ABSTRACT
Vaccination is considered the most effective way to reduce the impact of coronavirus disease 2019 (COVID-19). Several new vaccines have been manufactured. This study aimed to assess the current status and prospects of COVID-19 vaccine research using a bibliometric analysis. We analyzed 3,954 scientific articles on COVID-19 vaccines in the Web of Science Core Collection (WoSCC). CiteSpace and VOSviewer were used for bibliometric visualization. Original articles and reviews were used for the analysis. A total of 2,783 (70.38%) studies were published in 2021. The USA contributed the highest, publishing 1,390 articles with 41,788 citations, followed by China and the UK. The USA’s primary collaborators were the UK (n = 133), China (n = 87), and Canada (n = 65). The most active institutions were the University of Oxford and Harvard Medical School, while Emory University was the most influential. The Vaccines journal had the most number of publications (402). The most cited journal was the New England Journal of Medicine. In 2021, the focus was on RNA vaccines, attitudes toward vaccination, and hesitancy. In contrast, studies in 2022 focused on vaccine double-blind trials, viral mutations, and antibodies. In the context of rapid virus transmission, vaccine studies on immunogenicity, spike proteins, efficacy, safety, and antibody response have been prioritized. Additional phased clinical trials are needed to determine the effectiveness, acceptance, and side effects of vaccines against mutated strains of the virus.
Introduction
On 11 March 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic,Citation1 This was a medical issue that also raised a multidisciplinary discussion related to health, economics, and social systems,Citation2,Citation3 Owing to the high transmission rate of COVID-19, the public health system and global economy have been heavily burdened, highlighting the need for a rapid and effective method to prevent infections. Moreover, many therapies, such as antiviral drugs,Citation4,Citation5 antimalarial drugs,Citation6,Citation7 immunomodulators,Citation8 and cell- and plasma-based therapyCitation9 have been developed. Despite various emerging treatment approaches, there are no specific drugs available to treat COVID-19. Additionally, some studies have indicated reinfection after clinical recovery of patients.Citation10 Therefore, vaccination is considered to be the most economical and feasible means of preventing viral infections, especially in underdeveloped countries.Citation11–13 Given these challenging circumstances, governments have focused on vaccines as the only means of controlling COVID-19. It is suggested that 60%–70% of the global population should be vaccinated to completely control COVID-19.Citation14
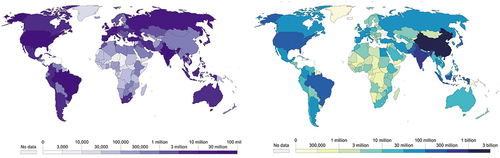
On December 11 and 18, 2020, the Food and Drug Administration (FDA) granted emergency approval to Pfizer/BioNTech and Moderna, respectively, for COVID-19 vaccines. Owing to the availability of genomic and structural information on SARS-CoV-2, vaccines are being developed at a remarkable pace and on an unprecedented scale. According to the latest global statistics, 497,960,492 COVID-19 cases, including 6,181,850 deaths, have been confirmed,Citation15 and 11,250,782,214 doses of COVID-19 vaccines have been administered (). Vaccines have been approved in 197 countries to date, 36 types of vaccines have been licensed and are in use, and 10 vaccines have been granted emergency approval by the WHO.Citation16 Further, it is necessary to highlight available information on the vaccines to provide references for their development and further research.
Figure 1. The statistics on the COVID-19 pandemic from Our World in Data. (a) the cumulative confirmed COVID-19 cases in the world. (b) the number of people who completed the initial COVID-19 vaccination protocol.
Research on COVID-19 vaccines is being prioritized. Thus, studies in this field should be scrutinized more rigorously. Mathematical and statistical methods are used in bibliometrics to quantify the current status, research hotspots, and trends.Citation17 The present study performed bibliometric analysis to assess the current status and prospects of COVID-19 vaccine research using papers indexed in the Web of Science Core Collection (WoSCC). This novel, comprehensive bibliometric analysis may help researchers and non-researchers to rapidly identify landmark studies and research topics of their interest. Additionally, information on the main vaccines approved by the WHO has been provided to inform future vaccine research.
