ABSTRACT
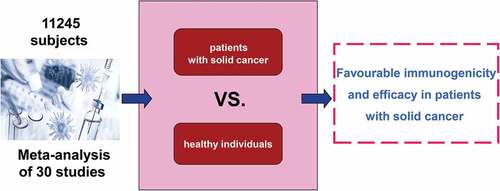
Patients with solid cancer have an increased risk of severe coronavirus disease 2019 (COVID-19) and associated mortality than the general population. This meta-analysis aimed to investigate the currently available evidence about the efficacy of COVID-19 vaccines in patients with solid cancer. We included prospective studies comparing the immunogenicity and efficacy of COVID-19 vaccines between patients with solid cancer and healthy individuals. Relative risks of seroconversion after the first and second dose of a COVID-19 vaccine were separately pooled with the use of random effects meta-analysis. Thirty studies with 11,245 subjects met the inclusion criteria. After first vaccine dose, the pooled RR of seroconversion in patients with solid cancer vs healthy individuals was 0.54 (95% CI 0.38–0.78, I2 = 94%). After a second dose, the pooled RR of seroconversion in patients with solid cancer vs healthy controls was 0.87 (0.86–0.88, I2 = 87%). Our review suggests that, compared with healthy individuals, COVID-19 vaccines show favorable immunogenicity and efficacy in patients with solid cancer. A second dose is associated with significantly improved seroconversion, although it is slightly lower in patients with solid cancer compared with healthy individuals.
Graphical abstract
Introduction
Transmission of SARS-CoV-2 has led to the ongoing global COVID-19 pandemic. By July 2022, more than 518 million have had confirmed COVID-19 and more than six million have died worldwide. In addition to large-scale economic disruption, COVID-19 has caused various manifestations in multiple organ systems,Citation1–5 and has increased disease severity and mortality in patients with solid cancer.Citation6–11
Among the proposed novel treatment strategies,Citation12–17 vaccination is the most effective strategy for preventing SARS-CoV-2 infection.Citation18 Fortunately, a concerted global effort has prompted an unprecedented pace in several highly effective vaccines development.Citation19–21 All of these vaccines were well tolerated in clinical trials and their proven efficacy was greater than 90% in preventing symptomatic laboratory-confirmed SARS-CoV-2 infection, except for the CoronaVac vaccine, which only had proven efficacy of 51%.Citation22–25 In many parts of the world, mass vaccination campaigns have considerably reduced the incidence of severe COVID-19 in the general population after at least two vaccine doses. Vaccine trials, however, have excluded patients with solid cancer, leading to a paucity of data on the efficacy and safety of currently available vaccines as well as the durability of vaccine responses remain in this population. Owing to the increased risk of COVID-19-related complications and mortality, patients with solid cancer were prioritized for vaccination.Citation26–28 These patients, which comprise only a minority of the global population, are of particular interest because of possible suppression or over-activation of the immune system attributable to the primary disease or concurrent treatment.Citation29 Data are urgently needed on patients with solid cancer, as infection and viral shedding have been reported to be more severe and persistent in this group.Citation30–32
This systematic review and meta-analysis aims to integrate the currently available evidence to assess the serologic response rate of COVID-19 vaccines in patients with solid cancer. Better understanding the overall efficacy of COVID-19 vaccines in solid cancer patients can improve clinical practice and protect this vulnerable patient group.
Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Citation33
Search strategy and selection criteria
A comprehensive electronic search (from inception to 30 May 2022) of PubMed/Medline, EMBASE, the Cochrane Library database, COVID-19 Open Research Dataset Challenge (CORD-19), and WHO COVID-19 databases was conducted to identify studies assessing the response to COVID-19 vaccination in patients with solid cancer.
We included prospective studies reporting the outcomes of COVID-19 vaccination in patients with solid cancer. No geographic or language restrictions were imposed. There were no restrictions regarding age, sex, or duration of the study. Studies reporting outcomes in patients with active or history of cancer were eligible. The databases were searched (JY) using the mapped terms [“cancer” OR “tumor” OR “malignancy”] AND “vaccine” AND [“COVID-19” or “SARS-CoV-2”] and the exploded MeSH terms “COVID-19 Vaccines.” To improve the validity of data, we excluded non-peer reviewed articles in preprint databases. The reference lists of all included articles were also manually searched to identify any potentially eligible studies.
Two reviewers double-screened independently each title and abstract (JY and CW). Discrepancy or uncertainty was resolved by a third independent reviewer (YL). Studies were limited to human participants and of any follow-up duration and time points.
We performed a meta-analysis of prospective studies that met the following criteria: human participants who received a COVID-19 vaccine of any brand and type; patients with solid cancer; studies that included and reported data on a control group comprising subjects who are not with solid cancer; and studies that reported at least one of seroconversion after COVID-19 vaccination or serological titers after COVID-19 vaccination.
We excluded studies that enrolled but did not report outcomes of a control group; reported seroconversion data in a form that prevented the calculation of proportions, risk of seroconversion, or number of seroconverted participants; and reported serological titers in a form from which neither mean nor median titers could be derived.
When studies did not provide available data, we contacted the corresponding authors via e-mail for information. We excluded studies only if data were not provided at the time of meta-analysis.
