ABSTRACT
In China, a free influenza vaccination policy is being implemented among individuals aged 70 years and over in Zhejiang province during the COVID-19 pandemic. The objective was to assess the effectiveness of influenza vaccine in reducing hospitalization and mortality in the elderly. We used data from the Regional Health Information Platform in Yinzhou located in Zhejiang province and applied a regression discontinuity design to estimate the intention-to-treat effect on admission and mortality rates by month of age in the population who was near the age of 70 years threshold. At age 70 years, the influenza vaccination rate increased by 29.1% (95% CI, 28.2% to 29.9%) compared to those under 70 in the study population. When turning age 70 years, the potential effectiveness of receiving influenza vaccine was 8.2% (95% CI, −36.8% to 51.3%) for total hospitalization and the evaluation of vaccine effectiveness was 13.1% (95% CI, −34.2 to 61.8) for the all-cause mortality. An increase in the influenza vaccination rate was associated with a weak decline in most outcomes, but no significance was found for all outcomes. Influenza vaccination had a limited effect on hospital admission and mortality for the free influenza vaccination program that can be related to the low vaccination rate among the Chinese elderly. Supplementation strategies and future studies may be needed to expand immunization coverage and validate this finding, and further provide a reference for other cities to promote the free influenza vaccination policy in China, especially under circumstances of the COVID-19 pandemic.
Introduction
World Health Organization (WHO) estimates that seasonal influenza causes about 3–5 million serve cases and 290–650 thousand respiratory-related deaths worldwide each year,Citation1 resulting in social costs of billions of dollars.Citation2 A study based on national influenza surveillance and cause-of-death surveillance data in China revealed that an annual mean of 88,100 excess deaths, of which elderly adults accounted for 80%, were caused by influenza-associated respiratory diseases during the 2010–11 to 2014–15 seasons.Citation3 Previous studies of general people have demonstrated that influenza vaccination is associated with lower risk respiratory disease [i.e. influenza, influenza-like illness (ILI) and chronic obstructive pulmonary disease (COPD)] and cardiovascular (i.e. acute myocardial infarction and ischemic cerebrovascular).Citation4,Citation5 In addition, several studies have suggested that influenza vaccination could reduce the all-cause mortalityCitation4 and the mortality risk in patients with cardiovascular diseaseCitation6 and COPD.Citation7,Citation8 Therefore, many countries implemented annual seasonal influenza vaccination to reduce the burden of influenza-related morbidity and mortality, especially in older adults who are usually at a higher risk of influenza, severe complication and death than others.Citation9 However, the association between influenza vaccination and hospitalization and mortality is inconsistent among the older adults. Recently, an updated meta-review including 8 RCTs or quasi-RCTs between 1965 and 2000 indicated that the evidence for a lower risk of influenza and ILI with vaccination in the elderly is limited by biases in the design or conduct of the studies and the providing data for mortality and pneumonia hospitalization were underpowered to detect difference.Citation10 Hence, good evidence of the effect of influenza vaccine on hospitalization and mortality still needs to be provided in the elderly. Due to the limitation of the ethics, new RCTs are not permitted, in turn, observational studies are more feasible, but they are inherently inclined to selection and confounding biases.Citation11
Observational studies, such as cohort and case-control studies, have found 20%−50% reductions in hospitalization and deaths in vaccinated elderly people,Citation12,Citation13 yet the results may be impacted by selection bias and confounding in the real-world setting. Recently, the regression discontinuity design (RDD), a relatively new quasi-RCT study design, has been applied to estimate the overall vaccination effectiveness (VE) for the vaccination program in the elderly people—in United KingdomCitation14 and Netherlands,Citation15 which can maximize controlling above bias. This is a powerful design that is analogous to a random assignment receiving the intervention for an individual,Citation16 which can protect against selection bias and are not available in the other observational research designs used to study this issue.
Currently, availability and improvement of high-quality electronic healthcare databases in China, such as Regional Health Information Platform (RHIP), has produced the prospect of providing real-world evidence in the condition when RCT is unfeasible.Citation17 Yinzhou is one of the earliest areas to build the RIHP, which has become the best regional community-based database and has been confirmed by multiple studies after more than 15 years of integration and development.Citation18 In addition, the free influenza vaccination program in Zhejiang Province was initiated in 2020 COVID-19 pandemic and targeted on the elderly permanent household residents aged 70 years or older,Citation19 which makes it possible to evaluate the overall VE of influenza vaccine using RDD.
Therefore, this study aimed to estimate the intention-to-treat effect of influenza vaccine on the hospital admission and mortality for people aged 70 and older, based on Yinzhou RHIP database.