Materials and methods
Data collection and search strategy
The WoSCC is one of the most common, authoritative scientific databases. Many researchers have analyzed the data coverage, quality of journals, and advantages and disadvantages of the WoSCC.Citation18 Hou et al. reported that the WoSCC has more standardized documents than other databases,Citation19 that is essential for bibliometric analysis. Falagas et al. suggested that data collected from a database may provide superior visualizations.Citation20 Therefore, the WoSCC was selected for literature search.
Bibliometric analysis was performed on 28 March 2022. We conducted a search using the WoSCC. “COVID-19 Vaccine” and “SARS-CoV-2 Vaccine” were used as title words – TI = “COVID-19 Vaccine” OR “SARS-CoV-2 Vaccine.” Only research articles and reviews were included in this study. There were no language limitations to this study. Two authors read the titles and abstracts, and if necessary, the full text to exclude irrelevant and duplicate articles. We obtained 3,954 records between 1 January 2020 to 28 March 2022. Data collection methodology used for the scientometric analysis is shown in . Furthermore, information on the type of vaccine was obtained from the website https://covid19.trackvaccines.org/(accessed on 7 April 2022).
Analysis tools
We analyzed all the extracted files using VOSviewer and CiteSpace. VOSviewer is a method of managing and visualizing knowledge structures.Citation21 With the capabilities of VOSviewer, data on authors, institutions, and countries were analyzed, and the status of scientific collaborations was determined.Citation22 “Countries” was selected as the unit of analysis, and full counting as the counting method. We selected a minimum of five documents per country. The same method was used to analyze “institutions” and “authors.” A minimum of 16 and 6 documents were selected for analyzing “institutions” and “authors,” respectively.
Professor Chen (Drexel University) created CiteSpace V, a Java-based information visualization program for bibliometric analysis.Citation23 The program allows researchers to assess a discipline’s evolution and identify frontier trends intuitively by providing data in the form of knowledge maps. It adopts a time-slicing technique to create a timeline of network models and integrates them to produce an overview network for the systematic analysis of relevant publications.Citation23 In this study, CiteSpace was used to track the research process and to develop a time zone map of the research trends.
Results
Publication output
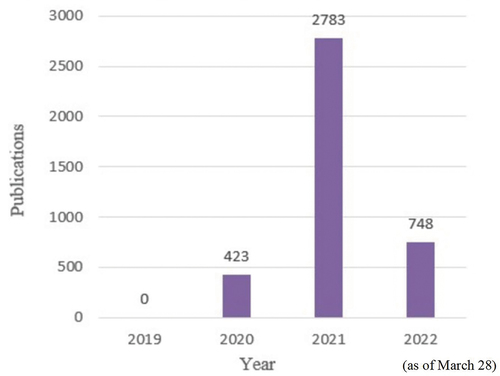
demonstrates the temporal trend and number of articles published per year. A total of 3,954 publications were found in the WoSCC. Of these, 423 (10.7%), 2,783 (70.38%), and 748 (19%) papers were published in 2020, 2021, and 2022 (as of March 28). Global widespread outbreaks of COVID-19, along with emergence of more mutations, have warranted more studies on vaccines. Additional relevant scientific output is expected by the end of 2022.