Data extraction
Two reviewers (JY and CW) synthesized data from all eligible studies and created graphs using a Microsoft Excel spreadsheet. At the end of the data extraction phase, all key extracted data were reviewed and quality checked by the same two reviewers.
Risk of bias assessment
The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was used to rate risk of bias for non-randomized included studies. This tool assesses seven domains: risk of bias from confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported results.Citation29 Two investigators (JY and CW) independently judged these domains as low, moderate, serious, or critical risk of bias, or no information. All discrepancies were first discussed between the investigators, then split by a third investigator (×S) in case of persistent discordance. A study would be judged as having an overall low risk of bias if all the domains were judged as low risk. A study would be considered as having critical risk of bias if one domain was judged at high risk of bias. A standardized method, namely, version 2 of the Cochrane risk-of-bias tool (RoB 2) was to be used for randomized trials.Citation34 During this study, however, no eligible randomized studies were found.
Outcome assessment
The primary endpoint was seroconversion after a first and second dose of SARS-CoV-2 vaccination in patients with solid cancer. As brand and type of assay, type of immunoglobulin, and definition of seroconversion differed across studies, reports the respective data for each included study. Secondary outcomes of interest were mean or median serological titers and cumulative incidence of seroconversion after a first and second dose of COVID-19 vaccines. As the type of antibodies measured and reported differed across studies, Table S1 and S2 show the titers after a first and second vaccine dose, respectively. The time points of serological assessment after COVID-19 vaccination and the different brands of serological kits are shown in . Subgroup analyses according to proportion of patients on anti-cancer medication/treatment and age of subjects were undertaken when data were available. Anti-cancer medications/treatments included chemotherapy, monoclonal antibodies, immune check-point inhibitors, radiotherapy, hormonal therapy, etc. were assessed separately.
Table 1. Characteristics of included studies.
Statistical analysis
We used random effects model to estimate the pooled risk ratios (RR) and corresponding 95% confidence intervals (CI) for the primary outcomes of interest. A RR < 1 indicates that patients with solid cancer had a lower risk of achieving seroconversion after COVID-19 vaccination compared with control groups. Statistical heterogeneity of the results in the enrolled studies was assessed by χ2 test and I2 statistic. We considered heterogeneity to be significant when the P value <0.10, or the I2 statistic was ≥50%.Citation35
We performed separate meta-analyses for the relative risk of seroconversion (measured as RR compared with healthy controls) after each vaccine dose. Generalized linear mixed effects models were used to pool the logit transformed proportions of patients with solid cancer who achieved seroconversion after a first and second COVID-19 vaccine dose.
Statistical analyses were performed using RevMan 5.4 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2020). Unless specified otherwise, we considered a two-sided P value of <0.05 to be statistically significant.
Publication bias was assessed by the visual inspection of funnel plot.Citation36 We performed subgroup analysis to determine if the results were influenced by the type of COVID-19 vaccines. Sensitivity analysis was conducted to assess the robustness of the synthesized results.
The certainty of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).Citation37
Results
Study characteristics
Thirty articles including 11,245 subjects met eligibility criteria () and were included for meta-analysis of seroconversion rates ().Citation38–67 Tables S1 and S2 present the serological antibody titers after a first and second dose of COVID-19 vaccines, respectively. In addition, four articles that met the inclusion criteria for meta-analysis were excluded because seroconversion rates among healthy controls could not be obtained in time from the corresponding authors.Citation68–71
In the 34 included studies, 32 (94.1%) used mRNA vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), in which eight (23.5%) studies used simultaneously with viral vector vaccines AZD1222 (ChAdOx1 nCoV-19; Oxford-AstraZeneca) and Ad26.CoV2.S (Janssen/Johnson & Johnson); and two (5.9%) inactivated vaccines CoronaVac (Sinovac, Biotech). Among the mRNA vaccines, BNT162b2 was used in 30 (88.2%) studies and as the sole vaccine in 16 (47.1%), and mRNA-1273 was used in 15 (44.1%) studies and as the sole vaccine in two (5.9%), therefore BNT162b2 featured more prominently.
Vaccine response
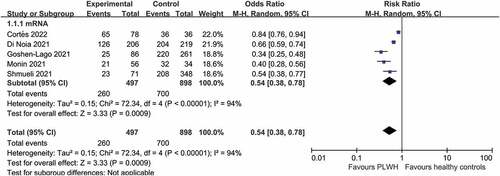
As shown in , five studies reported seroconversion after a first vaccine dose in patients with solid cancer (n = 497) compared with healthy controls (n = 898). There was significant difference in the seroconversion rate between patients with solid cancer and healthy controls after a first vaccine dose (RR 0.54, 95% CI 0.38–0.78, I2 = 94%) (moderate certainty of evidence).
Figure 2. Pooled risk ratios for patients with solid cancer compared with healthy controls after a first dose of COVID-19.
