ABSTRACT
This study provides a snapshot of the current vaccine business ecosystem, including practices, challenges, beliefs, and expectations of vaccine providers. Our team focused on providers’ firsthand experience with administering vaccines to determine if an oral vaccine (e.g. pill or oral-drop) would be well-received. We interviewed 135 healthcare providers and vaccine specialists across the US, focusing questions on routine vaccinations, not COVID-19 vaccines. Improving workflow efficiency is a top concern among vaccine providers due to shrinking reimbursement rates—determined by pharmacy benefit managers (PBMs)—and the time-intensiveness of injectable vaccines. Administering injectable vaccines takes 23 minutes/patient on average, while dispensing pills takes only 5 minutes/patient. An average of 24% of patients express needle-fear, which further lengthens the processing time. Misaligned incentives between providers and PBMs could reduce the quality and availability of vaccine-related care. The unavailability of single-dose orders prevents some rural providers from offering certain vaccines. Most interviewees (74%) believe an oral vaccine would improve patient–provider experience, patient-compliance, and workflow efficiency, while detractors (26%) worry about the taste, vaccine absorption, and efficacy. Additional research could investigate whether currently non-vaccinating pharmacies would be willing to offer oral vaccines, and the impact of oral vaccines on vaccine acceptance.
Introduction
Nearly all routine vaccines in the United States (US) are administered via needle-based injection, either intramuscularly or subcutaneously. Currently, the only exceptions to these routes are the oral (liquid) rotavirus vaccine (RV1 [Rotarix], RV5 [RotaTeq]) and intranasal live, attenuated influenza (LAIV [FluMist]) vaccine.Citation1 The CDC’s Advisory Committee on Immunization Practices (ACIP) recommends that infants receive their rotavirus series between 2 and 6 months of age,Citation2 and that everyone 6 months and older receive an annual flu vaccine. The intranasal LAIV vaccine may be administered to non-pregnant individuals 2 through 49 years of age.Citation3 Oral vaccines for adenovirus, cholera, and typhoid are also available in the US, but none of these are routinely administered. Adenovirus vaccine is only available for US military personnel—not the general public.Citation4 Cholera and typhoid vaccines may be recommended for individuals traveling to parts of the world where these diseases are common.Citation5,Citation6 The oral polio vaccine is no longer licensed or available in the US but may be administered in other parts of the world.Citation7
FruitVaccine, Inc., (http://www.fruitvaccine.org/) was founded in 2017 to develop plant-based (short-growth-cycle plants, e.g., tomatoes), mucosally (e.g., orally or nasally) administered subunit vaccines (for subunit vaccine technology review, see Quinlan et al.Citation8) against mucosally attacking viruses such as respiratory and gastrointestinal viruses. Our vaccine platforms in-development include a chewable/swallowable fruit-pill, oral-drop, and fruit-puree/paste, as alternatives to traditional needle-based immunizations. Vaccination via mucosal routes, e.g., oral administration (per os), has been shown to be more effective than parenterally delivered vaccines for immunization against infections primarily acquired through mucosal surfaces.Citation9–14 Our initial vaccine target is the globally problematic respiratory infection caused by the human respiratory syncytial virus (hRSV). hRSV is a flu-like disease that can cause severe illness in infants and young children, adults over 65, and individuals with compromised immune systems. Each year in the US, more than 100,000 infants (under 1 yr), approximately 58,000 children under 5 years old, and 177,000 older adults are hospitalized due to hRSV infection. An estimated 14,000 of these adults succumb to the disease. On a global scale, hRSV annually infects over 64 million individuals, resulting in 160,000 direct deaths and over 1.4 million additional deaths due to secondary infections.Citation15–18 Despite the urgent need, there is no prophylactic (preventive) vaccine against hRSV currently available on the market.
In late 2020, FruitVaccine received NSF-SBIR (National Science Foundation – Small Business Innovation Research) funding to develop an oral vaccine-pill against hRSV. This qualified us to take part in two of NSF’s entrepreneurial Customer Discovery training programs, each lasting approximately two months, and with the objective of determining whether there is a good product-market fit between a company’s value proposition and its anticipated customer segment(s): (1) NSF SBIR “Beat-the-Odds Boot Camp” (BC), which we completed in early 2021. Participating BC teams are required to interview a minimum of 30 potential direct-customers to learn about their current jobs, workflow, and experience with existing solutions. This should reveal the customers’ needs, pain points, and decision criteria, and allow teams to validate/invalidate their hypotheses regarding product-market fit. (2) Our Successful completion of BC qualified us for NSF’s more comprehensive All-SBIR Innovation Corps (I-Corps) Program (IC), which we completed in the summer of 2021. IC expands upon the BC curriculum by applying the interview process to validate/invalidate hypotheses across a company’s entire business model. IC teams are required to interview at least 100 decision-makers, influencers, economic buyers, and other key individuals from their respective industries. The patterns that emerged from these interviews gave us insight into the experiences, practices, behaviors, and challenges faced by different stakeholders in the vaccine business ecosystem. Being a business-to-business (B2B) entity, FruitVaccne primarily focussed on interviewing vaccine providers (), instead of patients (the vaccinees/end-users).