Co-authorship among countries, institutions, and researchers
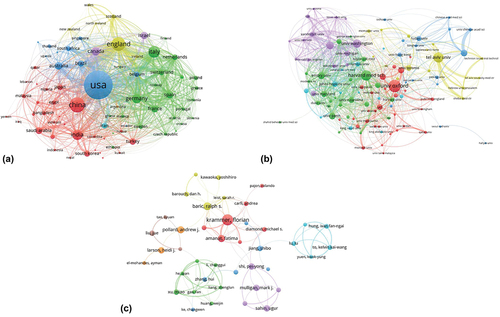
shows a co-authorship analysis of the most active countries, institutions, and authors in the field. A total of 137 countries have engaged in relevant research. Of these, 113 countries participated in at least two studies. The USA ranked first in the number of publications (n = 1,390), followed by China (n = 484), the UK (n = 409), and Italy (n = 309). lists the most prolific countries worldwide. shows 81 countries with a frequency of > 5 publications which had connections with other countries, distributed in seven clusters. The USA had 77 partners. Of them, the main partner was the UK (133 collaborative studies), followed by China (87 collaborative studies) and Canada (65 collaborative studies). The largest cluster (#1) is represented by Italy, Germany, France, Spain, and Switzerland. The second cluster (#2) is represented by China, India, Saudi Arabia, Turkey, and South Korea. The third cluster (#3) is represented by the USA, Australia, Brazil, Belgium, and South Africa.
Table 1. The most prolific countries in COVID-19 vaccine research.
A total of 6,173 institutions have shown an interest in COVID-19 vaccine research. However, a majority of the institutions only participated in one study. The details of the top ten institutions are listed in . Nine of them were universities. A network map of institutions with a frequency of > 16 publications, containing eight clusters, is shown in . The largest cluster (#1) is represented by the University of Oxford, Imperial College London, and London School of Hygiene and Tropical Medicine. The second cluster (#2) mainly comprised the Harvard Medical School, University of Pennsylvania, and University of North Carolina. The third cluster (#3) mainly comprised the Chinese Academy of Sciences, Fudan University, and University of Hong Kong. The fourth cluster (#4) mainly comprised the University of Washington, Stanford University, and University of Michigan. As the most active institutions – the University of Oxford, UK, and Harvard Medical School, USA – contributed more to publishing the studies than other institutions. Of the top 10 institutions listed in , five are from the USA, three from the UK, and one each from China and Israel. Universities in the USA and UK have played a significant role in the research on COVID-19 vaccines. Additionally, the Emory University and University of Oxford had the greatest citation impact.
Table 2. The most prolific institutions in COVID-19 vaccine research.
More than 23,000 authors have contributed to research publications on COVID-19 vaccines. The details of the top 10 most prolific authors in COVID-19 vaccine research are listed in . Forty-one authors who had connections with others constituted eight clusters (), in which the threshold was six publications. The red cluster, led by Krammer F, was the largest and included seven authors from the USA who collaborated closely. The second and third clusters, colored green and blue, respectively, mainly comprised Chinese authors. The fourth- and fifth-largest clusters in yellow and purple, respectively, mainly comprised American authors. Dhama K had the most number of publications, whereas Krammer F had the highest number of citations per paper. However, Hotez PJ obtained the highest h-index in the field, despite being at the third place in terms of scientific publications. Moreover, of the top 10 most cited papers, the only review published in Nature, titled “SARS-CoV-2 vaccine in development,” was by Krammer et al.
Table 3. The most prolific authors in COVID-19 vaccine research.
Journals
As shown in , the number of publications was the highest in the Vaccines journal (IF: 4.422, 402 articles, and 3,267 citations). Studies published in the New England Journal of Medicine (IF: 91.253) had the most extensive citation impact, receiving 14,730 citations, and the highest citations/article ratio. The Lancet (IF: 79.323) was not included in the top 10 journals in terms of the number of publications. However, the scientific impact of this academic journal cannot be ignored, as it received 6,434 citations and ranked second as per the citations/article ratio. All the top 10 journals have an IF and are among the most highly qualified journals in the field. Of them, two, three, three, and two journals were Q1, Q2, Q3, and Q4, respectively.
Table 4. The most prolific journals in COVID-19 vaccine research.
Research content
presents the subject areas of the studies on COVID-19 vaccines. More than 28% of the studies were conducted in the field of immunology, indicating that researchers have studied immunological responses to the vaccines. Furthermore, research and experimental medicine (19.07%), and public, environmental and occupational health (11.79%) areas were also active. Researchers working on these three areas have published >59% of the papers and have played a significant role in COVID-19 vaccine development.