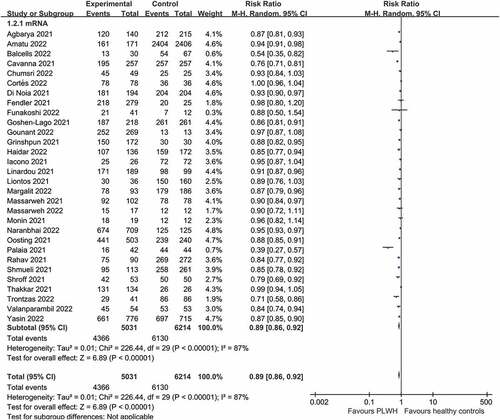
All 30 studies (5,031 patients with solid cancer and 6,214 healthy controls) assessed the serologic response after the second dose of COVID-19 vaccine in patients with solid cancer (). The seroconversion rate was lower among patients with solid cancer than that among healthy controls after a second vaccine dose (0.89 [0.86–0.92], I2 = 87%) (moderate certainty of evidence).
Figure 3. Pooled risk ratios for seroconversion among patients with solid cancer compared with healthy controls.
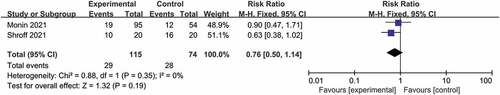
Only two prospective observational studies reported data after a third dose of COVID-19 vaccine, and both used mRNA vaccines as a third dose (). There was no significant difference in the pooled RR of seroconversion rate between patients with solid cancer and healthy controls after a third vaccine dose (RR 0.76, 95% CI 0.50–1.14, I2 = 0%) (moderate certainty of evidence).
Heterogeneity after the second doses
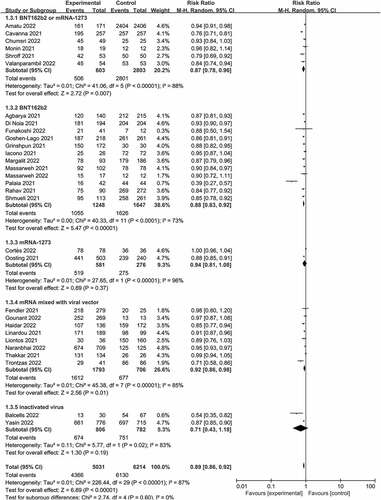
All the five studies reported seroconversion rates among patients with solid cancer after a first dose of COVID-19 vaccine used only mRNA vaccines. Therefore, subgroup analysis was only performed for studies involving mRNA vaccines and non-mRNA vaccines after the second dose. There were significant differences (P < .01 for test of subgroup effect, ) in effects on seroconversion among mRNA vaccines (risk ratio 0.88, 0.84 to 0.92), mRNA mixed with viral vaccines (0.92, 0.86 to 0.98), and inactivated vaccines (0.71, 0.43 to 1.18).
Figure 5. Subgroup analysis of vaccine type among patients with solid cancer patients after second dose.
The brand of serology kit for assays and country/region of study were of inconsistent significance across patients with solid cancer groups, and are therefore unlikely to be major confounders overall.
Mixed effects meta-regression of seroconversion against potential effect moderators (continuous and categorical study level characteristics), including country/region, race, mean age of patients, brand of serology kit, time points for assays after COVID-19 vaccination, and risk of bias of study showed no consistent effect moderation across patients with solid cancer after the second dose.
Risk of bias assessment
Twenty-three studies were assessed to be at low risk of bias and seven at moderate risk of bias (Table S3). No studies were considered at severe or critical risk of bias. Risk of bias was mainly associated with confounding effects or with controls not being age-matched.
Publication bias
Funnel plot of the studies included in the meta-analysis demonstrated no asymmetry by visual inspection. Therefore, no significant publication bias was found in our study (Figure S1).
Discussion
This meta-analysis was the first to assess and compare the efficacy of COVID-19 vaccines available at present for patients with solid cancer. In this systematic review and meta-analysis of 30 studies, we found that patients with solid cancer, compared with healthy controls, had a nearly half seroconversion rate after a first dose of COVID-19 vaccine (0.54[0.38–0.78], I2 = 94%), and seroconversion rates significantly increased after a second dose of COVID-19 vaccine (0.89[0.86–0.92], I2 = 94%). Among patients with solid cancer, our results cannot show an ideal seroconversion rate even after a second dose of COVID-19 vaccine, prompting the requirement for additional measures. Encouragingly, there was no significant difference in the seroconversion rate between patients with solid cancer and healthy controls after a third vaccination (RR 0.63, 95% CI 0.32–1.24, I2 = 56%). Shmueli et al showed that the booster of BNT162b2 vaccine may be efficacious in eliciting an antibody response in patients with solid cancer who remain seronegative despite two doses of BNT162b2.Citation72
Our meta-analyses show significant heterogeneity in immunogenicity between different patients with solid cancer groups after the second dose of COVID-19 vaccines. After the vaccination, the response noticeably varied in patients with solid cancer, which may be attributed to age, ethnicity, sex, smoking, the cancer types, comorbidities, treatments received, quantitative methods used in the studies, measurement kits, and cutoff points to determine a positive seroconversion.Citation73–75
To date, there is no international consensus on measures to determine immunogenicity. Surrogate measures, including seroconversion rates and geometric mean titers, were reported in many trials.Citation76 These surrogate measures involved parameters related to anti-SARS-COV-2 recombinant spike, receptor binding domain (RBD), neutralizing IgG or total antibodies. The use of immunomarkers to reflect the complexity and durability of protective immunity against COVID-19 has been the subject of much debate.Citation77–82 The neutralizing antibody (NAb) level has more recently been recognized as a reliable predictor of protection against symptomatic COVID-19. However, the measures taken in many studies varied. In this systematic review, only studies that compared measures of effect between patients with solid cancer and healthy controls were enrolled.