Table 1. Total number of contacts made (#: second column) and interviews completed (#: fourth column).
Interviews were composed of both closed- and open-ended questions, and we primarily used text mining and machine learning (ML) for analyzing the responses. Due to the COVID-19 pandemic restrictions in 2021, the interviews were conducted remotely on the Zoom platform,Footnotea and digital transcripts were generated using Zoom’s native annotation service and Google Cloud Platform’s Speech-to-Text API.Footnoteb Our automated text mining pipeline was fed with the complete text transcripts of the interviews composed of both the questions and answers to those questions. Therefore, the pipeline first identified the answers for each question, and then extracted various information useful for generating insight. This pipeline was powered by advanced Transformer language models, which have recently gained popularity in similar tasks.Citation19–25
In the past, text mining techniques have been used to analyze various types of survey data. Responses to an open-ended question are challenging to analyze and are often ignored or categorized into pre-defined labels by human coders.Citation26–28 Researchers use text mining to automatically analyze and categorize these responses.Citation26–32 Ford et al.Citation29 used text mining to analyze narrative responses to an open-ended question regarding improving employee performance. They identified four new topics that were not previously addressed. Moreo et al.Citation30 developed an ML model that interactively learns from user annotations to classify open-ended responses and showed that previously trained models can be successfully re-trained for a new related classification task. Schonlau et al.Citation27 annotated responses with multiple labels where all labels that are related to the responses are applied. SchwablCitation32 used text mining to categorize conversations where a user is asking for particular information related to an ingredient while cooking. He and SchonlauCitation26 found in their research that ML models make similar classification mistakes to human coders. They also found that ML models yield higher error rates than human coders. He and SchonlauCitation28 found that training data annotated by two coders and intercoder disagreements resolved by an expert lead to high classification accuracy.
For data extraction, we used fine-tuned language transformer models as available from the authors without additional tuning, because training from scratch is resource-consuming. There exist many fine-tuned models suitable for similar tasks, and in most cases, these provide satisfactory results. So, we evaluated several top-performing models over a subset of data and chose the best-performing models. In particular, we used two language models that performed exceptionally well in our case and allowed us to spend our time extracting and curating answers than training from scratch. As one of the secondary outcomes of this project, we highlight how researchers can use fine-tuned transformer models to analyze survey data effectively.
Our goal was to use the BC and IC interview data to discover meaningful patterns that would inform the development of FruitVaccine’s business model. As a B2B business, we placed an emphasis on interviewing point of care (POC) healthcare providers in pharmacy, clinic, and hospital settings to determine whether there is a good product-market fit for an orally-administered vaccine, such as a vaccine-pill, within different customer segments. In addition, we wanted to seek advice and learn industry best practices from a broader range of experts across the vaccine business ecosystem. Though we anticipated that many of the conversations with industry experts would yield unique questions/responses depending on their respective roles, we hoped to quantify the results of our POC interviews to highlight some of the vaccine-related experiences, challenges, and unmet needs that are common among healthcare providers.
Materials and methods
Survey conduction
Our BC team was composed of two members (Entrepreneurial Lead [EL], and Co-EL) who were responsible for soliciting and conducting interviews. These same two individuals led the four-member (EL, Co-EL, Industry Mentor [IM], and Technical Lead [TL]) IC team and were again responsible for soliciting and conducting interviews. At least one of these two individuals was present to direct each BC and IC interview. The IM and TL served in advisory roles to provide expertise and support during the program.
Interview leads were generated using three methods: (1) by selecting healthcare professionals and organizations from publicly available sources; (2) through the professional networks of the BC/IC team members; and (3) via referrals from prior interviewees. Most of our BC interviews were generated by cold calling healthcare organizations within FruitVaccine’s expected customer segments. Publicly available data was used to contact pharmacies (both chain and independent), local health departments, and individuals within regional health systems (i.e., hospitals) in Central Illinois and the surrounding region.
For the IC program, we expected that the bulk of our interviews would again need to be generated through cold leads. We began by compiling a list of 500 POC healthcare professionals and organizations from across the United States. Based on our experience during BC and the input from our IM and TL, our initial IC contact list was made up of independent pharmacies, local health departments, regional health systems, pediatricians, geriatricians, and immunologists, mainly using publicly available information. In addition, LinkedInFootnotec was used to quickly and efficiently contact many industry experts from the broader vaccine business ecosystem (e.g., researchers; marketers; NGOs).
Interview leads were contacted via e-mail, phone, or LinkedIn message. When able, multiple communication methods were used to follow up on initial requests. We followed up with most individuals/organizations at least two to three times. Due to time limitations, we abandoned attempts to interview individuals who initially declined to participate or made no effort to respond to multiple requests.