Table 5. Active research areas in COVID-19 vaccine research.
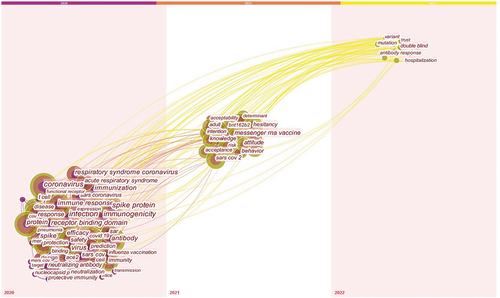
shows a keyword cloud map that presents the focus of the research. Cloud tags describe how much attention researchers pay to specific topics. The higher the frequency of the keywords used, the larger the number of tags, and vice versa. Topics such as infection, immunogenicity, COVID-19 vaccination, antibodies, vaccine hesitancy, immune response, COVID-19 vaccine efficacy, and safety have received high attention.
Based on the timezone view, shows the trend of research on COVID-19 vaccines. The nodes move from left to right indicating the different topics that researchers tended to focus on in each period. Additionally, the size of each node reflects the interest of the researchers in a given topic. In 2020, topics such as infection, immunogenicity, antibody hesitancy, immune response, efficacy and safety of COVID-19 vaccines, spike protein, and receptor-binding domain were more visible. It is evident from the trend of research in 2021 that researchers focused on topics such as risk, acceptance and attitude toward vaccines, COVID-19 vaccines, BNT162b2, and mRNA vaccines. However, the research trends till 2022 suggest that topics such as vaccine double-blind trials, viral mutations, antibody responses, variants, hospitalization, and uncertainty have received more attention than other topics.
Vaccines
lists the details of the 36 vaccines approved by at least one country. There are 14 protein subunit vaccines, 11 inactivated vaccines, six non-replicating viral vector vaccines, three RNA vaccines, and one DNA and one virus-like particle (VLP)-based vaccine each. Comirnaty (BNT162b2), Spikevax (mRNA-1273), CoronaVac, Vaxzevria (AZD1222), Ad26.COV2.S, Covaxin (BBV152), Covishield (Oxford/AstraZeneca formulation), Covilo (BBIBP-CorV), Nuvaxovid (NVX-CoV2373), and COVOVAX (Novavax formulation) are approved by the WHO for emergency use. Additionally, the three vaccines, Comirnaty (BNT162b2), Spikevax (mRNA-1273), and CoronaVac had the most number of published studies in the WoSCC. Moreover, of the 36 vaccines, China has eight, India has six, Russia and Iran have five each, and the USA has four vaccines. They are the leading producers of COVID-19 vaccines. Covifenz (MT-2766, plant-based VLP) is the only VLP-based vaccine developed jointly by Canada and Japan. ZyCoV-D, a DNA vaccine developed in India, has also been approved.
Table 6. Information on 36 vaccines approved by at least one country.
Most-cited articles and co-cited references
The top 10 most-cited articles are listed in . The most cited paperCitation24 was by Polack et al., published in the New England Journal of Medicine, that also has the highest IF. Moreover, six of the top ten articles were published in the New England Journal of Medicine, two in The Lancet, one in Nature, and one article on the potential acceptance of the COVID-19 vaccine published in Nature Medicine. Highly co-cited references are regularly cited by other articles, and thus, can be considered as knowledge bases in a specific field. The top 10 co-cited references with their co-citation counts are listed in . The top co-cited reference, titled “Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine,” was cited >1,000 times and was also the most cited article.Citation24 Most of the highly cited and co-cited articles focused on the safety and efficacy of vaccines.
Table 7. The top 10 most cited articles on COVID-19 vaccine research.
Table 8. The top 10 co-cited references.
