ABSTRACT
A 74-yr-old man underwent thoracic laparoscopy combined with radical gastrectomy, and the postoperative pathological diagnosis was esophageal and gastric cardia cancer pT3N1M0, pStage IIB. Immunohistochemical staining for HER2 (3+) and PD-L1 (<5%) was positive. Adjuvant chemotherapy was not performed because the patient developed severe thrombocytopenia (platelet counts <30 × 109/L), which was never cured throughout the reporting period. At 10.7 months post-surgery, he suffered metastases in multiple organs, including the peritoneum, liver, lung, and bone. Following two cycles of first-line trastuzumab and pembrolizumab (200 mg), he developed immune-related myositis (G2), myocarditis (G2), and hepatitis (G1). Therefore, pembrolizumab was discontinued. Trastuzumab was administered as a monotherapy; meanwhile, adoptive cytokine-induced killer (CIK) cell infusions were initiated. Eight months after the initial immunotherapy, a solitary brain metastasis was detected, and the patient underwent CyberKnife radiosurgery. For second-line therapy, adoptive CIK cell immunotherapy plus trastuzumab was still used. At the time of reporting, the patient had achieved a complete response (CR) in the brain and liver and a partial response (PR) in the ilium, and he had been followed-up for 36.6 months, much longer than the median survival time for patients with advanced GEJ cancer. We suggest that HER2-targeted therapy and immunotherapy with pembrolizumab or CIK adoptive cell infusions prolonged the overall survival of an elderly patient with HER2-positive GEJ cancer with multiple metastases.
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed cancer in the world and the fourth-leading cause of cancer-related death.Citation1 Approximately 10–22% of patients with GC have HER2 overexpression.Citation2 For these patients, trastuzumab plus chemotherapy significantly increased overall survival (OS).Citation3 However, the prognosis of patients with advanced gastric or gastroesophageal junction (GEJ) cancer is still not optimistic. Immune checkpoint inhibitors (ICIs) have been used in the treatment of multiple advanced cancers and have achieved considerable response. In May 2021, the US Food and Drug Administration (FDA) accelerated the approval of pembrolizumab plus trastuzumab combined with chemotherapy as a first-line treatment in locally advanced unresectable or metastatic HER2-positive gastric or GEJ adenocarcinoma.Citation4 In addition, cytokine-induced killer (CIK) cell immunotherapy has shown tumor-killing effects in the treatment of gastrointestinal cancer.Citation5 Here, we report the case of a patient with advanced GEJ cancer with multiple organ metastasis after surgery. After receiving HER2-targeted therapy and immunotherapy with pembrolizumab or CIK adoptive cell infusion, the patient has survived for 36.6 months.
Patient presentation
A 74-yr-old man was admitted to the hospital due to dysphagia. Lower esophageal and gastric cardia cancer was diagnosed by gastroscopic biopsy. His clinical stage was defined as cT3N1M0, stage IIIA, and neoadjuvant chemotherapy was recommended.Citation6
The patient’s clinical course, imaging changes () and tumor marker CEA () are shown in . One month after diagnosis, he received a cycle of SOX chemotherapy (S1, 60 mg, twice daily + oxaliplatin, 130 mg/m2), but he suffered severe grade 4 myelosuppression: his white blood cell count was 2.3 × 109/L, and his platelet count was 13.8 × 109/L. Fortunately, intravenous immunoglobulin and recombinant human thrombopoietin injection successfully treated this condition.
Figure 1. Imaging data and CEA changes during the treatment of a patient with advanced GEJ cancer with multiple organ metastasis after surgery. The surgery is taken as the origin, and – or + indicates the month before or after the surgery, respectively (m: months). (a) the patient was diagnosed with gastroesophageal junction cancer. After receiving neoadjuvant therapy, surgery, pembrolizumab (P) for 2 cycles, trastuzumab (T)-targeted therapy for 36 cycles, and CIK cell immunotherapy 9 times, he survived 36.6 months after the diagnosis of postoperative metastases to the time at which this manuscript was submitted. (b) After the patient received two cycles of trastuzumab and pembrolizumab, his CEA decreased significantly and remained within the normal range during subsequent treatments.