We ended up conducting extensive interviews with 135 individuals across the US, including healthcare and vaccine providers and professionals from within the vaccine business ecosystem. Questions were focused on routine vaccinations (e.g., influenza, MMR, and rotavirus), rather than COVID-19 vaccines. We attempted to conduct all interviews using the teleconferencing software Zoom Meetings. Zoom was selected for its familiarity of use and ability to record interviews. During IC, we took the additional step of purchasing a Zoom Business account to enable the auto-generation of interview transcripts. Transcripts of our BC interviews were generated from our Zoom audio recordings, using Google Cloud Speech-to-Text API. All interviewees were asked for permission prior to recording.
Our BC and IC instructors recommended that teams interview one customer at a time to focus on each person’s unique experience. The instructors also suggested that teams limit each interview to 20 minutes. However, many interviews went longer when the conversation was going well and there were no scheduling conflicts with any of the parties. Our team developed a general questionnaire that we believed could be applied to most interviews. If a particular question was not relevant to an interviewee, it was skipped. Conversely, our team often asked additional follow-up questions and had unique questions for certain experts from different areas of the vaccine business ecosystem.
BC interviews were conducted between January 22 and 18 February 2021; and IC interviews were conducted from July 21 to 31 August 2021. Our interview question lists were composed of 12 and 21 questions, respectively. Some of these were modified/improved over the course of this process. The following are the questions used for the analysis presented in this paper:
How long does the total vaccination process take per patient?
If you could improve one step/thing in your workflow process, what would it be?
Do you purchase your vaccines via a Buyers’ Club/Pharmacy Buying Group?
How do you learn about new vaccine products?
Based on your experience, what percentage of patients are afraid of needles?
Do you see any benefits in using oral vaccines over injectable?
Have you encountered any problems using oral vaccines over injectable?
Do you have any unmet needs regarding the vaccination process? If yes, what are those?
Demographics and other variables
shows the breakdown of the organizations/professionals that were contacted during the BC and IC programs, and the number of interviewees by role within their organizations. The categorizations differ because we often spoke with multiple individuals who each held different roles within a single type of organization (e.g., physicians, nurses, and administrators from hospitals). Seven interviewees in the “Others” category include vaccine experts from pharmaceutical companies, epidemiologists, academics, representatives from professional healthcare associations, regional healthcare coordinators, and veterinary vaccine specialists.
The number (#) of contacts for each type of healthcare professional/organization is reflective of our success with follow-up communications and scheduling interviews. For example, individuals at chain pharmacies were extremely reluctant to speak with us without first obtaining permission from their regional/corporate supervisors, and our subsequent follow-ups were largely unanswered. Due to the time constraints, it was necessary for us to focus on contacting organizations with a higher chance of yielding an interview.
There were three cases when our team interviewed two people during the same interview. In these instances, we requested that each person answer our questions independently so that we could generate two sets of answers. There were also three cases where interviewees did not consent to being recorded, one interview could not be transcribed due to technical error, and two interviews were conducted by phone due to technical error and interviewee request. In total, we were able to generate 126 transcripts by interviewing 135 individuals.
Text mining analysis
We achieved the goal of extracting exact answers from the transcripts by dividing the overall problem into two independent sub-problems: 1) identifying questions from the questionnaire in the transcripts, and 2) extracting exact answers from relevant context. The questionnaire included questions, such as Q1 and Q5 (see the section Survey Conduction above), that yielded similar answers such as quantity. Providing a whole transcript as a context makes the context noisy and results in a high false-positive rate. So, we first divided each transcript into segments to limit the context to relevant sentences by identifying the questions of the questionnaire present in the transcript.
We used a fine-tuned model called SRoBERTa (paraphrase distilroberta base v2),Citation25 specially fine-tuned for semantic textual similarity tasks, to identify the questions in the transcripts. We used cosine similarity to calculate sentence similarity among questions in the questionnaire and each dialogue turn spoken by the interviewers in the transcript to match the questions between the questionnaire and a transcript. Typically, interviewers ask one question at a time from the questionnaire and do not proceed to the next question until the interviewee is done answering the current question. Thus, a dialogue turn should match with only one question. If a dialogue turn matches multiple questions, we paired the dialogue turn with the question having the highest similarity score and found new matches for the other questions removing this dialogue turn from the corpus. To improve the performance, we paraphrased similar questions and elaborated on the short questions in the questionnaire. In some cases, we included both the original question and the paraphrased questions for better performance.
Once all the questions were identified in a transcript, we split the transcript into segments by the matched dialogue turns and assigned each segment as the context to the preceding question. Sometimes, interviewers repeat part of the answer given by the interviewee for confirmation or clarity which should not be considered as answers. So, we refined the context by keeping the utterances only spoken by the interviewees. Once this step was completed, we applied various techniques for extracting answers to the list of questions as briefly described below.