Materials and methods
Data source and study population
This study was based on the data warehouse of Yinzhou RHIP (YRHIP), which located in a developed area of Ningbo City, Zhejiang province, the eastern coastal region of China, and had a total of more than 1.6 million permanent residents in 2020. This platform was originally designed in 2006 by the Yinzhou District Center for Disease Control and Prevention (CDC) to facilitate routine primary care services for local general practitioners, and gradually integrated with information on public health surveillance, population screen, disease management, health information system in hospital and other healthcare services. Since 2009, the YRHIP has covered nearly all health-related activities of residents throughout life, from birth to death, including children, adolescents, pregnant women, adults and elderly people in this district. Until 2017, 98% of permanent residents in Yinzhou have registered in the health information system. YRHIP contains all electronic medical records (EMR) data of outpatient, emergency and inpatient visits, primary and secondary diagnosis, laboratory services and medications usage from a network of all five hospitals (public and private hospital) and 289 primary care institutions across Yinzhou.Citation20 In 2020, the database was approved and awarded a Five Grade Class B, which was the second-leading level of the Standardization and Maturity Measurement of Regional Health Information Interconnection by the National Health Commission of China. Furthermore, the high-quality, practical data in this platform has already been applied for studies of chronic noncommunicable diseaseCitation20–22 and safety and effectiveness of vaccineCitation23,Citation24 and drugs,Citation18,Citation25 proving the reliability and accurateness of the data source for performing future researches.
In this study, free influenza vaccination policy was initiated in September 2020 and targeted no the permanent household residents aged 70 years and older or born prior 31 December 1950. All other individuals had to pay for the full cost of influenza vaccine. Since the actual free influenza vaccination has initiated in September, the study period is defined as the influenza season from 1 September 2020 to 31 March 2021. The study population consisted of adults born between 1 January 1930 to 31 December 1970 who had at least 180 days of continuous enrollment in this database and had a local household registration. Simultaneously, these populations who are alive on 1 September 2020 were included in the study.
Procedures and outcomes
We used the integrated data from four data sources. The vaccination data set (a total of 36068 individuals aged 55 to 85 years) was derived from the specified free influenza vaccination data sheet and the immunization administration registry, which provided influenza vaccine uptake data, including NID, gender, birth date, vaccine name, and vaccination date vaccination. The admission data set (a total of 91113 discharges diagnostic records, 19083 patients aged 55 to 85 years and hospitalized during influenza season) comprised the healthcare data of all hospitals and community health centers in Ningbo region. Key variables of each record include NID, gender, birth date, discharge date, and discharge diagnosis name along with the International Classification of Diseases 10th Revision (ICD10). Mortality data set (a total of 2545 individuals aged 55 to 85 years and died during influenza season) was derived from the death surveillance information system which included identity information, sex, birth date, medical certificates of the death and the ICD10 code of leading cause. The electronic health archive data set (a total of 287770 individuals aged 55 to 85 years; age is computed as age on 31 December 2020) was used to define the denominator of all study populations who meet the inclusion criteria. We linked the various data sets using the unique NID number to select the permanent household residents.
Vaccination rate was calculated by age using the integrated data from vaccination data set and electronic health archive data set. For each hospital admission record, if an outcome was extracted in discharge records, we identified the cause of admission by the ICD10 code: any cause, including all hospital admission records; pneumonia and influenza, including ICD-10 codes of J09 to J18; respiratory disease, including J00 to J99; circulatory disease, including I00 to I99; respiratory or circulatory disease, including J00 to J99 and I00 to I99. For each mortality record, we identified the above categories of mortality outcomes by the ICD10 code of leading cause of death. We calculated the admission and mortality rates at the month level for each month-of-birth cohort, using the panel data of electronic health archive data set integrated with the admission data set or mortality data set, respectively. The denominator of each birth cohort was the mean number of persons who were alive in a particular month-of-birth.
Study design
Regression discontinuity design (RDD), a kind of quasi-experimental design, has consistently been demonstrated to closely approximate the findings of RCTs,Citation26,Citation27 and can provide valuable information on the overall effects (or impacts) of vaccination programs in real-world settings, vaccine safety, the effectiveness of interventions to increase vaccine uptake.Citation16,Citation28–31 The rationale of RDD is that an assignment variable is used to determine whether or not an individual is assigned to receive treatment or exposure (exposure variable) based on a threshold rule. Individuals just above the threshold are expected to be similar in their distribution of measured and unmeasured baseline covariates to individuals just below the threshold, emulating in the effect of random grouping. Two criteria should be met when using this method: 1) the exposure variable has an apparent jump at the threshold; 2) the relation between the outcomes and assignment variable at the threshold is smooth, before the start of an actual study. RDD divides into sharp RDD and fuzzy RDD according to whether the assignment variable completely determines the exposure probability, and the latter is analogous to the noncompliance setting of RCT within a small local range of the assignment variable.