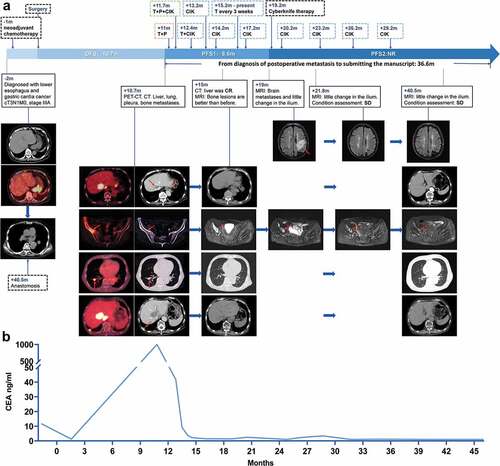
After approximately 2 months, he underwent thoracoscopic and laparoscopic esophagectomy combined with radical gastrectomy (pT3N1M0, pStage IIB). Immunohistochemical staining findings were positive PD-L1 (<5%, 22C3),Citation7 microsatellite stable (MSS) tumor, cMet (-), EGFR (2+), and HER2 (3+).Citation8 In situ hybridization showed a negative Epstein–Barr encoding region (EBER). After surgery, the patient’s platelet counts progressively decreased. Despite a series of treatments (transfusions and eltrombopag/ciclosporin/testosterone/undecanoate/prednisone), his platelet counts were maintained at only 20–30 × 109/L and did not return to normal levels. Therefore, the adjuvant chemotherapy was not performed because he developed severe thrombocytopenia (platelet counts <30 × 109/L), which was never cured thereafter.
At 10.7 months post-surgery, the patient was readmitted to the hospital because of abdominal pain. Abdominal ultrasonography revealed solid liver masses. Abdominal computed tomography (CT) showed multiple liver masses, enlarged abdominal and diaphragmatic lymph nodes, pleural nodules, and pleural thickening. Positron emission tomography-computed tomography (PET/CT) revealed metastases in multiple organs, including the lymph node, peritoneum, liver, lung, bilateral pleura, and bone (ilium, acetabulum, and ischia on the right side). Laboratory tumor markers were dramatically elevated (carcinoembryonic antigen (CEA) 1870 ng/ml; carbohydrate antigen 19–9 (CA19–9) 168 U/ml). Genetic analysis showed ERBB2 amplification, low TMB (2.74 mutations/Mb), MSS tumors, and negative EBER. Pathological findings were HER2 (3+), PD-L1 (+ <5%), and ERBB2 amplification. Unfortunately, the patient’s platelets remained low (26 × 109/L), and therefore, he was considered unable to tolerate conventional first- and second-line therapy; we considered pembrolizumab for the third-line treatment of patients with advanced or metastatic gastric or GEJ cancer with high PD-L1 expression, which was approved by the FDA in September 2017.Citation9 Meanwhile, the patient and their families have a strong willingness to treat, after sufficient communication and signed informed consent, we adjusted the patient’s therapeutic regimen with HER2-targeted therapy (trastuzumab 8 mg/kg D1) and anti-PD-1 antibody (pembrolizumab 200 mg D2). In addition, after the second cycle of therapy (trastuzumab 6 mg/kg D1, pembrolizumab 200 mg D2), the patient participated in a clinical trial (NCT02585908) and was treated twice with half-matched CIK cell infusions. However, before the third cycle of therapy, the patient felt fatigued, and his Karnofsky Performance Status (KPS) was 50. We considered this to be an adverse effect of pembrolizumab through a multidisciplinary treatment (MDT) discussion since adverse effects of trastuzumab and CIK cell infusions are rare,Citation10 so pembrolizumab was discontinued, and monotherapy of trastuzumab (6 mg/kg) and CIK cell immunotherapy were continued.
Two weeks later, the patient returned due to blurred vision, blepharoptosis, and lower extremity weakness. There was no myalgia, precordial discomfort, chest pain and congestion, dyspnea, or other symptoms. Laboratory tests for myocardial enzymes revealed aspartate aminotransferase (AST) 94 U/L, creatine kinase (CK) 912 U/L, creatine kinase isoenzyme MB (CK-MB) 66 U/L, α-hydroxybutyrate dehydrogenase (α-HBDH) 456 U/L, and lactate dehydrogenase (LDH) 522 U/L. Serum cardiac markers were myoglobin (Mb) 1034 ng/ml, high-sensitivity troponin (hs-TnT) 633.9 pg/ml, and brain natriuretic (BNP) 1520 pg/ml. The electrocardiogram showed a T wave abnormality. The echocardiography results were normal, the Holter electrocardiogram was further checked, and the changes in myocardial zymogram and serum cardiac markers were monitored. Since the patient had no typical chest pain, we performed radionuclide myocardial perfusion imaging and eliminated the diagnosis of myocardial infarction. We considered him to have immune-related myositis (G2), immune-related myocarditis (G2), and immune-related hepatitis (G1) due to pembrolizumab (10). Then, he received treatment with intravenous methylprednisolone. Approximately 10 d later, his symptoms improved, and various enzyme indicators gradually decreased. The dose of methylprednisolone was gradually reduced within 4 weeks.
