ABSTRACT
To evaluate the safety of the 15-valent pneumococcal conjugate vaccine (PCV15 (by LvZhu & Co. Ltd)) in healthy infants aged 2 months (minimum to 6 weeks) and 3 months old. This phase I clinical trial enrolled 80 subjects in Laishui County, Hebei Province, China. The total population was divided into 4 age groups on average: 20 adults (≥18 years) and 20 children (1–5 years) all received one vaccine dose; 20 infants (3 months) received the vaccine according to a 3-dose schedule at 0, 1, and 2 months. Twenty infants (2 months, minimum of 6 weeks old) received the vaccine according to a 3-dose schedule of 0, 2, and 4 months. The adverse events (AEs) until 30 days after each dose and serious adverse events (SAEs) until 6 months after the whole dose were reported. The solicited and unsolicited AE frequencies and laboratory indices were similar among the treatment groups. No vaccine-related SAEs were reported. Most vaccine-related adverse events consisting of systemic and local reactions were fever and pain. One hypersensitivity manifested as systemic urticaria that occurred on the third day after the second dose in the 2-month group. The 15-valent pneumococcal conjugate vaccine was generally well tolerated in infants.
1. Introduction
Streptococcus pneumoniae is the leading cause of community-acquired pneumonia (CAP) in all age groups in both developing and developed countries.Citation1,Citation2 Pneumococci can also cause invasive infections, such as pneumonia with empyema or bacteremia, febrile bacteremia and meningitis, which are the most common invasive pneumococcal diseases (IPDs).Citation3 According to the World Health Organization (WHO), more than 1.2 million people die from pneumococcal infections each year, and most of them occur in developing countries. Children under 2 years make up the population that is most susceptible to pneumococcal disease, with a particularly high fatality rate.Citation4,Citation5 Several studies have reported that mortality and hospitalization rates, particularly for pneumonia, are higher in older adults than in younger adults.Citation6–8 Currently, vaccination is the most effective way to prevent pneumococcal disease.
At present, only a few of conjugate vaccines are in the commercialized pneumococcal conjugate vaccines (PCVs). The first available vaccine against this microorganism was the pneumococcal polysaccharide vaccine (PPSV23) which was found not to be very effective in the children’s population, and in the adult population the results were found to be discordant.Citation9,Citation10 Since 2001, a 7-valent pneumococcal conjugate vaccine (PCV7) targeting seven serotypes (4, 6B, 9 V, 14, 18C, 19F, and 23F),Citation11 PCV10 (PCV7 serotypes plus 1, 5, and 7F) (21–23) and PCV13 vaccines (PCV10 serotypes plus 3, 6A, and 19A) Citation12,Citation13 was successfully developed and became commercially available, highly effective in children.Citation11 In addition, a significant increase of nonvaccine serotypes was observed.Citation14 PCV20 and PCV15 were both licensed by the Food and Drug Administration in 2021, but just for adults. At present, pneumococcal polysaccharide vaccines (e.g., 23-valent pneumococcal polysaccharide vaccines) are used for children aged 2 years and older, pneumococcal conjugate vaccines (e.g., 13-valent pneumococcal conjugate vaccines) are used for infants aged under 2 years internationally.Citation6,Citation15–21 As observed in PCV7 and PCV10, incorporation of the PCV13 vaccine appeared to be very effective in decreasing the number of IPD cases caused by serotypes included in the vaccine in all of the age groups (12). Still, there was an unforeseen increase of non-PCV13 serotypes causing IPD. These facts call for the incorporation of more serotypes in future vaccines.
The new vaccine used in our research has been shown to be safe and highly immunogenic in animals and was approved for human trials by the China Medical Products Administration. Each dose of this vaccine contains 15 pneumococcal serotypes, including types 1, 2, 3, 4, 5, 6A, 6B, 7F, 9 V, 12F, 14, 18C, 19A, 19F, and 23F. In addition to the serotype of the pneumococcal 13-valent conjugate vaccine mentioned above, types 2 and 12F were added. These two serotypes are more common in Asia and prevalent in China.Citation22 The covered serotypes can account for more than 85% of the serotypes isolated from pneumococcal cases under 5 years of age in 70 countries,Citation22 providing more widespread protection for the population against Streptococcus pneumoniae infection. Here, we report the findings from a phase I clinical trial conducted in China on a vaccine candidate.