Discussion
With a high prevalence of COVID-19 and continuous evolution of the causative virus, many studies have been conducted on treatment and pharmacological methods. Therefore, safe and effective vaccines are urgently needed to control the pandemic. The fight against this disease is ongoing. According to the latest global statistics, >400 million cases of COVID-19 have been confirmed, including >6 million deaths. To date, 197 countries have approved vaccines, and approximately 36 vaccines have received the necessary licenses, including 10 vaccines granted emergency use authorization by the WHO; Citation16 and 11,250,782,214 doses of vaccines have been administered. Four countries – the USA, China, the UK, and Italy – have made significant contributions to this field. The USA – UK and USA – China had the highest levels of collaboration. We hypothesize that an increase in relevant research outputs will continue until the pandemic is under control.
Several studies have been conducted on COVID-19 vaccines. The “Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine” published in 2020 is the most cited article.Citation24 Polack et al. included >40,000 subjects to verify the safety and efficacy of BNT162b2. This has had a profound impact on subsequent studies. In 2021, Baden et al. published a phase 3 randomized, controlled, clinical trial on the safety and efficacy of mRNA-1273.Citation25 However, the main limitation of this study was the short follow-up period for evaluating safety and efficacy. Further, the emergence of Omicron variants has led to numerous studies on the treatment of mutant virus strains. The 2022 study by Garcia-Beltran et al. highlighted the importance of additional mRNA doses to broaden neutralizing antibody responses against highly divergent SARS-CoV-2 variants.Citation26 This has contributed to a reduction in the emergence and spread of the highly mutated SARS-CoV-2 variants.
Ahmad et al. conducted the first bibliometric study on 12 January 2020 to provide details of the published literature on COVID-19 vaccines.Citation27 The study shows that vaccine development and therapy are major research hotspots. The results provided a reference for researchers and policymakers. Furthermore, Xu et al. included relevant literature before 11 August 2021, and updated the research trends of global COVID-19 vaccines.Citation28 The research highlights hotspots centered on vaccine side effects and public attitudes toward vaccination. The study findings will be updated in the future.
The following research hotspots were identified: infection, immunogenicity, vaccination, antibodies, vaccine hesitancy, immune response, efficacy, and vaccine safety. The current analysis, based on time zone maps, shows the focus of the different phases of research. In 2020, topics such as infection, immunogenicity, antibodies, hesitancy, immune response, efficacy and safety of vaccines, spike protein, and receptor-binding domain were predominantly highlighted. However, in 2021, researchers conducted studies on topics focused on the risk, acceptance, and attitude toward vaccines, COVID-19 vaccines, BNT162b2, and mRNA vaccines. Vaccine hesitancy negatively affects vaccine coverage and is still a major issue that cannot be ignored. Vaccine hesitancy is a multifactorial phenomenon. Misinformation and unsubstantiated rumors regarding the side effects of vaccination limit its acceptance.Citation29 Additionally, education level and trust in the government have been reported to influence responses to vaccination.Citation30,Citation31 A majority of countries have implemented vaccination programs and attitudes toward COVID-19 vaccination are changing positively, but targeted multipronged efforts are still required to maximize vaccination coverage, especially in underdeveloped countries. Finally, the research trends to date suggest that topics such as vaccine double-blind trials, viral mutations, antibody responses, and variants are more prominent than other topics.
The knowledge base comprises references co-cited by the research community,Citation32 that are not entirely equivalent to highly cited articles.Citation33 However, there is a considerable overlap between the collections of highly cited and co-cited articles in the present study. The top three cited articles and top three co-cited references all focused on the safety and efficacy of vaccines,Citation24,Citation25,Citation34 indicating the research hotspots. Additionally, more phased clinical trials are needed to determine the safety, immunogenicity, and efficacy of candidate vaccines in response to the emergence of new mutant strains. Of the 36 vaccines currently approved for use, there are 14 protein subunit vaccines, 11 inactivated virus vaccines, six non-replicating viral vector vaccines, three RNA vaccines, one DNA vaccine, and one VLP-based vaccine. Of these, two RNA vaccines, Comirnaty (BNT162b2) and Spikevax (mRNA-1273), have been the most extensively studied. Further research should be conducted on other types of vaccines. The effectiveness of vaccines against mutated strains of the virus and their side effects should be thoroughly studied.The world will eventually steer away from the impact of COVID-19, but vaccination remains a vital safeguard against this disease. The USA, China, the UK, and Italy have made significant contributions to COVID-19 vaccine research. In the context of rapid virus transmission, vaccine studies on immunogenicity, spike proteins, efficacy, safety, antibody response, and acceptance have received much attention. Additional clinical trials are required to determine the ability of potential vaccines to confer protection against emerging mutant strains. The findings of the present study may help funding agencies to better assess ongoing research and future research trends in COVID-19 vaccines. Moreover, the study will help vaccine researchers to determine the next research direction. Furthermore, the economic benefits should also be considered.