In the general population, the adaptive immune response to SARS-CoV-2 consists of T cells that support antibody production while also directly killing virus-infected cells, and B cells that produce different classes of antibodies in order to neutralize the virus.Citation83 Although memory B and T cells have been described both in naturally infected individuals and in vaccinated populations, their specific roles in achieving protective immunity remain to be determined.Citation84–86 However, T cells are considered to play a crucial part in reducing the severity of COVID-19.Citation87–89 Several observational studies suggest that early SARS-CoV-2 T-cell responses are associated with milder COVID-19.Citation88,Citation89 In this regard, data from a phase III clinical trial investigating COVID-19 vaccines suggest that protection may require low levels of neutralizing antibodies and might involve other immune effector mechanisms, including non-neutralizing antibodies, T cells and innate immunity.Citation86 Circulating antibody titers did not predict T-cell memory.Citation85,Citation87,Citation90 Furthermore, real-world data indicate that vaccine protection against SARS-CoV-2 infection wanes over time. However, protection against hospitalization and severe disease appears to remain.Citation91–93 Figuring out the complexity of a protective immune response against SARS-CoV-2 is challenging for patients with solid cancer, given both the biological differences among cancer types and the different treatments received.Citation94 Additionally, patients with solid cancer have been largely excluded from phase III clinical trials testing vaccine candidates, thus evidence about protective immune responses came from highly heterogeneous single-center observational studies.
Javadinia et al. confirmed that inactivated vaccine is safe and effective in patients with malignancies,Citation95,Citation96 and that vaccination against COVID-19 in patients with active malignancies using activated or inactivated vaccines is a safe, tolerable, and highly effective procedure.Citation97 Over the last decade, mRNA has emerged as a promising platform for developing vaccines against infectious disease and cancer.Citation98 Compared with traditional vaccines such as live attenuated vaccines, inactivated virus vaccines, and protein subunit vaccines, mRNA vaccines have the advantages of versatility, rapid development, good safety profiles, and potent immunogenicity.Citation99–101 Therefore, multiple researchers and companies have chosen this platform to develop vaccines against COVID-19. It is difficult to directly compare the seroconversion rates of the COVID-19 mRNA vaccines with more traditional, frequently used vaccines. In our study, no significant difference was found in a subgroup analysis of mRNA vs. conventional vaccines in patients with solid cancer. Fan et al. concluded that two mRNA vaccine doses prevent SARS-COV-2 infection most effectively than non-replicating viral vector or inactivated vaccines. However, mRNA vaccines showed more relevance to serious adverse events (SAEs) than the other two vaccine platforms.Citation102
This study has several limitations. First, most of the enrolled studies are observational, but one is randomized controlled trial. Factors that might influence the immune response to the vaccine, such as differences in study design and sample size, may not be controlled for between patients with solid cancer and the healthy control group. To address this limitation, we performed a subgroup analysis and found no significant effect modification between studies of different designs. Second, in our study, the seroconversion rate was pooled after the first and second doses of a COVID-19 vaccine. However, the seroconversion rate, an indicator of an immune response to a vaccine, is only a proxy for the effects of the vaccine on infection rate and COVID-19 severity. Data on clinical efficacy endpoints, such as the COVID-19 infection rate in vaccinated patients with solid cancer, are still lacking. Last, the results may be imbalanced, because 20 of the 30 publications enrolled were on mRNA and other eight were mRNA mixed with viral vector. However, in view of the fact that the studies included in this review predominantly used mRNA vaccines, the possible differential analyses were limited.
Conclusions
In conclusion, this meta-analysis has shown that, compared with healthy individuals, COVID-19 vaccines show favorable immunogenicity and efficacy in patients with solid cancer. A second dose was associated with improved seroconversion, although it is slightly lower in patients with solid cancer compared with healthy individuals. Additional strategies, such as the administration of a third (booster) vaccination with mRNA COVID-19 vaccines, might improve seroprotection for these patients.
Supplemental Material
Download TIFF Image (44.9 MB)Supplemental Material
Download MS Word (32.5 KB)Supplemental Material
Download MS Word (83 KB)Supplemental Material
Download MS Word (56 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2119763
Additional information
Funding
References
- Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA, Pagenstecher A, Kupke A, Enquist LW, Perlman S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20(9):1–11. doi:10.1016/s1474-4422(21)00182-4.
- Spudich S, Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267–269. doi:10.1126/science.abm2052.
- Sansoè G, Aragno M, Wong F. COVID-19 and liver cirrhosis: focus on the nonclassical Renin-Angiotensin system and implications for therapy. Hepatology. 2021;74(2):1074–1080. doi:10.1002/hep.31728.
- Thompson CK, Lee MK, Baker JL, Attai DJ, DiNome ML. Taking a second look at neoadjuvant endocrine therapy for the treatment of early stage estrogen receptor positive breast cancer during the COVID-19 outbreak. Ann Surg. 2020;272(2):e96–e7. doi:10.1097/sla.0000000000004027.
- Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, Park J, Foox J, Hether T, Warren S, et al. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021;593(7860):564–569. doi:10.1038/s41586-021-03475-6.
- Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi:10.1158/2159-8290.Cd-20-0422.
- Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu CY, Shyr Y, Li X, Choueiri TK, Painter CA, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and cancer consortium. Ann Oncol. 2021;32(6):787–800. doi:10.1016/j.annonc.2021.02.024.
- Pinato DJ, Zambelli A, Aguilar-Company J, Bower M, Sng C, Salazar R, Bertuzzi A, Brunet J, Mesia R, Segui E, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10(10):1465–1474. doi:10.1158/2159-8290.Cd-20-0773.
- Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi:10.1016/s1470-2045(20)30309-0.
- Shahidsales S, Aledavood SA, Joudi M, Molaie F, Esmaily H, Javadinia SA. COVID-19 in cancer patients may be presented by atypical symptoms and higher mortality rate, a case-controlled study from Iran. Cancer Rep (Hoboken). 2021;4(5):e1378. doi:10.1002/cnr2.1378.
- Taghizadeh-Hesary F, Porouhan P, Soroosh D, PeyroShabany B, Shahidsales S, Keykhosravi B, Rahimi F, Houshyari M, Forouzanfar MM, Javadinia SA. COVID-19 in cancer and non-cancer patients. Int J Manage. 2021;14(4):e110907. doi:10.5812/ijcm.110907.
- Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe COVID-19 pneumonia: pathogenesis and clinical management. Bmj. 2021;372:n436. doi:10.1136/bmj.n436.
- Sidebottom DB, Gill D. Ronapreve for prophylaxis and treatment of COVID-19. BMJ. 2021;374:n2136. doi:10.1136/bmj.n2136.
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi:10.1056/NEJMoa2116044.
- Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–393. doi:10.1038/s41577-021-00542-x.
- Young B, Tan TT, Leo YS. The place for remdesivir in COVID-19 treatment. Lancet Infect Dis. 2021;21(1):20–21. doi:10.1016/s1473-3099(20)30911-7.
- Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, Metzendorf MI, Fischer AL, Kopp M, Stegemann M, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8(8):Cd014963. doi:10.1002/14651858.Cd014963.
- Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–329. doi:10.1056/NEJMoa2107058.
- Berlin DA, Gulick RM, Martinez FJ, Solomon CG. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi:10.1056/NEJMcp2009575.
- Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi:10.1126/science.abm8108.
- Piccaluga PP, Di Guardo A, Lagni A, Lotti V, Diani E, Navari M, Gibellini D. COVID-19 vaccine: between myth and truth. Vaccines (Basel). 2022;10(3):349. doi:10.3390/vaccines10030349.
- Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. doi:10.1038/s41541-021-00292-w.
- Thomas SJ, Moreira ED,sJr., Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi:10.1056/NEJMoa2110345.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389.
- Mallapaty S. China’s COVID vaccines have been crucial - now immunity is waning. Nature. 2021;598(7881):398–399. doi:10.1038/d41586-021-02796-w.
- Silveira MM, Moreira G, Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2021;267:118919. doi:10.1016/j.lfs.2020.118919.
- Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, Jaffee EM, Wherry EJ, Soria JC, D’Souza G. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11(2):233–236. doi:10.1158/2159-8290.Cd-20-1817.
- Giesen N, Sprute R, Rüthrich M, Khodamoradi Y, Mellinghoff SC, Beutel G, Lueck C, Koldehoff M, Hentrich M, Sandherr M, et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur J Cancer. 2021;147:154–160. doi:10.1016/j.ejca.2021.01.033.
- Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, Tay SH, Teo CB, Tan BKJ, Chan YH, et al. Efficacy of Covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi:10.1136/bmj-2021-068632.
- Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102(3a):2–9; discussion 25–6. doi:10.1016/s0002-9343(97)00003-x.
- Cederwall S, Påhlman LI. Respiratory adenovirus infections in immunocompetent and immunocompromised adult patients. Epidemiol Infect. 2020;147:e328. doi:10.1017/s0950268819002176.
- Manuel O, Estabrook M. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13511. doi:10.1111/ctr.13511.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi:10.1002/sim.1186.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi:10.1136/bmj.39489.470347.AD.
- Agbarya A, Sarel I, Ziv-Baran T, Agranat S, Schwartz O, Shai A, Nordheimer S, Fenig S, Shechtman Y, Kozlener E, et al. Efficacy of the mRNA-Based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers (Basel). 2021;13(16):4191. doi:10.3390/cancers13164191.
- Amatu A, Pani A, Patelli G, Gagliardi OM, Loparco M, Piscazzi D, Cassingena A, Tosi F, Ghezzi S, Campisi D, et al. Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment. Eur J Cancer. 2022;163:16–25. doi:10.1016/j.ejca.2021.12.006.
- Balcells ME, Le Corre N, Durán J, Ceballos ME, Vizcaya C, Mondaca S, Dib M, Rabagliati R, Sarmiento M, Burgos PI, et al. Reduced immune response to inactivated severe acute respiratory syndrome Coronavirus 2 vaccine in a cohort of immunocompromised patients in Chile. Clin Infect Dis. 2022;75(1):ciac167. doi:10.1093/cid/ciac167.
- Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, Cremona G, Muroni M, Bernuzzi P, Lo Cascio G, et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: seropositivity and safety. A prospective observational study in Italy. Eur J Cancer. 2021;157:441–449. doi:10.1016/j.ejca.2021.08.035.
- Chumsri S, Advani PP, Pai TS, Li Z, Mummareddy A, Acampora M, Reynolds GA, Wylie N, Boyle AW, Lou Y, et al. Humoral responses after SARS-CoV-2 mRNA vaccination and breakthrough infection in cancer patients. Mayo Clin Proc Innov Qual Outcomes. 2022;6(2):120–125. doi:10.1016/j.mayocpiqo.2021.12.004.
- Cortés A, Casado JL, Longo F, Serrano JJ, Saavedra C, Velasco H, Martin A, Chamorro J, Rosero D, Fernández M, et al. Limited T cell response to SARS-CoV-2 mRNA vaccine among patients with cancer receiving different cancer treatments. Eur J Cancer. 2022;166:229–239. doi:10.1016/j.ejca.2022.02.017.
- Di Noia V, Pimpinelli F, Renna D, Barberi V, Maccallini MT, Gariazzo L, Pontone M, Monti A, Campo F, Taraborelli E, et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Cancer Res. 2021;27(24):6815–6823. doi:10.1158/1078-0432.Ccr-21-2439.
- Fendler A, Shepherd STC, Au L, Wilkinson KA, Wu M, Byrne F, Cerrone M, Schmitt AM, Joharatnam-Hogan N, Shum B, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2(12):1321–1337. doi:10.1038/s43018-021-00274-w.
- Funakoshi Y, Yakushijin K, Ohji G, Hojo W, Sakai H, Takai R, Nose T, Ohata S, Nagatani Y, Koyama T, et al. Safety and immunogenicity of the COVID-19 vaccine BNT162b2 in patients undergoing chemotherapy for solid cancer. J Infect Chemother. 2022;28(4):516–520. doi:10.1016/j.jiac.2021.12.021.
- Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi:10.1001/jamaoncol.2021.2675.
- Gounant V, Ferré VM, Soussi G, Charpentier C, Flament H, Fidouh N, Collin G, Namour C, Assoun S, Bizot A, et al. Efficacy of severe acute respiratory syndrome Coronavirus-2 vaccine in patients with thoracic cancer: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J Thorac Oncol. 2022;17(2):239–251. doi:10.1016/j.jtho.2021.10.015.
- Grinshpun A, Rottenberg Y, Ben-Dov IZ, Djian E, Wolf DG, Kadouri L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open. 2021;6(6):100283. doi:10.1016/j.esmoop.2021;6(6):100283.
- Haidar G, Agha M, Bilderback A, Lukanski A, Linstrum K, Troyan R, Rothenberger S, McMahon DK, Crandall MD, Sobolewksi MD, et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clin Infect Dis. 2022;19(6):385–401. doi:10.1093/cid/ciac103.
- Iacono D, Cerbone L, Palombi L, Cavalieri E, Sperduti I, Cocchiara RA, Mariani B, Parisi G, Garufi C. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021;12(8):1253–1255. doi:10.1016/j.jgo.2021.06.002.
- Linardou H, Spanakis N, Koliou GA, Christopoulou A, Karageorgopoulou S, Alevra N, Vagionas A, Tsoukalas N, Sgourou S, Fountzilas E, et al. Responses to SARS-CoV-2 vaccination in patients with cancer (Recover study): a prospective cohort study of the hellenic cooperative oncology group. Cancers. 2021;13(18):4621. doi:10.3390/cancers13184621.
- Liontos M, Terpos E, Markellos C, Zagouri F, Briasoulis A, Katsiana I, Skafida E, Fiste O, Kunadis E, Andrikopoulou A, et al. Immunological response to COVID-19 vaccination in ovarian cancer patients receiving PARP inhibitors. Vaccines (Basel). 2021;9(10):1148. doi:10.3390/vaccines9101148.
- Margalit O, Shacham-Shmueli E, Itay A, Berger R, Halperin S, Jurkowicz M, Levin EG, Olmer L, Regev-Yochay G, Lustig Y, et al. Seropositivity and neutralising antibodies at six months after BNT162b2 vaccination in patients with solid tumours. Eur J Cancer. 2022;168:51–55. doi:10.1016/j.ejca.2022.03.013.
- Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, Benouaich-Amiel A, Ben-Zvi H, Moskovits N, Brenner B, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–1140. doi:10.1001/jamaoncol.2021.2155.
- Massarweh A, Tschernichovsky R, Stemmer A, Benouaich-Amiel A, Siegal T, Eliakim-Raz N, Stemmer SM, Yust-Katz S. Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients with primary brain tumors: a prospective cohort study. J Neurooncol. 2022;156(3):483–489. doi:10.1007/s11060-021-03911-7.
- Monin L, Laing AG, Munoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi:10.1016/S1470-2045%2821%2900213-8.
- Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, Isakoff SJ, Farmer JR, Zubiri L, Hobbs GS, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol. 2022;40(1):12–23. doi:10.1200/jco.21.01891.
- Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22(12):1681–1691. doi:10.1016/s1470-2045(21)00574-x.