2. Methods
2.1. Study design and participants
This study was a single-center, non-controlled phase I clinical trial. Healthy adults aged >18 years, children aged 1–5 years, and healthy infants aged 3 months and 2 months old (minimum of 6 weeks) living in Laishui County, Hebei Province, China, were enrolled. Subjects were excluded if they were allergic to diphtheria toxoid and any other vaccinations or drugs; were immunocompromised; had severe congenital malformations, developmental disorders, severe chronic disease, severe cardiovascular disease, liver and kidney disease, malignant tumors, skin disease, serious respiratory disease or any acute infection; had a history of invasive disease caused by Streptococcus pneumoniae confirmed by a bacterial culture; had a history or family history of psychiatric illness, convulsions, seizures, or encephalopathy; had a history of asphyxia, nerve organ damage, pathological jaundice, or coagulopathy; or received blood products or immunoglobulins within 3 months prior to enrollment (hepatitis B immunoglobulin is acceptable). In addition, infants (<1 year old) with a birth weight <2.5 kg, pregnant or lactating women, and adults (>18 years old) with an abnormal laboratory collection indicator (blood, urine, or biochemical) were also excluded.
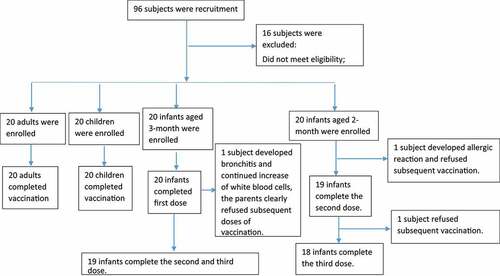
Eighty subjects aged over 2 months (minimum of 6 weeks) were divided into 4 age groups on average: 20 adults and 20 children all received one vaccine dose; 20 infants (aged 3 months) received the vaccine according to a 3-dose schedule of 0, 1, and 2 months. It should be shaken well and intramuscular injection. The injection site is anterolateral area of the thigh for infants, the deltoid muscle of the upper arm for adults and children. The order of inclusion was adult group, then children group and then infant group. First, 20 adults (aged over 18 years) received one dose of vaccine if the safety of this vaccination was confirmed after 7 days of observation. Then, 20 children (aged 1–5 years) started to receive one dose of vaccine. After 7 days of safety observation, infants aged 3 months and 2 months old (minimum of 6 weeks old) received the vaccine according to a 3-dose schedule of 0, 1, and 2 months and 0, 2, and 4 months, respectively. The researchers assessed the safety findings and approved enrollment at every stage in the absence of contraindications until the vaccination of all dose groups was completed.
This study was approved by the Institutional Review Board of the Hebei Center for Disease Control and Prevention and was conducted in compliance with Good Clinical Practice, the Declaration of Helsinki, and local regulations in China. Parents/guardians of eligible study participants or the subjects themselves signed written informed consent forms before any study-related procedures were performed. The clinical trial is registered at the National Medical Products Administration (NMPA) under number CRT20150878.
2.2. Vaccines
The experimental vaccine used in the study was the 15-valent pneumococcal conjugate vaccines (1, 2, 3, 4, 5, 6A, 6B, 7F, 9 V, 12F, 14, 18C, 19A, 19F, and 23F) manufactured by Zhifei Lvzhu Biopharmaceutical Co., Ltd., Beijing, People’s Republic of China. Vaccine lot number was 20,110,401. Strains were obtained by culturing fifteen species of pneumococcus. Pneumococcal polysaccharide was dissolved, hydrolyzed by acetic acid solution, and oxidized by sodium periodate to obtain oxidized polysaccharide. Adipic hydrazide and Sodium cyanoborohydride were added to the oxidized polysaccharide to prepare derivatives. The derivative and DT were mixed in a certain proportion, and added the EDAC to prepare pneumococcal conjugate. The pneumococcal conjugate was purified by chromatography column and filtered by removing bacteria to get the stock solution of pneumococcal polysaccharide conjugate. PCV15 was prepared by adding aluminum phosphate adjuvant to the stock solution of different types of conjugates. Following extraction and purification, the capsular polysaccharide was obtained, combined with carrier protein, adsorbed by aluminum phosphate adjuvant, and prepared as a milky white uniform suspension to obtain this experimental vaccine. The strains used in the production of pneumococcal conjugate vaccine were obtained from the Chinese Medical Bacterial Preservation and Management Center and were consistent with the relevant provisions of the Management Regulations of Strains used in the Production and Verification of Biological Products in The Chinese Pharmacopoeia (2015 Edition iii). The active component of this vaccine was the conjugate of pneumococcal polysaccharide and diphtheria toxoid. The ingredients were aluminum phosphate adjuvant and sodium chloride. Each dose of vaccine consisted of 0.5 ml, with 2 μg of 15 pneumococcal polysaccharides (6B = 4 μg) and 30 75 μg of diphtheria toxoid.