We used a fine-tuned question-answering model called RoBERTa-base for QA model (second revision) for extracting exact answers from the contexts.Citation33 We simplified, paraphrased, and/or elaborated on the questions to improve the performance of the model. The main purpose of changing the questions was to make them easily understandable to the model. Answers including numbers in words were replaced with digits by a human. Answers that were given in ranges, e.g. 5 to 10, were replaced with the average value, such as 7.5 in this example. The model may always return an answer even though there is none. In some rare cases, the context did not include an exact answer due to not being answered by the interviewees, or an incorrect segment is matched with the question by the previous model. In such cases, the model returned an answer that has a low matching score and did not relate to the question at all. These answers have been manually reviewed and removed, when applicable, by a human curator. For generating the word clouds using answers to some of the open-ended questions (e.g., Q2 in the section Survey Conduction), we manually replaced each of the extracted answers with keywords or shorter terms for improving the quality of the cloud.
We developed the data extraction pipeline strictly focusing on extracting answers from the IC transcripts. Later, we decided to include answers from the BC interviews as well. For IC interviews, we utilized the Zoom annotation service to acquire the transcripts. However, the Zoom annotation service was not at hand during the BC interviews and only the audio recordings were available. So, we utilized the Google Cloud Speech-to-Text API to transcribe the BC interviews.
Results and discussion
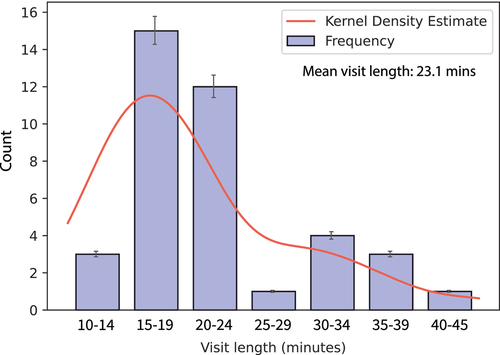
When asked about the one thing that an interviewee would like to improve in the vaccination process, we received a wide variety of responses (). However, interview discussions often turned toward time-saving measures and workflow efficiency, which we interpreted to be the underlying issue for most healthcare providers. This was especially true among independent pharmacists who generally expressed the need to streamline patient services wherever possible. Many pharmacists explained that this need is driven by ever-shrinking reimbursement rates, as contracted with their respective pharmacy benefit managers (PBMs). This has reduced the margin on services that pharmacies provide, requiring independent pharmacists to provide a higher volume of patients served to remain profitable. However, the administration of routineFootnoted intramuscular/injectable vaccines is one of the most time-consuming services a pharmacist can provide, taking an average of 23 minutes per patient () according to our respondents.Footnotee This may become especially troublesome during multiple vaccinations in one visit, especially for children.Citation34 Conversely, several prescriptions could be filled during this time, and most pharmacists said they make little to nothing—or even lose money—on each vaccine.
Figure 1. Word-Clouds generated from interviewee responses: (a) one step that can be improved in the workflow process, and (b) unmet needs regarding the vaccination process.

When we asked the interviewees about any unmet needs regarding the vaccination process, a frequent frustration among healthcare providers was the combination of low demand and vaccine wastage due to multi-dose packaging (). That is, the minimum order size for a particular vaccine might be a 10-dose pack, but the provider may be unable to use all those doses before they expire. Many of our interviewees described having good relationships with other local healthcare providers and the ability to transfer single doses within these networks. However, there were many interviewees who did not have the ability to transfer doses and were unable to offer multi-pack vaccines due to the financial risk, especially with relatively expensive vaccines such as the shingles vaccine (Shingrix). Therefore, multi-dose packaging may be making it difficult for some patients to access certain vaccines, especially in rural areas.
We had candid discussions with several pharmacists to ask why they would continue to provide an unprofitable and cumbersome vaccination service. Their response was a genuine desire to improve the health of patients in their communities, and a belief that a significant healthcare gap would develop if pharmacies stopped providing vaccines, i.e., the convenience and frequency of patient visits at pharmacy locations result in much higher vaccine compliance rates than what clinics and hospitals could achieve on their own. These discussions indicate a current misalignment of incentives between independent pharmacists and PBMs, generating a perception that the two prioritize patients and profits, respectively. Most of the pharmacists we spoke with believed strongly that the practices and amount of control exerted by PBMs are detrimental to the healthcare industry. Some independent pharmacists revealed that they became members of a pharmacy buying group (), to leverage large networks of pharmacies to contract for better pharmaceutical prices. While these groups manage to relieve some of the pricing pressure, they fail to resolve the underlying issues stated above. Our interviewees stated that patients on average visit their pharmacist once a month, compared with seeing their doctor once a year, and that they trust their pharmacists just as much as their doctor. Convenient, alternative locations to hospitals and clinics, such as pharmacies, are very promising for maintaining and improving vaccination rates.Citation13,Citation35s
Figure 3. Distribution of response type of the interviewees with counts (# of interviewees) and respective percentages: (a) Independent Pharmacy Owners on joining a Pharmacy Buying Group to strengthen their bargaining power, (b) problems in using oral vaccines, (c) polarity regarding benefits in using oral vaccines, and (d) benefits of using oral vaccines, categorized based on interviewee-responses.