In this study, we used the two-stage fuzzy RDD since age was not the only determinate of vaccination status. First, we plotted age profile with vaccination rate using the local polynomial regression model. Second, we tested for discontinuous changes in hospital admission and mortality at 70 years to identify the impact of elevation in vaccination rate at 70 years on these outcomes. Finally, we converted these coefficients into a percentage term relative to baseline admission or mortality rates at age of 69 to calculate the effectiveness of the vaccine and their 95% confidence intervals (CI). Additional details on this computation refer to the description of methods by Michael and colleagues.Citation14
Statistical analysis
For the free-vaccination-policy effect, we plotted the relation of vaccination rate following the age profile using the local polynomial regression and estimated change of vaccination rate by controlling an indicator variable for age 70 years and older, a quadratic in age, an interaction between that quadratic and the indicator for age 70 years and older. We restricted the sample to a bandwidth of 10 years of age around the age-70 threshold (that is, individuals aged 60 to 80 years) and then checked the robustness of using various alternative bandwidths. The regression was weighted by the number of individuals of that age.
The regression discontinuity analysis was used to test whether the increase in vaccination rate was associated with a corresponding decrease in admission and mortality rates. The regression fitted the admission and mortality rate of month-of-event level (dependent variables) with a month-of-birth indicator, an indicator of age 70 years and above, a quadratic indicator and an interaction between that quadratic and the age indicator. We restricted the sample to a bandwidth of 5 years of age around the age-70 threshold (that is, individuals aged 65 to 75 years) and then checked the robustness of using alternative bandwidths. The regression is weighted by the number of individuals of that month-of-age. This method was analogous to taking the age-70 discontinuity as an instrument variable to identify the causal effect of vaccination. Besides, we conducted sensitivity analyses to examine the validity of RDD using the linear regression model (exclude quadratic of age). The effectiveness of vaccination was calculated as the change in admission or mortality rate at the age-70 threshold divided by the change in vaccination rate when turning age 70 years. Because some individuals aged below 70 years received the influenza vaccine, the raw admission or mortality rate underestimated the true admission rate or mortality rate for unvaccinated persons in this age range when computing confidence interval boundaries. Therefore, the actual admission or mortality rate below 70 years was defined that the raw rate subtracted the vaccination rate below age 70 multiplied by the raw effect estimate, with the assumption that the vaccination effect is similar for vaccinated persons near the age of 70 years.Citation14 We found that the optimal bandwidth of age was 3.8 years for hospital admission, and 4.3 years for mortality. Whether the 95% CI of the estimate of vaccination effect includes the null value was used to determine whether it is statistically significant. Data management and data link were performed using the Hive SQL. All data analyses were conducted using Stata SE, Version 15.0 (Stata Corp).
Results
Change of influenza vaccination rate at age of 70 years
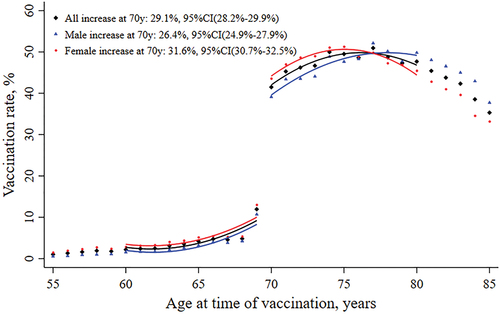
At age of 70 years, the seasonal influenza vaccination rate increased by 29.1% (95% CI 28.2%–29.9%) in the study population (). Changes in vaccination rate were higher in women (31.6%, 95% CI 30.7%–32.5%) than in men (26.5%, 95% CI 24.9%–27.9%). The increase in vaccination rate at age of 70 years was jumped discontinuously in the selected individuals aged 60–80 years, and the regression function showed sufficiently flexible to fit the age profiles (). The estimate of the variation in the percentage of vaccination at age 70 was robust against the bandwidth of age with years (Suplementary Figure S1).
Table 1. The change of influenza vaccination rate at age 70 years.