2.3. Assessment of safety
Subjects were observed for 30 min to monitor for any immediate adverse events (AEs) after the administration of the vaccine. Thereafter, the subjects were given a thermometer and a diary card covering days 0–7 for safety follow-up. They were instructed to observe and record their axillary temperature daily and any AEs on the card for 7 days. The subjects were asked to return to the research site on day 8 after vaccination. Thereafter, contact cards were given to the subjects to collect the safety information for days 8–30. Serious adverse events (SAEs) were collected 6 months after receiving the full course of vaccination. Additionally, the laboratory indices of healthy adults were determined before the first dose and 4 days after vaccination, including routine blood, blood biochemical and urine tests. The collected indicators were routine blood (white blood cell count, hemoglobin, and platelet count), biochemical blood (glutamic-pyruvate transaminase, glutamic-oxaloacetic transaminase, creatinine, total bilirubin, direct bilirubin, and indirect bilirubin), and routine urine measurements (urine protein, urine glucose, and red blood cell count). The normal range of indicators would refer to the corresponding index testing standards of the laboratory of the testing hospital. Systemic and local reactions after vaccination were determined in accordance with the “Guiding Principles for the Classification Standards of Adverse Reactions in Clinical Trials of Prophylactic Vaccines” issued by the State Medical Products Administration.
2.4. Statistical analysis
All the analyzes were performed using SAS version 9.4. All the statistical tests were two-sided, and differences resulting in p values of 0.05 or less were considered statistically significant. Student’s t test or the Mann-Whitney U test was used to assess dimensional outcomes, and the chi-square or Fisher’s exact test was used to analyze dichotomous outcomes.
3. Results
3.1. Demographic and other baseline characteristics
In total, 96 volunteers participated in interviews and physical examinations for this trial. Of these volunteers, 16 were excluded due to a failure to sign the informed consent form, meeting the exclusion criteria, and other reasons. Eighty subjects (20 adults, 20 children, 20 infants aged 3 months and 20 infants aged 2 months old) were enrolled in the trial (). All the subjects in the adult and child groups completed the follow-up. One infant aged 3 months and 2 infants aged 2 months old were lost to follow-up, resulting in a final total of 95% (19/20) of infant participants aged 3 months old and 90% (18/20) of infant participants aged 2 months old who received three doses of the vaccine. shows the demographic characteristics of these 4 groups.
Table 1. Summary of demographic characteristics.
3.2. Safety
A summary of the AEs (including solicited and unsolicited AEs) reported during the 0–30-day postvaccination period in the whole group is provided in . Similar incidence rates of solicited AEs were observed in the adult (65%) and the toddler groups (50%). No grade 3 solicited AEs or SAEs were reported in the adult group except for 1 grade 3 vaccination-related solicited AE, which was reported to have a high increased white blood cell count on the fifth day after the first dose of the vaccine. Most vaccine-related adverse events consisting of systemic and local reactions in adults and toddlers were fever and pain, respectively (). Redness, pruritus and swelling at the site of inoculation were all reported in the toddler group. In addition, two grade 3 vaccination-related solicited AEs were reported in the pediatric group; one subject showed swelling 2 days after inoculation, and their temperature reached 39.8°C 6 days after vaccination. The other showed a temperature that reached 39.2°C 1 day after vaccination. Among these changes in the hematology, biochemistry, and urine analysis results, three adults presented abnormalities (one with an abnormal routine blood analysis, two with an abnormal urine routine analysis), and no children presented abnormalities.
Table 2. Summary of adverse events (AEs) in the adult, toddler, and infant cohorts.
Table 3. Summary of vaccination-related adverse events (AEs) in the adult and toddler cohorts.