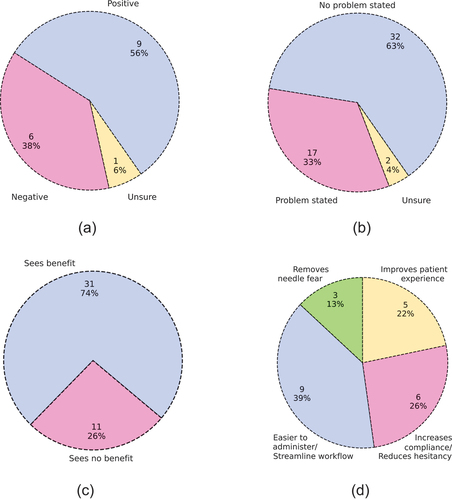
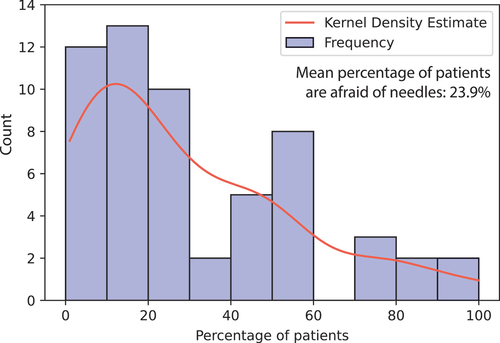
When asked about the perceived benefits of using oral vaccines, most (74%) of the interviewees expressed one or more benefits (). Providers most frequently cited oral vaccines’ ease of administration, better vaccine compliance, and their ability to streamline workflow (). Several pharmacists spoke of their experience with the oral typhoid vaccine, which can be distributed to a patient in approximately five minutes. The patient can then ingest the vaccine at home, like most other prescriptions.Footnotef Other interviewees suggested that oral vaccines would increase patient compliance by reducing vaccine hesitancy, improve patients’ experience while getting vaccinated, and remove the fear and anxiety that some patients face with needles. An average of 24% of our interviewees’ patients express needle-fear (). These factors can have a significant impact on vaccine services and availability, as highlighted by one pharmacy manager we interviewed: their chain of pharmacies does not offer vaccines to young children due to needle fear and the disruption a screaming child can cause at their supermarket locations. Alternative low-pain/pain-free vaccination methods can be viable solutions to resolve these discomfort issues surrounding needle-based vaccinations.Citation34,Citation36,Citation37
Figure 4. The percentage of patients who are afraid of needles, as estimated by the interviewees (vaccine providers).
Most interviewees did not assert problems with their current vaccine storage, preparation, administration spaces, or waste disposal arrangements, but we believe oral vaccines would provide ancillary benefits in each of these areas. In general, interviewees possessed sufficient cold storage space for their vaccine stocks, especially due to widespread capacity increases to accommodate the COVID-19 vaccines. However, several expressed frustration with inventory management requirements such as logging temperatures and ensuring that required cold temperatures are maintained with contingency plans (e.g., in the event of a power outage). Oral vaccine pills that are stable at room-temperature could provide relief toward managing and reporting on vaccine stocks. In addition, ACIP guidelines state that vaccine preparation and administration should be done in separate areas, emphasize care when using multi-dose vials, and stress the safe use of needles and syringes—recognizing that bloodborne diseases are an occupational hazard for healthcare providers.Citation38 One pharmacist described their ongoing remodeling effort to increase patient privacy with a new vaccine administration area. Here again, oral vaccines can provide relief by freeing-up dedicated vaccination spaces and eliminating patient/provider risks associated with syringes. Also, oral vaccines would reduce needle and related accessory waste, easing disposal compliance for healthcare providers. Each of these areas is an inextricable element of the vaccination process, and we believe the widespread development and adoption of oral vaccines will benefit providers by reducing storage, preparation, administration, and waste disposal requirements—further streamlining their vaccination workflow (). On a global scale, it should be noted that vaccine production which facilitates easy scaling-up (e.g., locally producible) and expedited supply chains are needed to improve vaccine availability/accessibility and address the current vaccine shortages, especially during epidemic and pandemic situations.Citation39–41
When asked if they could see any problems with oral vaccines, most (63%) interviewees did not raise any concerns (). Of those who did, the most common issues raised were those of taste, efficacy, and absorption—whether an oral vaccine’s mucosal route would be as effective at triggering an immune response as a traditional intramuscular injection (). Most of the remaining responses had to do with concerns over patient experience during the administration of the vaccine or the potential for new side effects related to the digestive system. Several individuals voiced concern over the idea that younger patients (infants or children) could spit out partial doses of an oral vaccine, which might lead healthcare professionals to question whether the patient had received an adequate amount. In addition, a small number of respondents believed that an oral vaccine would not be superior to current intramuscular injections. One reason is that healthcare workers are already well-trained in administering needle-based injections, and oral administration could introduce new uncertainties for healthcare workers. Furthermore, the “newness” of an oral vaccine could generate vaccine hesitancy among patients who are otherwise used to—and trust the safety of—intramuscular vaccines, even if they find the experience with a needle unpleasant. High vaccine hesitancy rates,Citation42–44—especially following the release of the COVID-19 vaccines—could serve as a cautionary predictor of public reaction toward novel vaccination methods,Citation45,Citation46 with side effects and safety cited as the top concerns.Citation47
We also wanted to determine how healthcare providers learn about new vaccine products (). While many first become aware of new products through publications, subscriptions, or meetings with professional associations, most interviewees found visits from a pharmaceutical sales rep to be the most helpful. We learned that most interviewees prefer a short, in-person session to see a product demonstration and have an opportunity to ask questions. However, the frequency of these rep visits varied widely among our interviewees, with some reporting regular visits from multiple manufacturer reps, and others only called on when a new product is launched.