Change of hospital admission at age of 70 years
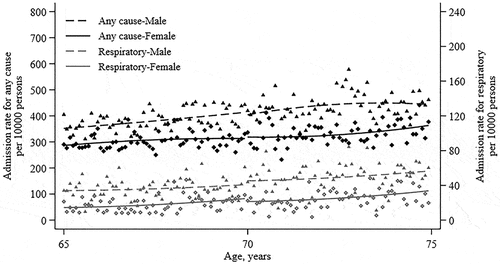
There was a higher hospital admission rate in men than women and the rate increased with age; meanwhile, the age profiles of admission for any cause and for respiratory appeared smooth across the age threshold of 70 years in both sexes (). Hospital admission rates did not change significantly at the age of 70 years (). At age of 70 years, the admission rate for any cause changed by −8.8 (95% CI, −20.2 to 4.7) per 10000 persons compared with the baseline rate of 368.4. The overall effectiveness of the decrease in hospital admission was estimated to be 8.2% (95%CI, −36.8 to 51.3). Further, the admission rate for pneumonia or influenza, respiratory, circulatory, and respiratory or circulatory changed by −2.0 (95% CI, −8.2 to 4.1) per 10000 persons, −3.4 (95% CI, −18.2 to 11.5) per 10000 persons, −17.3 (95% CI, −50.7 to 16.0) per 10000 persons, and −18.1 (95% CI, −47.6 to 13.5) per 10000 persons compared with the baseline rate of 6.8, 33.7, 145.3, 158.3, respectively. When we changed the bandwidth with years around the age-70 threshold, the changes of admission rate were consistent with the primary analyses (Supplementary Figure S2). Taking the vaccination rate into account, the potential effectiveness of receiving seasonal influenza vaccine was 8.2% (95% CI, −36.8% to 51.3%) for total admission, 8.3% (95% CI, −40.4% to 69.2%) for pneumonia and influenza admissions, 23.8% (95% CI, −16.9% to 67.5%) for respiratory admission, 30.2% (95% CI, −27.8% to 83.4%) for circulatory admission, and 31.5% (95% CI, −25.7% to 78.3%) for respiratory or circulatory. Results were similar in both men and women for any category of admission. We did not find a strong difference when using a linear function to fit age (Suplementary Table S1).
Table 2. Effectiveness of influenza vaccination on hospital admission.
Change of mortality rate at age of 70 years
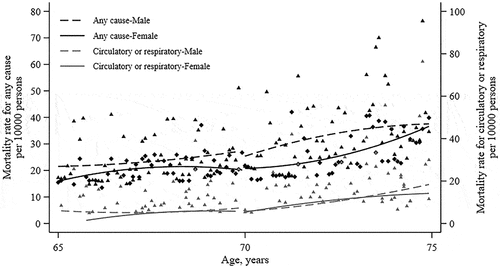
The age profiles of mortality for any cause and for respiratory or circulatory generally appeared smooth across the age threshold of 70 years in both sexes (). Mortality rate did not change significantly at age of 70 years (). At age of 70 years, the mortality rate for any cause and for respiratory or circulatory changed by −2.7 (95% CI, −16.4 to 9.9) per 10000 persons and −1.6 (95% CI, −10.9 to 7.8) per 10000 persons compared with the baseline rate of 14.5 and 3.0, respectively. The results were robust against various bandwidths with years around the age-70 threshold (Supplementary Figure S3). The evaluation of vaccine effectiveness was 13.1% (95% CI, −34.2 to 61.8) for the all-cause mortality, 20.1% (95% CI, −43.7% to 87.3%) for respiratory or circulatory death after retrimming the impact of influenza vaccination rate. There were opposite effects of receiving influenza vaccine on mortality between men and women, although the change also exhibited insignificant impact on the mortality in both genders (). Further, we conducted the sensitivity analyses using the linear fitting model for age, and no strong difference was found (Suplementary Table S2).
Table 3. Effectiveness of influenza vaccination on mortality.
Discussion
Our results found that the free vaccination program contributed to a 29.1% sharp increase in influenza vaccination rates at age of 70 years, which corresponded to a merely slight decrease in hospital admission and mortality. The influenza vaccination also presented certain protective effects on the overall admission rate and admission rate from specific diseases, as well as total mortality and mortality due to respiratory or circulatory disease. But there was no statistical significance in any of the above findings.