A summary of vaccination-related adverse events (AEs) in the infant cohort is listed in . In total, 33 (82.5%) infant participants reported at least one AE within 30 days following any vaccination, 16 (80%) in the 3-month group, and 17 (85%) in the 2-month group. At least one solicited AE was recorded in 26 infants (65%) among the whole group. Twelve infants (60%) were from the 3-month group, and 14 infants (70%) were from the 2-month group. No vaccination-related serious adverse events (SAEs) were reported, and all vaccination-related adverse events in the infant group were solicited. The main symptom was fever (11 cases at 3 months old and 12 cases at 2 months old), which primarily occurred after the first dose. The secondary symptom was diarrhea, which occurred after 3 doses. There were 1 case of anorexia and 1 case of nausea/vomiting in the 3-month-old group, all of which occurred after the first dose of the inoculation. Local reactions such as pain (1 case), redness (1 case), swelling (1 case) and induration (1 case) all occurred after the first dose of inoculation. There were two grade 3 adverse events in the 3-month-old group, including diarrhea and redness, which occurred after the first and third doses of inoculation, respectively. There were no local adverse reactions in the 2-month-old group, but hypersensitivity occurred, which manifested as systemic urticaria on the third day after the second dose. There were three serious adverse events in the infant group, which were all diagnosed as infectious pneumonia and were unrelated to vaccination.
Table 4. Summary of vaccination-related adverse events (AEs) in the infant cohort.
4. Discussion
Numerous data demonstrate that in infants, pneumococcal conjugate vaccines (PCVs) are highly effective against invasive pneumococcal disease (IPD) and reduce the risk of all-cause pneumonia.Citation20,Citation23,Citation24 As of May 2017, PCV13 had been incorporated into the routine pediatric immunization schedules of > 100 countries. The continued use of PCVs in national immunization programmes has been supported by high-quality national surveillance systems documenting reductions in invasive pneumococcal disease (IPD) incidence.Citation25–29 In older adults, PCVs have shown efficacy against vaccine-type community-acquired pneumonia and invasive disease as well as effectiveness against hospitalized vaccine-type community-acquired pneumonia.Citation30–32 The development of novel PCVSs that cover more serotypes and are more suitable for the Asian population is crucial. At present, pneumococcal polysaccharide vaccines are used for children aged 2 years and older, and pneumococcal conjugate vaccines (e.g., 13-valent pneumococcal conjugate vaccines) are used for infants aged under 2 years. PCV20 (Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.) and PCV15 (Merck Sharp & Dohme Corp.) were both licensed by the Food and Drug Administration just for adults aged ≥18 years.
This phase I clinical trial is the first study to evaluate the safety and immunogenicity of PCV15 (types 2 and 12F were added to PCV13) in healthy adults, toddlers, and infants. According to the requirements of clinical trials, studies to explore vaccine safety should be conducted in a strict sequence from adult to child to infant due to low infant tolerance to adverse reactions. The safety of the vaccine was first evaluated in healthy adults and toddlers to provide sufficient safety data to allow the study to proceed in the target population of infant subjects. This new vaccine used in our research is not indicated for adults and toddlers; therefore, only a single dose of vaccine was assigned to evaluate safety.
This study showed that the most common vaccination-related AEs in both the adult and toddler groups were fever and injection-site pain. PCV15 is well tolerated with an overall safety profile comparable to PCV13 in adults 18 years of age and older reported in a phase III trial of another PCV15 (types 22 F and 33F were added to the PCV13) compared with PCV13 in adults 50 years of age and older. These data are consistent with previous reports for PCV vaccinations in adults.Citation8 The majority of vaccination-related adverse events were reported as mild in severity and of short duration, except fever and myalgia in the adult group. In the current study, 1 grade 3 vaccination-related solicited AE was reported, with a high increased white blood cell count appearing on the fifth day after the first vaccine dose. In addition, a total of three adults presented abnormalities in their blood and urinary routine index, which were not reported in other studies. For children aged 1–5 years old, the most common vaccination-related AEs were injection site local reactions, such as pain, pruritus, redness, swelling and induration, but all were reported as mild in severity. No abnormal changes in the hematology, biochemistry, or urine analysis results were reported in the toddler group.