Conclusion and future work
The FruitVaccine team conducted comprehensive interviews with 135 healthcare and vaccine providers and professionals across the US to learn about their experiences, practices, and challenges with providing vaccinations. Our questions were focused on routine vaccinations (e.g., influenza, MMR, and rotavirus), rather than COVID-19 vaccines. We spoke with a diverse set of individuals who fill different roles within independent pharmacies, hospitals, public health departments, university clinics, pharmaceutical companies, NGOs, and other organizations. We recorded these interviews and later analyzed the transcripts to see if meaningful patterns could be derived from the questions we asked.
We discovered that the greatest challenge many healthcare providers face is the need to improve workflow efficiency. In the pharmacy space, this is being driven by shrinking reimbursement rates from pharmacy benefit managers, necessitating that a higher volume of services be provided to patients. As margins continue to tighten, pharmacists could be forced to discontinue relatively time-intensive services such as intramuscular/injectable vaccine administration. Oral vaccine pills would provide relief as a time-saving measure by streamlining providers’ workflow, improve the patient-provider experience by removing needle anxiety/fear, and could increase vaccine compliance by reducing vaccine hesitancy. Other benefits include a reduction in providers’ storage, preparation, administration, and waste disposal requirements related to vaccines. Additional research could investigate the trends and factors influencing vaccine availability in pharmacies, whether availability is slowing—or even reversing—and the reason(s) why some pharmacists choose not to offer vaccines. If these pharmacists/providers indicate a willingness to offer oral vaccines (e.g., pill or oral-drop) over needle-based injections, the resulting positive impact on vaccine availability and accessibility could be quantified.
Contributors
IR and JK conceived and planned this study. IR was in charge of overall direction and planning. IR and JK carried out the surveys. NK, supervised by IK, designed the computational framework and analyzed the survey data. All authors contributed to the interpretation of the results. JK and NK took the lead in writing the manuscript supervised by IR and IK. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Acknowledgment
We thank Dr. Edwin G. Moore (BioPhia Consulting) and Dr. Brenda Anne Wilson (University of Illinois, Urbana-Champaign, IL) for joining our I-Corps team and providing their expertise and guidance during the program. We also thank Haidong Wang for his assistance with contacting leads and scheduling interviews. We thank the NSF, and the Boot Camp and I-Corps instructors, for supporting and guiding this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
[a]. https://zoom.us/.
[d]. Most of the independent pharmacists we interviewed had significantly decreased patient processing time during dedicated COVID-19 vaccine clinics. While some pharmacists expressed an improvement in scheduling due to these clinics (i.e., moving from paper to electronic), their vaccination process time for routine vaccinations remained unchanged.
[e]. For most vaccinations, the CDC recommends that healthcare providers observe patients for 15 minutes after injection.Citation1,Citation38
[f]. Several interviewees pointed out that there is always the potential for patients to mishandle their prescriptions (e.g., improper storage; not taking doses as instructed, etc.) and administration by a healthcare provider removes this uncertainty.
References
- Centers for Disease Control and Prevention. Vaccine administration route and site. Atlanta (GA): Office of the Associate Director for Communication; 2021 [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/hcp/admin/administer-vaccines.html.
- Centers for Disease Control and Prevention. Birth-18 years immunization schedule. Atlanta (GA): Office of the Associate Director for Communication; 2022 [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- Centers for Disease Control and Prevention. Live intranasal influenza vaccine information statement. Atlanta (GA): Office of the Associate Director for Communication; 2021 [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/flulive.html.
- Centers for Disease Control and Prevention. Adenovirus vaccine information statement. Atlanta (GA): Office of the Associate Director for Communication; 2020 [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/adenovirus.html.
- Centers for Disease Control and Prevention. Cholera vaccine information statement. Atlanta (GA): Office of the Associate Director for Communication; 2019a [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/cholera.html.
- Centers for Disease Control and Prevention. Typhoid vaccine information statement. Atlanta (GA): Office of the Associate Director for Communication; 2019b [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/typhoid.html.
- Centers for Disease Control and Prevention. Polio vaccination: what everyone should know. Atlanta (GA): Office of the Associate Director for Communication; 2018 [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/vpd/polio/public/index.html.
- Quinlan EJ, Chubet R, Leonardi P. A novel SARS-CoV-2 subunit vaccine engineered on an immune-activating platform technology. Hum Vaccines Immunother. 2022:1–10. doi:10.1080/21645515.2022.2062971. PMID: 35801956.