This study has some limitations. First, we considered the WoSCC as a reputable and reliable service for publications and citations; hence, we extracted data only from it, limiting the coverage of all possible articles, that led to a smaller number of documents included in the analysis. Second, there were no limitations on languages and the WoSCC contains literature in non-English languages, but its coverage may be incomplete. This bias should be considered in future studies. Third, the search strategy may be insufficient, that may lead to a lack of articles due to other terms. Finally, we selectively analyzed the information. Thus, some important points and details may have been overlooked. These reasons may have led to a bias in the results. Therefore, the results should be interpreted with caution.
Conclusions
Given the rapid transmission of SARS-CoV-2, vaccine studies on immunogenicity, spike proteins, efficacy, safety, and antibody response have undoubtedly received much attention. Additional phased clinical trials are needed to determine the effectiveness, acceptance, and side effects of vaccination against mutated strains of the virus. Currently, the safety and efficacy of vaccines are the focus of COVID-19 research. Other aspects may also receive attention, including studies on vaccine effectiveness against mutated strains of the virus, acceptability, and side effects.
Availability of data and material
All data generated or analyzed are included in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- WHO. Director-general’s opening remarks at the media briefing on COVID-19 - 11 March 2020. [accessed 2022 April 7]. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- Moradian N, Ochs HD, Sedikies C, Hamblin MR, Camargo CA, Martinez JA, Biamonte JD, Abdollahi M, Torres PJ, Nieto JJ, et al. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J Transl Med. 2020;18(1):1. doi:10.1186/s12967-020-02364-2.
- Moradian N, Moallemian M, Delavari F, Sedikides C, Camargo CA, Torres PJ, Sorooshian A, Mehdiabadi SP, Nieto JJ, Bordas S, et al. Interdisciplinary approaches to COVID-19, Adv exp med biol. 2021;1318:923–12. doi:10.1007/978-3-030-63761-3_52.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCov) in vitro. Cell Res. 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382(19):1787–1799. doi:10.1056/NEJMoa2001282.
- Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi:10.5582/bst.2020.01047.
- Campos DMO, Fulco UL, de Oliveira CBS, Oliveira JIN. SARS-CoV-2 virus infection: targets and antiviral pharmacological strategies. J Evid Based Med. 2020;13(4):255–260. doi:10.1111/jebm.12414.
- Twomey JD, Luo S, Dean AQ, Bozza WP, Nalli A, Zhang B. COVID-19 update: the race to therapeutic development. Drug Resist Updat. 2020;53:100733. doi:10.1016/j.drup.2020.100733.
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi:10.14336/ad.2020.0228.
- Xing Y, Mo P, Xiao Y, Zhao O, Zhang Y, Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveillance : Bulletin Euro Sur Les Maladies Transmissibles = Euro Commun Dis Bulletin. 2020;25(10). doi:10.2807/1560-7917.Es.2020.25.10.2000191.
- Su Z, McDonnell D, Li X, Bennett B, Šegalo S, Abbas J, Cheshmehzangi A, Xiang YT. COVID-19 vaccine donations-vaccine empathy or vaccine diplomacy? A narrative literature review. Vaccines. 2021;9. doi:10.3390/vaccines9091024.
- Basak P, Abir T, Al Mamun A, Zainol NR, Khanam M, Haque MR, Milton AH, Agho KE. A global study on the correlates of gross domestic product (GDP) and COVID-19 vaccine distribution. Vaccines. 2022;10(2). doi:10.3390/vaccines10020266.