- Palaia I, Caruso G, Di Donato V, Vestri A, Napoli A, Perniola G, Casinelli M, Alunni Fegatelli D, Campagna R, Tomao F, et al. Pfizer-BioNtech COVID-19 vaccine in gynecologic oncology patients: a prospective cohort study. Vaccines (Basel). 2021;10(1):21. doi:10.3390/vaccines10010012.
- Rahav G, Lustig Y, Lavee J, Ohad B, Magen H, Hod T, Noga S-T, Shmueli ES, Drorit M, Ben-Ari Z, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine. 2021;41:101158. doi:10.1016/j.eclinm.2021.101158.
- Shmueli ES, Itay A, Margalit O, Berger R, Halperin S, Jurkowicz M, Levin EG, Levy I, Olmer L, Regev-Yochay G, et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy - a single centre prospective study. Eur J Cancer. 2021;157:124–131. doi:10.1016/j.ejca.2021.08.007.
- Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, Peyton KL, Uhrlaub JL, Ripperger TJ, Jergović M, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27(11):2002–2011. doi:10.1038/s41591-021-01542-z.
- Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, Rahman S, Kim SY, Ko B, Sica RA, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081. doi:10.1016/j.ccell.2021.06.002.
- Trontzas IP, Vathiotis I, Economidou C, Petridou I, Gomatou G, Grammoustianou M, Tsamis I, Syrigos N, Anagnostakis M, Fyta E, et al. Assessment of seroconversion after SARS-CoV-2 vaccination in patients with lung cancer. Vaccines (Basel). 2022;10(4):618. doi:10.3390/vaccines10040618.
- Valanparambil R, Carlisle J, Linderman S, Akthar A, Millett RL, Lai L, Chang A, McCook A, Switchenko J, Nasti T, et al. Antibody response to SARS-CoV-2 mRNA vaccine in lung cancer patients: reactivity to vaccine antigen and variants of concern. medRxiv. 2022. doi:10.1101/2022.01.03.22268599.
- Yasin AI, Aydin SG, Sumbul B, Koral L, Simsek M, Geredeli C, Ozturk A, Perkin P, Demirtas D, Erdemoglu E, et al. Efficacy and safety profile of COVID-19 vaccine in cancer patients: a prospective, multicenter cohort study. Future Oncol. 2022;18(10):1235–1244. doi:10.2217/fon-2021-1248.
- Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wörmann B, Turajlic S, von Lilienfeld-Toal M. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19(6):385–401. doi:10.1038/s41571-022-00610-8.
- Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Alessio N, Comolli G, Sarasini A, Sammartino JC, Ferrari A, et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors on active treatment: a prospective cohort study. ESMO Open. 2022;7(2):100458. doi:10.1016/j.esmoop.2022.100458.
- Peeters M, Verbruggen L, Teuwen L, Vanhoutte G, Vande Kerckhove S, Peeters B, Raats S, Van der Massen I, De Keersmaecker S, Debie Y, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5):100274. doi:10.1016/j.esmoop.2021.100274.
- Thomas SJ, Perez JL, Lockhart SP, Hariharan S, Kitchin N, Bailey R, Liau K, Lagkadinou E, Türeci Ö, Şahin U, et al. Efficacy and safety of the BNT162b2 mRNA COVID-19 vaccine in participants with a history of cancer: subgroup analysis of a global phase 3 randomized clinical trial. Vaccine. 2022;40(10):1483–1492. doi:10.1016/j.vaccine.2021.12.046.
- Shmueli ES, Lawrence YR, Rahav G, Itay A, Lustig Y, Halpern N, Boursi B, Margalit O. Serological response to a third booster dose of BNT162b2 COVID-19 vaccine among seronegative cancer patients. Cancer Rep (Hoboken). 2022;5(8):e1645. doi:10.1002/cnr2.1645.
- Mehrabi Nejad MM, Moosaie F, Dehghanbanadaki H, Haji Ghadery A, Shabani M, Tabary M, Aryannejad A, SeyedAlinaghi S, Rezaei N. Immunogenicity of COVID-19 mRNA vaccines in immunocompromised patients: a systematic review and meta-analysis. Eur J Med Res. 2022;27(1):23. doi:10.1186/s40001-022-00648-5.
- Urakawa R, Isomura ET, Matsunaga K, Kubota K, Ike M. Impact of age, sex and medical history on adverse reactions to the first and second dose of BNT162b2 mRNA COVID-19 vaccine in Japan: a cross-sectional study. BMC Infect Dis. 2022;22(1):179. doi:10.1186/s12879-022-07175-y.
- Notarte KI, Ver AT, Velasco JV, Pastrana A, Catahay JA, Salvagno GL, Yap EPH, Martinez-Sobrido L, Torrrelles JB, Lippi G, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNtech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022;28:1–18. doi:10.1080/10408363.2022.2038539.
- Javadinia SA, Ariamanesh M, Nabavifard M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Hatami F, Ghasemi A, Lyman GH, Welsh JS, et al. Multicenter study of antibody seroprevalence against COVID-19 in patients presenting to iranian cancer centers after one year of the COVID-19 pandemic. Cancer Invest. 2022;40(2):115–123. doi:10.1080/07357907.2021.1995742.
- Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, et al. COVID-19-Neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–88.e11. doi:10.1016/j.cell.2020.12.015.