Many previous observational studies, including cohort and case-control studies as well as test-negative design studies, have reported that influenza vaccine can prevent seasonal influenza (VE, 12%–58%)Citation32–34 and reduce influenza-related hospitalization (VE, 24%–73%) and mortality (VE, 18%–56%) for the elderly.Citation35–39 Moreover, influenza vaccine also showed a protective effect against respiratory and cardiovascular disease. Some studies reported that influenza vaccination can reduce acute infections and hospitalizations for COPD, chronic bronchitis and asthma attacks,Citation40,Citation41 as well as lower the mortality risk in patients with COPD.Citation4,Citation6 For the elderly people, influenza vaccination was associated with a decrease of the acute cardiovascular events and cardiopathy-related mortality in patients with coronary heart disease, and reduce 18% all-cause death and 18% death from cardiovascular disease in patients with heart failure.Citation42 Nevertheless, our results showed that influenza vaccination appeared a weak VE for overall hospital admission and mortality, and no significance was found even there were a relatively high VE for the hospitalization and mortality of respiratory or circulatory disease Several reasons may be considered as follows. One explanation for these differences was that RDD based on observational data had a more conservative conclusion, due to the fact that selection and confounding bias can be controlled at the lowest level in the real-world settings.Citation16 Another factor that may help explain our results was immune-response attenuated with age.Citation43 The selected population at near age-70 years was older than the average age for most previous studies (usually threshold at age of 65 years). Moreover, due to the impact of the COVID-19 pandemic, the health behavior changes, e.g. wearing masks, keeping social distancing and restricting the number of people in the public areas, reduced the occurrence of influenza-like-illness, which may offset the effect of influenza vaccine. A recent study using the RDD method and General Practitioners Research Database (GPRD) revealed that the free influenza vaccination program did not bring the moderate effectiveness for severe outcomes in elderly people.Citation14 Although all results did not reach statistical significance in our study which was similar to the GPRD study, there were higher point estimates of VE for the hospitalization and mortality. Possible reasons, such as lower vaccination rates in the entire population (Suplementary Table S3) and ineffective herd immunity effect, might explain higher effect when a sharp increase of the vaccination rate at age 70 years in our study. Additionally, a relatively small sample size of analytical data set for the near age-70 years (admission data set or mortality data set converted to ecological panel data set) led to wider CIs compared with the GPRD study,Citation14 which simultaneously provided an explanation for the absence of an effect on mortality caused by influenza/pneumonia and respiratory.
Influenza vaccination for the elderly adults is recommended in most developed countries,Citation44–46 but only several developed areas or regions in China provided free influenza vaccination. A national cross-sectional survey showed that the overall influenza vaccination rate was 4.5% for Chinese urban population aged 60 years or older.Citation47 Even Yinzhou, a developed urban area in China, had a higher proportion (15.3% for population aged 60 or older after the implementation of the policy) of influenza vaccination than other most regions, the vaccination rate for the targeted elderly population was far lower than the WHO recommended 75%Citation48 and the vaccination rate for the developed countriesCitation49–52 like South Korea (75.6%), Australia (70.9%), United States (71.5%) and United Kingdom (70.8%). Therefore, low vaccination coverage showed a worse herd immunity. Furthermore, because the cross-epidemic of COVID-19 and influenza will bring a burden of cascading effect, it will complicate the differential diagnosis of COVID-19 cases in healthcare facilities and, in turn, increase the risk of COVID-19 transmission. Hence, taking free vaccination policy into account in the administrative plan is particularly important under the circumstance of the COVID-19 pandemic.
Although the first free influenza vaccination program was urgently implemented before 2020 2021 influenza season, the overall vaccine uptake rate is still not very high in the elderly. The following supplementary strategies, including improvement of vaccination services, promoting vaccination facilitation, strengthening the policy promotion and boosting the influenza vaccine deployment, could increase the vaccination coverage. Primary healthcare institutions should increase the number of primary influenza vaccination sites, appropriately early initiate vaccination process, extend of vaccination time, increase daily service duration to provide the convenient services for the old people. Some specific measures, such as centralized vaccination in the nursing home, making an appointment with Party and government organs and enterprises and public institutions for collective vaccination, should be considered to improve vaccination efficiency. The communities should step up the promotion of free influenza vaccination policy and enhance the public’s scientific understanding of influenza prevention. In addition, for CDC, it is necessary to assess the influenza vaccine demand, closely track of the status of vaccine procurement, supply, distribution and vaccination, and timely strengthen the dynamic deployment of influenza vaccine to ensure balanced vaccine supply at vaccination sites. On the basis of increasing vaccination rate, expanding the free policy to the children and others who are most likely to spread influenza, may also be necessary to address the high burden of influenza-related complications among older adults.Citation53
In this study, the results showed a different or contrary effectiveness of influenza vaccination on hospital admission and mortality between men and women. Potential reasons may be the immune-response heterogeneity, the discrepancy of health awareness, the willingness of medical consultations and the disease spectrum between the genders. In addition, a small sample size for panel data near the age threshold in both men and women is possible to lead to a large variation of point estimates and a wide range of CI from the point view of statistics. The study with a large sample size of panel data is necessary to validate and complete our findings.