- Ahn DG, Shin HJ, Kim MH, Lee S, Kim H-S, Myoung J, Kim B-T, Kim S-J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19. J Microbiol Biotechnol. 2020;30(3):313–324. doi:10.4014/jmb.2003.03011.
- Graham BS. Rapid COVID-19 vaccine development. Science (New York, NY). 2020;368(6494):945–946. doi:10.1126/science.abb8923.
- WHO. Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Zimmer C, Corum J, Wee SL. COVID-19 vaccine tracker. [accessed 2022 April 7]. https://covid19.trackvaccines.org/vaccines/approved/.
- Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: how great is the impact? Scientometrics. 2015;105(3):1809–1831. doi:10.1007/s11192-015-1645-z.
- Martín-Martín A, Thelwall M, Orduna-Malea E, Delgado López-Cózar E. Google Scholar, Microsoft Academic, Scopus, dimensions, Web of Science, and opencitations’ COCI: a multidisciplinary comparison of coverage via citations. Scientometrics. 2020:1–36. doi:10.1007/s11192-020-03690-4.
- Hou Q, Mao G, Zhao L, Du H, Zuo JJIJOLCA. Mapping the scientific research on life cycle assessment: a bibliometric analysis. Int J Life Cycle Assess. 2015;20:541–555. https://doi.org/10.1007/s11367-015-0846-2.
- Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2008;22(2):338–342. doi:10.1096/fj.07-9492LSF.
- Chen S, Lu Q, Bai J, Deng C, Wang Y, Zhao Y. Global publications on stigma between 1998-2018: a bibliometric analysis. J Affect Disord. 2020;274:363–371. doi: 10.1016/j.jad.2020.05.006.
- van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3.
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5303–5310. doi:10.1073/pnas.0307513100.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389.
- Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell. 2022;185(3):e454. doi:10.1016/j.cell.2021.12.033.
- Ahmad T, Murad MA, Baig M, Hui J. Research trends in COVID-19 vaccine: a bibliometric analysis. Hum Vaccines Immunotherapeutics. 2021;17(8):2367–2372. doi:10.1080/21645515.2021.1886806.
- Xu Z, Qu H, Ren Y, Gong Z, Ri HJ, Zhang F, Chen X, Zhu W, Shao S, Chen X, et al. Update on the COVID-19 vaccine research trends: a bibliometric analysis. Infect Drug Resist. 2021;14:4237–4247. doi:10.2147/idr.S335745.
- Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine : a survey of U.S. adults. Ann Intern Med. 2020;173(12):964–973. doi:10.7326/m20-3569.
- Xu Y, Zhang R, Zhou Z, Fan J, Liang J, Cai L, Peng L, Ren F, Lin W. Parental psychological distress and attitudes towards COVID-19 vaccination: a cross-sectional survey in Shenzhen, China. J Affect Disord. 2021;292:552–558. doi: 10.1016/j.jad.2021.06.003.
- Wang Q, Yang L, Jin H, Lin L. Vaccination against COVID-19: a systematic review and meta-analysis of acceptability and its predictors. Prev Med. 2021;150:106694. doi:10.1016/j.ypmed.2021.106694.
- Lu X, Lu C, Yang Y, Shi X, Wang H, Yang N, Yang K, Zhang X. Current status and trends in peptide receptor radionuclide therapy in the past 20 years (2000-2019): a bibliometric study. Front Pharmacol. 2021;12:624534. doi: 10.3389/fphar.2021.624534.
- Lu C, Liu M, Shang W, Yuan Y, Li M, Deng X, Li H, Yang K. Knowledge mapping of angelica sinensis (Oliv.) Diels (Danggui) research: a scientometric study. Front Pharmacol. 2020;11:294. doi: 10.3389/fphar.2020.00294.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdox1 nCov-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England). 2021;397(10269):99–111. doi:10.1016/s0140-6736(20)32661-1.