- Roozendaal R, Solforosi L, Stieh DJ, Serroyen J, Straetemans R, Dari A, Boulton M, Wegmann F, Rosendahl Huber SK, van der Lubbe JEM, et al. SARS-CoV-2 binding and neutralizing antibody levels after Ad26.COV2.S vaccination predict durable protection in rhesus macaques. Nat Commun. 2021;12(1):5877. doi:10.1038/s41467-021-26117-x.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi:10.1038/s41591-021-01377-8.
- Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, Dull P, Plotkin SA. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi:10.1016/j.vaccine.2021.05.063.
- Vidal SJ, Collier AY, Yu J, McMahan K, Tostanoski LH, Ventura JD, Aid M, Peter L, Jacob-Dolan C, Anioke T, et al. Correlates of neutralization against SARS-CoV-2 variants of concern by early pandemic sera. J Virol. 2021;95(14):e0040421. doi:10.1128/jvi.00404-21.
- Jin P, Li J, Pan H, Wu Y, Zhu F. Immunological surrogate endpoints of COVID-2019 vaccines: the evidence we have versus the evidence we need. Signal Transduct Target Ther. 2021;6(1):48. doi:10.1038/s41392-021-00481-y.
- Corti C, Crimini E, Tarantino P, Pravettoni G, Eggermont AMM, Delaloge S, Curigliano G. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer. 2021;148:316–327. doi:10.1016/j.ejca.2021.01.046.
- Barrière J, Re D, Peyrade F, Carles M. Current perspectives for SARS-CoV-2 vaccination efficacy improvement in patients with active treatment against cancer. Eur J Cancer. 2021;154:66–72. doi:10.1016/j.ejca.2021.06.008.
- Mairhofer M, Kausche L, Kaltenbrunner S, Ghanem R, Stegemann M, Klein K, Pammer M, Rauscher I, Salzer HJF, Doppler S, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39(9):1171–1172. doi:10.1016/j.ccell.2021.08.001.
- Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi:10.1038/s41577-021-00578-z.
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi:10.1126/science.abf4063.
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi:10.1016/j.cell.2020.09.038.
- Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, Zhuang Y, Tham CYL, Chia A, Smith GJD, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6):108728. doi:10.1016/j.celrep.2021.108728.
- Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27(7):1147–1148. doi:10.1038/s41591-021-01432-4.
- Tenforde MW, Self WH, Naioti EA, Ginde AA, Douin DJ, Olson SM, Talbot HK, Casey JD, Mohr NM, Zepeski A, et al. Sustained effectiveness of Pfizer-BioNtech and moderna vaccines against COVID-19 associated hospitalizations among adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1156–1162. doi:10.15585/mmwr.mm7034e2.
- Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, Li Q, Bagchi S, Dubendris H, Link-Gelles R, et al. Effectiveness of Pfizer-BioNtech and moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1163–1166. doi:10.15585/mmwr.mm7034e3.
- Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E, Backenson B, Hoefer D, Morne J, Bauer U, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status - New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1150–1155. doi:10.15585/mmwr.mm7034e1.
- Giuliano AR, Lancet JE, Pilon-Thomas S, Dong N, Jain AG, Tan E, Ball S, Tworoger SS, Siegel EM, Whiting J, et al. Evaluation of antibody response to SARS-CoV-2 mRNA-1273 vaccination in patients with cancer in Florida. JAMA Oncol. 2022;8(5):748–754. doi:10.1001/jamaoncol.2022.0001.
- Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Dehghani M, Nabavifard M, Hatami F, Fereidouni M, Welsh JS, Javadinia SA. Immunogenicity and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in patients with malignancy. Cancer Invest. 2022;40(1):26–34. doi:10.1080/07357907.2021.1992420.
- Joudi M, Moradi Binabaj M, Porouhan P, PeyroShabany B, Tabasi M, Fazilat-Panah D, Khajeh M, Mehrabian A, Dehghani M, Welsh JS, et al. A cohort study on the immunogenicity and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in patients with breast cancer; does Trastuzumab interfere with the outcome? Front Endocrinol (Lausanne). 2022;13:798975. doi:10.3389/fendo.2022.798975.
- Javadinia SA, Alizadeh K, Mojadadi MS, Nikbakht F, Dashti F, Joudi M, Harati H, Welsh JS, Farahmand SA, Attarian F. COVID-19 vaccination in patients with malignancy; a systematic review and meta-analysis of the efficacy and safety. Front Endocrinol (Lausanne). 2022;13:860238. doi:10.3389/fendo.2022.860238.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi:10.1038/nrd.2017.243.
- Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O’Connell S, Bock KW, Minai M, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383(16):1544–1555. doi:10.1056/NEJMoa2024671.
- Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390(10101):1511–1520. doi:10.1016/s0140-6736(17)31665-3.
- Feldman RA, Fuhr R, Smolenov I, Mick Ribeiro A, Panther L, Watson M, Senn JJ, Smith M, Almarsson Ӧ, Pujar HS, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37(25):3326–3334. doi:10.1016/j.vaccine.2019.04.074.
- Fan YJ, Chan KH, Hung IF. Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3. Vaccines (Basel). 2021;9(9):989. doi:10.3390/vaccines9090989.