The major strength of this study included the use of population-based electronic healthcare database and the utilization of RDD that allows mitigating selection and confounding bias, as well as unmeasured or unknown bias.Citation54 To the best of our knowledge, this is the first study that evaluates the effect of influenza vaccine using an electronic healthcare database in China. Some limitations should be considered. First, the inherent locality of RDD means that it can only explain the causal effect of those observations near the age of 70 years threshold, and it was difficult to generalize to the whole older population. Second, the medical certificates of death contained only the ICD10 codes for the primary cause of death. As a result, some causes of death cannot be captured, thus the effect of vaccination on a particular cause of death could not be fully assessed. Third, due to the data use policy that there was a data six-month cache period to ensure data security, we cannot access the latest data when conducting data clean and analysis in March 2022. Additionally, the age threshold of the free vaccination was set at 65 years and older in the next free programs (2021–2022, 2022–2023 influenza seasons), hence, the evaluation of the effectiveness of influenza vaccination had to included just one influenza season data (2020–2021 influenza season) in this study. A single season data resulted in a small sample size of ecologic panel data near the threshold and then led to a large confidence interval range. However, sensitivity analysis with similar results using linear regression could enhance the reliability of this study. Further studies that included the data of more than one influenza season are needed to validate and assess the more comprehensive and accurate effect of influenza vaccine for the elderly.
Conclusions
In summary, these findings revealed a limited effect on hospital admission and mortality for the free influenza vaccination program, which could be related to the low vaccination rate among the elderly. The supplementation strategies of expending immunization coverage and the future studies that included more influenza seasons data and multiple data source are needed to validate the effect of vaccination in the real-world setting, especially under the circumstances of the COVID-19 pandemic, and further provide a more scientific reference for the promotion of free influenza vaccination project to other cities in China.
Supplemental Material
Download MS Word (588.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data can be shared upon request to the corresponding authors in a collaborative research approach.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2115751.
Additional information
Funding
References
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1–9. doi:10.1016/S0140-6736(17)33293-2.
- Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson MA. Influenza cost and cost-effectiveness studies globally – a review. Vaccine. 2013;31(46):5339–5348. doi:10.1016/j.vaccine.2013.09.013.
- Li L, Liu Y, Wu P, Peng Z, Wang X, Chen T, Wong JYT, Yang J, Bond HS, Wang L, et al. Influenza-associated excess respiratory mortality in China, 2010–15: a population-based study. Lancet Public Health. 2019;4(9):e473–e81. doi:10.1016/S2468-2667(19)30163-X.
- Cheng Y, Cao X, Cao Z, Xu C, Sun L, Gao Y, Wang Y, Li S, Wu C, Li X. Effects of influenza vaccination on the risk of cardiovascular and respiratory diseases and all-cause mortality[J]. Ageing Res Rev. 2020;62:101124. doi:10.1016/j.arr.2020.101124.
- Pang Y, Wang Q, Lv M, Yu M, Lu M, Huang Y, Wu J, Xie Z. Influenza vaccination and hospitalization outcomes among older patients with cardiovascular or respiratory diseases[J]. J Infect Dis. 2021;223(7):1196–1204. doi:10.1093/infdis/jiaa493.
- Yedlapati SH, Khan SU, Talluri S, Lone AN, Khan MZ, Khan MS, Navar AM, Gulati MS, Johnson HS, Baum S, et al. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta‐analysis[J]. J Am Heart Assoc. 2021;10(6):e019636. doi:10.1161/JAHA.120.019636.
- Schembri S, Morant S, Winter JH, MacDonald TM. Influenza but not pneumococcal vaccination protects against all-cause mortality in patients with COPD[J]. Thorax. 2009;64(7):567–572. doi:10.1136/thx.2008.106286.
- O’Reilly J, Jones MM, Parnham J, Jones M, Rudolf M. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance[J]. BMJ. 2010;340:340. doi:10.1136/bmj.c3134.
- Troeger CE, Blacker BF, Khalil IA, Zimsen SRM, Albertson SB, Abate D, Abdela J, Adhikari TB, Aghayan SA, Agrawal S, et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017[J]. Lancet Respir Med. 2019;7(1):69–89. doi:10.1016/S2213-2600(18)30496-X.
- Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S, Thomas R, Rivetti A. Vaccines for preventing influenza in the elderly[J]. Cochrane Database Syst Rev. 2018;2020(2). doi:10.1002/14651858.CD001269.pub6
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337–344. doi:10.1093/ije/dyi274.
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366(9492):1165–1174. doi:10.1016/S0140-6736(05)67339-4.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi:10.1056/NEJMoa070844.
- Anderson ML, Dobkin C, Gorry D. The effect of influenza vaccination for the elderly on hospitalization and mortality: an observational study with a regression discontinuity design. Ann Intern Med. 2020;172(7):445–452. doi:10.7326/M19-3075.
- Van Ourti T, Bouckaert N. The Dutch influenza vaccination policy and medication use, outpatient visits, hospitalization and mortality at age 65. Eur J Public Health. 2020;30(2):275–280. doi:10.1093/eurpub/ckaa016.
- Lopez Bernal JA, Andrews N, Amirthalingam G. The use of Quasi-experimental designs for vaccine evaluation. Clin Infect Dis. 2019;68(10):1769–1776. doi:10.1093/cid/ciy906.
