng Y, Zhang Y, He Z, Huang H, Tian X, Wang G, Chen D, Ren Y, Jia L, Wang W, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinMed. 2022;43:101226. doi:10.1016/j.eclinm.2021.101226.