- Farhadian A, Dounighi NM, Avadi M. Enteric trimethyl chitosan nanoparticles containing hepatitis B surface antigen for oral delivery. Hum Vaccines Immunother. 2015;11(12):2811–2818. doi:10.1080/21645515.2015.1053663. PMID: 26158754.
- Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14(2):430–445. doi:10.1128/CMR.14.2.430-445.2001.
- Kim SH, Jang YS. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res. 2017;6(1):15–21. doi:10.7774/cevr.2017.6.1.15.
- Hijano DR, Vu LD, Kauvar LM, Tripp RA, Polack FP, Cormier SA. Role of type I interferon (IFN) in the respiratory syncytial virus (RSV) immune response and disease severity. Front Immunol. 2019;10:566. doi:10.3389/fimmu.2019.00566.
- McRee AL, Reiter PL, Pepper JK, Brewer NT. Correlates of comfort with alternative settings for HPV vaccine delivery. Hum Vaccines Immunother. 2013;9(2):306–313. doi:10.4161/hv.22614. PMID: 23291948.
- Kichaev G, Mendoza JM, Amante D, Smith TR, McCoy JR, Sardesai NY, Broderick KE. Electroporation mediated DNA vaccination directly to a mucosal surface results in improved immune responses. Hum Vaccines Immunother. 2013;9(10):2041–2048. doi:10.4161/hv.25272. PMID: 23954979.
- McLaurin K, Farr A, Wade S, Diakun D, Stewart D. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36(11):990–996. doi:10.1038/jp.2016.113. PMID: 27490190.
- Centers for Disease Control and Prevention. RSV in infants and young children. Atlanta (GA): Office of the Associate Director for Communication; 2020 [accessed 2022 Mar 1]. https://www.cdc.gov/rsv/high-risk/infants-young-children.html.
- Centers for Disease Control and Prevention. Older adults are at high risk for severe RSV infection. Atlanta (GA): Office of the Associate Director for Communication; 2017 [accessed 2022 Mar 1]. https://www.cdc.gov/rsv/factsheet-older-adults.pdf.
- World Health Organization. Respiratory syncytial virus (RSV) disease. Geneva (Switzerland); 2022 [accessed 2022 Mar 1]. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/respiratory-syncytial-virus-disease.
- Devlin J, Chang MW, Lee K, Toutanova K. BERT: pre-training of deep bidirectional transformers for language understanding. arXiv preprint arXiv:181004805. 2018. doi:10.48550/arXiv.1810.04805.
- Yang Z, Dai Z, Yang Y, Carbonell J, Salakhutdinov RR, Le QV. Xlnet: Generalized autoregressive pretraining for language understanding. Adv Neural Inf Process Syst. 2019;32:5753–5763.
- Liu Y, Ott M, Goyal N, Du J, Joshi M, Chen D, Levy O, Lewis M, Zettlemoyer L, Stoyanov V. RoBERTa: a robustly optimized Bert pretraining approach. arXiv preprint arXiv:190711692. 2019. doi:10.48550/arXiv.1907.11692.
- Lan Z, Chen M, Goodman S, Gimpel K, Sharma P, Soricut R. ALBERT: a lite BERT for self-supervised learning of language representations. arXiv preprint arXiv:190911942. 2019. doi:10.48550/arXiv.1909.11942.
- Lee J, Yoon W, Kim S, Kim D, Kim S, So CH, Kang J. BioBERT: A pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36(4):1234–1240. doi:10.1093/bioinformatics/btz682.
- Sanh V, Debut L, Chaumond J, Wolf T DistilBERT, a distilled version of BERT: smaller, faster, cheaper and lighter. arXiv preprint arXiv:191001108. 2019. doi:10.48550/arXiv.1910.01108.
- Reimers N, Gurevych I Sentence-BERT: sentence embeddings using siamese BERT-networks. arXiv preprint arXiv:190810084. 2019. doi:10.48550/arXiv.1908.10084.
- He Z, Schonlau M Coding text answers to open-ended questions: human coders and statistical learning algorithms make similar mistakes. Methods Data Anal. 2021;15(1):17. doi:10.12758/mda.2020.10.
- Schonlau M, Gweon H, Wenemark M. Automatic classification of open-ended questions: check-all-that-apply questions. Soc Sci Comput Rev. 2021;39(4):562–572. doi:10.1177/0894439319869210.
- He Z, Schonlau M. Automatic coding of open-ended questions into multiple classes: whether and how to use double coded data. Surv Res Methods. 2020;14(3):267–287. doi:10.18148/srm/2020.v14i3.7639.
- Ford J, Nierle D, Leeds P, Stetz T. Text mining narrative survey responses to develop engagement scale items. In: Proceedings of the 51st Hawaii International Conference on System Sciences; 2018 Jan 3-6. p. 607–614; Waikoloa Village, Honolulu (HI): University of Hawaiʻi at Mānoa.