In the target population of infant subjects, the most commonly solicited AE was fever in both the 3-month and 2-month groups, which primarily occurred after the first dose. In addition, gastrointestinal symptoms, such as anorexia, nausea and diarrhea, were reported in the 3-month group and primarily occurred after the first dose; no other systemic reactions, such as drowsiness, myalgia or allergies, were reported. In the 2-month group, only mild diarrhea and twitchiness were reported. The main local symptoms were pain, redness, swelling and induration at the vaccination site in the 3-month-old group, which were not reported in the 2-month-old group. Overall, most local reactions and systemic events were of mild or medium intensity and typical of any injected vaccine, which was consistent with one meta-analysis exploring the safety and immunogenicity of PCV13 in infants.Citation33 The differences between age groups were not statistically significant. However, the meta-analysis only evaluated the safety of vaccines for the first dose in the infant series, which was different from this study. It is remarkable that one infant aged 2 months old had grade 3 hypersensitivity, which manifested as systemic urticaria on the third day after the second dose, which was not reported in other PCVs. Correspondingly, no cases of death were reported in the study.
The inability to the immunogenicity assessment may be due to the limited sample size. Additionally, ` probability of capturing rare adverse AEs, so a phase III trial is underway for further appraisal to evaluate its safety, immunogenicity, and efficacy. In addition, the co-administration of the study vaccine with other vaccines could have affected the assessment of the safety and immunogenicity of the study vaccine, so further study on simultaneous administration must be continued.
4.1. Clarification for the roles of the authors
For this phase I clinical trial, Zhao Yu-Liang, the corresponding author, and the coauthors of Pan Lu-Lu, Gao Zhao, Zhou Wei-Wei, Li Min-Jie, Ji Wen-Juan prepare of the field trial study scheme, organize the implementation, monitoring the assigned field trial following company authorized SOPs and in accordance with GCP, collect and analyses experimental data and draft this manuscript. Coauthors of Fang Wen-Jian and Zhao Ying are currently employees of Zhifei Lvzhu Biopharmaceutical Co., Ltd, the manufacturer of the vaccine. They participate in drafting the vaccine clinical trial protocols and provide technical guidance for researchers.
Institutional review board statement
The study protocols were approved by the Institutional Review Board of Hebei Center for Disease Control and Prevention and conducted in compliance with Good Clinical Practice, the Declaration of Helsinki, and local regulations in China.
Acknowledgement
We greatly appreciate all the researchers who were involved in this clinical trial and the participants who participated this study and their parents or legal guardians.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Hamborsky J, Kroger A, S W. Centers for disease control and prevention. epidemiology and prevention of vaccine-preventable diseases. Washington D.C: Public Health Foundation; 2015.
- Pick H, Daniel P, Rodrigo C, Bewick T, Ashton D, Lawrence H, Baskaran V, Edwards-Pritchard RC, Sheppard C, Eletu SD, et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013–18. Thorax. 2020;75(1):1–7. doi:10.1136/thoraxjnl-2019-213725. PMID: 31594801.
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, et al. Burden of disease caused by streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6. PMID: 19748398.
- Well-Olivier C, Van der Linden M, Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era. A European perspective. BMC Infect Dis. 2012;12(1):207. doi:10.1186/1471-2334-12-207. PMID: 22954038.
- World Health Organization. Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec. 2012;87(14):129–144. PMID: 24340399.
- Niederman MS, Folaranmi T, Buchwald UK, Musey L, Cripps AW, Johnson KD. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and non-invasive pneumococcal disease and related outcomes. A review of available evidence. Expert Rev Vaccines. 2021;20(3):243–256. doi:10.1080/14760584.2021.1880328. PMID: 33478306.
- Simonetti AF, Viasus D, Garcia-Vidal C, Carratalà J. Management of community-acquired pneumonia in older adults. Ther Adv Infect Dis. 2014;2(1):3–16. doi:10.1177/2049936113518041. PMID: 25165554.
- Platt HL, Cardona JF, Haranaka M, Schwartz HI, Narejos Perez S, Dowell A, Chang CJ, Dagan R, Tamms GM, Sterling T, et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults 50 years of age and older (PNEU-AGE). Vaccine. 2022;40:162–172. doi:10.1016/j.vaccine.2021.08.049. PMID: 34507861.
- Redin A, Ciruela P, de Sevilla MF, Gomez-Bertomeu F, Gonzalez-Peris S, Benitez MA, Trujillo G, Diaz A, Jou E, Izquierdo C, et al. Serotypes and clonal composition of streptococcus pneumoniae isolates causing IPD in children and adults in Catalonia before 2013 to 2015 and after 2017 to 2019 systematic introduction of PCV13. Microbiol Spectr. 2021;9(3):e0115021. Dec 22. doi: 10.1128/Spectrum.01150-21. PMID: 34878302.