- Zhang L, Wang H, Li Q, Zhao MH, Zhan QM. Big data and medical research in China. BMJ. 2018;360:j5910. doi:10.1136/bmj.j5910.
- Yang Y, Zhou X, Gao S, Lin H, Xie Y, Feng Y, Huang K, Zhan S. Evaluation of electronic healthcare databases for post-marketing drug saf surveillance and pharmacoepidemiology in China. Drug Saf. 2018;41(1):125–137. doi:10.1007/s40264-017-0589-z.
- Ningbo Municipal Government. Elderly people aged70 years and older with permanent residence in Ningbo can receive free influenza vaccine; 2020.
- Lin H, Tang X, Shen P, Zhang D, Wu J, Zhang J, Lu P, Si Y, Gao P. Using big data to improve cardiovascular care and outcomes in China: a protocol for the Chinese Electronic health Records Research in Yinzhou (CHERRY) study. BMJ Open. 2018;8:e019698. doi:10.1136/bmjopen-2017-019698.
- Wang J, Bao B, Shen P, Kong G, Yang Y, Sun X, Ding G, Gao B, Yang C, Zhao M, et al. Using electronic health record data to establish a chronic kidney disease surveillance system in China: protocol for the China Kidney Disease Network (CK-NET)-Yinzhou study. BMJ Open. 2019;9(8):e030102. doi:10.1136/bmjopen-2019-030102.
- Lin CY, Li D, Lu JM, Yu ZB, Zhu Y, Shen P, Tang ML, Jin MJ, Lin HB, Shui LM, et al. Short-Term associations between ambient fine particulate matter pollution and hospital visits for chronic obstructive pulmonary disease in Yinzhou District, China. Environ Sci Pollut Res Int. 2020;27(17):21647–21653. doi:10.1007/s11356-020-08448-2.
- Huang K, Tao S, Zhou X, Mo J, Zhu B, Shen P, Lin H, Arena PJ, He N. Incidence rates of health outcomes of interest among Chinese children exposed to selected vaccines in Yinzhou electronic health records: a population-based retrospective cohort study. Vaccine. 2020;38(18):3422–3428. doi:10.1016/j.vaccine.2020.03.013.
- Zhou X, Lee EWJ, Wang X, Lin L, Xuan Z, Wu D, Lin H, Shen P. Infectious diseases prevention and control using an integrated health big data system in China. BMC Infect Dis. 2022;22(1):344. doi:10.1186/s12879-022-07316-3.
- Li H, Zhao H, Lin H, Shen P, Liu C, Zhan S. Utilization of intravenous ribavirin among reproductive age adults in 2010-2017: a population-based study in the Yinzhou district, Ningbo city of China. Front Public Health. 2021;9:678785. doi:10.3389/fpubh.2021.678785.
- Fretheim A, Soumerai SB, Zhang F, Oxman AD, Ross-Degnan D. Interrupted time-series analysis yielded an effect estimate concordant with the cluster-randomized controlled trial result. J Clin Epidemiol. 2013;66(8):883–887. doi:10.1016/j.jclinepi.2013.03.016.
- Fretheim A, Zhang F, Ross-Degnan D, Oxman AD, Cheyne H, Foy R, Goodacre S, Herrin J, Kerse N, McKinlay RJ, et al. A reanalysis of cluster randomized trials showed interrupted time-series studies were valuable in health system evaluation. J Clin Epidemiol. 2015;68(3):324–333. doi:10.1016/j.jclinepi.2014.10.003.
- Leitmeyer K, Buchholz U, Kramer M, Schenkel K, Stahlhut H, Köllstadt M, Haas W, Meyer C. Influenza vaccination in German health care workers: effects and findings after two rounds of a nationwide awareness campaign. Vaccine. 2006;24(47–48):7003–7008. doi:10.1016/j.vaccine.2006.04.040.
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. doi:10.1016/S0140-6736(14)60686-3.
- Amirthalingam G, Andrews N, Keel P, Mullett D, Correa A, de Lusignan S, Ramsay M. Evaluation of the effect of the herpes zoster vaccination programme 3 years after its introduction in England: a population-based study. Lancet Public Health. 2018;3(2):e82–e90. doi:10.1016/S2468-2667(17)30234-7.
- Lau WC, Murray M, El-Turki A, Saxena S, Ladhani S, Long P, Sharland M, Wong IC, Hsia Y. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–5079. doi:10.1016/j.vaccine.2015.08.022.
- Flannery B, Chung JR, Thaker SN, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, et al. Interim estimates of 2016–17 seasonal influenza vaccine effectiveness – United States, February 2017. MMWR Morb Mortal Wkly Rep. 2017;66(6):167–171. doi:10.15585/mmwr.mm6606a3.
- Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med. 2017;377(6):534–543. doi:10.1056/NEJMoa1700153.
- Dawood FS, Chung JR, Kim SS, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Martin ET, Belongia EA, et al. Interim estimates of 2019–20 seasonal influenza vaccine effectiveness – United States, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(7):177–182. doi:10.15585/mmwr.mm6907a1.
- Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–6033. doi:10.1016/j.vaccine.2013.09.063.
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951. doi:10.1016/S1473-3099(16)00129-8.
- Smetana J, Chlibek R, Shaw J, Splino M, Prymula R. Influenza vaccination in the elderly. Hum Vaccin Immunother. 2018;14(3):540–549. doi:10.1080/21645515.2017.1343226.
- Rolfes MA, Flannery B, Chung JR, O’Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis. 2019;69(11):1845–1853. doi:10.1093/cid/ciz075.
- Chung JR, Rolfes MA, Flannery B, Prasad P, O’Halloran A, Garg S, Fry AM, Singleton JA, Patel M, Reed C. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis. 2020;71(8):e368–e76. doi:10.1093/cid/ciz1244.
- Huang YD, Zhao XP, Wan T, Zhang S Effects of influenza vaccination in chronic obstructive pulmonary disease. Hai-Nan Med J. 2011;22:29–31.
- Vasileiou E, Sheikh A, Butler C, Ferkh KE, Wissmann B, McMenamin J, Ritchie L, Schwarze J, Papadopoulos NG, Johnston S. Effectiveness of influenza vaccines in Asthma: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(8):1388–1395. doi:10.1093/cid/cix524.
- Modin D, Jørgensen ME, Gislason G, Jensen JS, Køber L, Claggett B, Hegde S, Solomon S, Torp-Pedersen C, Biering-Sørensen T. Influenza vaccine in heart failure. Circulation. 2019;139(5):575–586. doi:10.1161/CIRCULATIONAHA.118.036788.
- Yung RL. Changes in immune function with age. Rheum Dis Clin North Am. 2000;26(3):455–473. doi:10.1016/S0889-857X(05)70151-4.
- Grohskopf LA, Alyanak E, Broder KR, Blanton LH, Fry AM, Jernigan DB, Atmar RL. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices – United States, 2020–21 influenza season. MMWR Recomm Rep. 2020;69(8):1–24. doi:10.15585/mmwr.rr6908a1.
- Trucchi C, D’Amelio M, Amicizia D, Orsi A, Loiacono I, Tosatto R, Piazza MF, Paganino C, Pitrelli A, Icardi G, et al. Lowering the recommended age for the free and active offer of influenza vaccination in Italy: clinical and economic impact analysis in the Liguria region. Hum Vaccin Immunother. 2021;17(5):1387–1395. doi:10.1080/21645515.2020.1810494.
- Seo J, Lim J, Yon DK. The impact of free vaccination policies under the Korean influenza national immunization program: trends in influenza vaccination rates in South Korea from 2010 to 2019. PLoS One. 2022;17(1):e0262594. doi:10.1371/journal.pone.0262594.
- Fan J, Cong S, Wang N, Bao H, Wang B, Feng Y, Lv X, Zhang Y, Zha Z, Yu L, et al. Influenza vaccination rate and its association with chronic diseases in China: results of a national cross-sectional study. Vaccine. 2020;38(11):2503–2511. doi:10.1016/j.vaccine.2020.01.093.
- World Health Assembly. Prevention and control of influenza pandemics and annual epidemics; 2003.
- Giese C, Mereckiene J, Danis K, O’Donnell J, O’Flanagan D, Cotter S. Low vaccination coverage for seasonal influenza and pneumococcal disease among adults at-risk and health care workers in Ireland, 2013: the key role of GPs in recommending vaccination. Vaccine. 2016;34(32):3657–3662. doi:10.1016/j.vaccine.2016.05.028.
- Karki S, Dyda A, Newall A, Heywood A, MacIntyre CR, McIntyre P, Banks E, Liu B. Comparison of influenza vaccination coverage between immigrant and Australian-born adults. Vaccine. 2016;34(50):6388–6395. doi:10.1016/j.vaccine.2016.10.012.
- Kwon DS, Kim K, Park SM. Factors associated with influenza vaccination coverage among the elderly in South Korea: the fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). BMJ Open. 2016;6(12):e012618. doi:10.1136/bmjopen-2016-012618.
- Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. doi:10.15585/mmwr.ss6501a1.
- Ward CJ. Influenza vaccination campaigns: is an ounce of prevention worth a pound of cure? Am Econ J Appl Econ. 2014;6:38–72.
- Nelson JC, Jackson ML, Weiss NS, Jackson LA. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol. 2009;62(7):687–694. doi:10.1016/j.jclinepi.2008.06.014.