- Moreo A, Esuli A, Sebastiani F. Building automated survey coders via interactive machine learning. Int J Mark Res. 2019;61(4):408–429. doi:10.1177/1470785318824244.
- Meidinger M, Aßenmacher M. A new benchmark for NLP in social sciences: evaluating the usefulness of pre-trained language models for classifying open-ended survey responses. In: Rocha A, Steels L, van den Herik H, editors. Proceedings of the 13th International Conference on Agents and Artificial Intelligence (ICAART 2021); vol. 2; 2021 Feb 4-6; Online. Setúbal (Portugal): SciTePress; 2021. p. 866–873.
- Schwabl P. Classifying user information needs in cooking dialogues–an empirical performance evaluation of transformer networks [ master’s thesis]. Regensburg (Germany): University of Regensburg; 2021.
- Chan B, Pietsch M. RoBerta-base for QA; 2020 [accessed 2022 Mar 1]. https://huggingface.co/deepset/roberta-base-squad2.
- Kaaijk P, Kleijne DE, Knol MJ, Harmsen IA, Ophorst OJ, Rots NY. Parents’ attitude toward multiple vaccinations at a single visit with alternative delivery methods. Hum Vaccines Immunother. 2014;10(8):2483–2489. doi:10.4161/hv.29361. PMID: 25424960.
- Enri LR, Baratta F, Pignata I, Brusa P. How to promote vaccinations: a pilot study in the north-west of Italy. Hum Vaccines Immunother. 2019;15(5):1075–1079. doi:10.1080/21645515.2019.1581540. PMID: 30779685.
- Diehl MC, Lee JC, Daniels SE, Tebas P, Khan AS, Giffear M, Sardesai NY, Bagarazzi ML. Tolerability of intramuscular and intradermal delivery by CELLECTRA® adaptive constant current electroporation device in healthy volunteers. Hum Vaccines Immunother. 2013;9(10):2246–2252. doi:10.4161/hv.24702. PMID: 24051434.
- McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J Adv Nurs. 2019;75(1):30–42. doi:10.1111/jan.13818.
- Kroger A, Bahta L, Hunter P General best practice guidelines for immunization. Atlanta (GA): Centers for Disease Control and Prevention. 2022. [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html.
- Donnelly RF. Vaccine delivery systems. Hum Vaccines Immunother. 2017;13(1):17–18. doi:10.1080/21645515.2016.1259043. PMID: 28125375.
- Jarrahian C, Myers D, Creelman B, Saxon E, Zehrung D. Vaccine vial stopper performance for fractional dose delivery of vaccines. Hum Vaccines Immunother. 2017;13(7):1666–1668. doi:10.1080/21645515.2017.1301336. PMID: 28463054.
- Kumraj G, Pathak S, Shah S, Majumder P, Jain J, Bhati D, Hanif S, Mukherjee S, Ahmed S. Capacity building for vaccine manufacturing across developing countries: the way forward. Hum Vaccines Immunother. 2022;18(1):2020529. doi:10.1080/21645515.2021.2020529. PMID: 35086416.
- Sun LX, Chen LL, Chen WY, Zhang MX, Yang MG, Mo LC, Zhu JJ, Tung TH, Li FP. Association between health behaviours and the COVID-19 vaccination: risk compensation among healthcare workers in Taizhou, China. Hum Vaccines Immunother. 2022;18(1):2029257. doi:10.1080/21645515.2022.2029257. PMID: 35175866.
- Kulkarni S, Harvey B, Prybylski D, Jalloh MF. Trends in classifying vaccine hesitancy reasons reported in the WHO/UNICEF joint reporting form, 2014-2017: Use and comparability of the vaccine hesitancy matrix. Hum Vaccines Immunother. 2021;17(7):2001–2007. doi:10.1080/21645515.2020.1859319. PMID: 33534626.
- Jaca A, Iwu-Jaja CJ, Balakrishna Y, Pienaar E, Wiysonge CS. A global bibliometric analysis of research productivity on vaccine hesitancy from 1974 to 2019. Hum Vaccines Immunother. 2021;17(9):3016–3022. doi:10.1080/21645515.2021.1903294. PMID: 33939571.
- Dou K, Yang J, Wang LX, Li JB. Theory of planned behavior explains males’ and females’ intention to receive COVID-19 vaccines differently. Hum Vaccines Immunother. 2022:2086393. doi:10.1080/21645515.2022.2086393. PMID: 35749588.
- Moat SJ, Hillier S, de Souza S, Perry M, Cottrell S, Lench A, Payne H, Jolles S. Maternal SARS-CoV-2 sero-surveillance using newborn dried blood spot (DBS) screening specimens highlights extent of low vaccine uptake in pregnant women. Hum Vaccines Immunother. 2022;0(0):2089498. doi:10.1080/21645515.2022.2089498. PMID: 35731129.
- Centers for Disease Control and Prevention. COVID behaviors dashboard. Atlanta (GA): Office of the Associate Director for Communication; 2022 [accessed 2022 Mar 1]. https://covidbehaviors.org/.