- Pitsiou GG, Kioumis IP. Pneumococcal vaccination in adults: does it really work? Respir Med. 2011;105(12):1776–1783. doi:10.1016/j.rmed.2011.07.008. PMID: 21816596.
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California kaiser permanente vaccine study center group. Pediatr Infect Dis J. 2000;19(3):187–195. doi:10.1097/00006454-200003000-00003. PMID: 12907008.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population based surveillance. Lancet Infect Dis. 2015;15(3):301–309. doi:10.1016/S1473-3099(14)71081-3. PMID: 25656600.
- Domínguez Á, Ciruela P, Hernández S, García-García JJ, Moraga F, de Sevilla MF, Selva L, Coll F, Muñoz-Almagro C, Planes AM, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7-59 months. A matched case-control study. PLoS One. 12:e018319115. doi:10.1371/journal.pone.0183191. PMID: 28806737.
- Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia J, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46:174–182. doi:10.1086/524660. PMID: 18171247.
- Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS One. 2017;12(1):e0169368. doi:10.1371/journal.pone.0169368. eCollection 2017. PMID: 28061505.
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D’Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. Bmj. 2010;340(1):c1004. doi:10.1136/bmj.c1004. PMID: 20211953.
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;(1):Cd000422. doi:10.1002/14651858.CD000422.pub2. PMID: 18253977.
- Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, Hospital I, Gomez-Bertomeu F, Raga X. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine. 2009;27(10):1504–1510. doi:10.1371/journal.pmed.1003326. PMID: 33095759.
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325(21):1453–1460. doi:10.1056/NEJM199111213252101. PMID: 1944423.
- Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccines Immunotherapeutics. 2013;9(12):2505–2523. doi:10.4161/hv.26109. PMID: 23955057.
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. doi:10.1056/NEJMoa035060. PMID: 14523142.
- O’Brien, KL. Pneumococcal regional serotype distribution for pneumococcal AMC TPP (Codebook to assess whether a pneumococcal vaccine meets the pneumococcal AMC target product profile for regional vaccine serotype coverage). Prepared by GAVI’s PneumoADIP. et al.11/30/2008.
- Black SB, Shineford HR, Ling J, Hansen S, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–815. doi:10.1097/00006454-200209000-00005. PMID: 12352800.
- French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362(9):812–822. PMID: 27895845.
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a post licensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–846. doi:10.1016/S1473-3099(14)70822-9. PMID: 25042756.
- Weinberger R, van der Linden M, Imöhl M, von Kries R. Vaccine effectiveness of PCV13 in a 3+1 vaccination schedule. Vaccine. 2016;34(18):2062–2065. doi:10.1016/j.vaccine.2016.02.043. PMID: 26920471.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4(5):399–406. doi:10.1016/S2213-2600(16)00052-7. PMID: 26987984.
- Pichichero M, Kaur R, Scott DA, Gruber W, Trammel J, Almudevar A, Center KJ. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. 2018;2(8):561–568. doi:10.1016/S2352-4642(18)30168-8. PMID: 30119715.
- Guevara M, Barricarte A, Torroba L, Herranz M, Gil-Setas A, Gil F, Bernaola E, Ezpeleta C, Castilla J. Direct, indirect and total effects of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children in Navarra, Spain, 2001 to 2014: cohort and case-control study. Euro Surveillance. 2016;21(14). doi:10.2807/1560-7917.ES.2016.21.14.30186. PMID: 27103428.
- Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. doi:10.1056/NEJMoa1408544. PMID: 25785969.
- McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018;67(10):1498–1506. doi:10.1093/cid/ciy312. PMID: 29790925.
- Cannon K, Elder C, Young M, Scott DA, Scully IL, Baugher G, Peng Y, Jansen KU, Gruber WC, Watson W, et al. A trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in populations of adults≧65 years of age with different prior pneumococcal vaccination. Vaccine. 2021;39(51):7494–7502. doi:10.1016/j.vaccine.2021.10.032. PMID: 34839993.
- Ruiz-Aragón J, Márquez Peláez S, Molina-Linde JM, Grande-Tejada AM. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in infants: a meta-analysis. Vaccine. 2013;31(46):5349–5358. doi:10.1016/j.vaccine.2013.09.008. PMID: 24055349.