At 15 months post-surgery, the patient underwent comprehensive assessments. The KPS was 90, CEA decreased from greater than 1000 to normal, and all the examinations were normal except for low platelets. Therefore, we considered a complete response in his liver and stable disease in his bone. After communication with the family, a regimen with fewer adverse effects was selected, he was treated with trastuzumab (every 3 weeks) and CIK cell immunotherapy (every quarter, total cellular score: (1.19 ± 0.02) ×1010 pcs/150 ml) and was followed-up every 3 months. Four months later, cranial CT revealed a solitary brain metastasis. We commenced local CyberKnife radiotherapy based on his progressive disease. The cranial CT revealed the disappearance of the brain metastasis 3 months later. After that, he repeated the maintenance therapy (trastuzumab every 3 weeks; CIK adoptive immunotherapy every quarter until 18 months ago; routine follow-up every 3 months).
At 40.5 months after surgery, the patient had stable disease after our evaluation. At the time of reporting, his disease-free survival was 10.7 months, and a total of 36.6 months had passed since the diagnosis of his postoperative metastasis ().
Discussion
In recent years, trastuzumab has been added to routine chemotherapy for patients with advanced or metastatic gastric or GEJ cancer and has achieved benefits, but the prognosis of patients with advanced GC is still not optimistic.Citation3 With the approval of pembrolizumab for the treatment of patients with PD-L1-positive advanced gastric or GEJ cancer,Citation9 researchers have tried to add pembrolizumab to the first-line therapy of patients with HER2-positive advanced gastric or GEJ cancer and found a better response rate and improved survival.11-13 Dual-antibody treatment combined with conventional chemotherapy for the first-line treatment of patients with advanced HER2-positive GC has shown good survival benefits (median PFS 8.6 months, median OS 19.3 months).Citation12 The ongoing phase 3 KEYNOTE-811 trial is a large sample, multicenter, randomized, double-blind trial that compares pembrolizumab/placebo plus trastuzumab and chemotherapy as the first-line treatment for patients with advanced gastric or GEJ cancer.Citation13 At present, this trial has reached the secondary endpoint, and a considerable amount of disease response data has been obtained. Therefore, dual-antibody combination chemotherapy has been approved by the FDA for the first-line treatment of patients with locally advanced unresectable or metastatic HER2-positive gastric or GEJ cancer.Citation4
Trastuzumab, a monoclonal antibody targeting HER2, inhibits the proliferation and survival of tumor cells by inhibiting HER2-mediated signal transduction, and patients with HER2-expression may have a better response targeted for trastuzumab therapy.Citation3 ICIs block the PD-1/PD-L1 pathway to enhance the activity of T cells. In previous studies, pembrolizumab was used in patients with PD-L1-positive advanced gastric or GEJ cancer and has good disease responses, and studies have indicated that anti-HER2 therapy may increase the expression of PD-L1 on tumor cells, further enhancing the potential synergy between the two.Citation11,Citation14 CIK cell immunotherapy, a cell therapy composed of dendritic cells (DCs) and CIKs, have the strong anti-tumor activity of T lymphocytes as well as non-major histocompatibility antigen (MHC)-restricted tumor killing ability of natural killer cells (NK cells),which can directly or indirectly lyse tumor cells by releasing perforin, granzyme, cytokine,Citation15 and studies have reported that combining anti-PD-1 therapy improves the survival of patients with GC. The mechanism of action may be that the combination of CIK cells and tumor cells leads to the upregulation of PD-L1/PD-1 expression and the upregulation of PD-1 expression on tumor cells and inhibits the tumoricidal activity of CIK cells through lentiviral transduction; thus, blocking PD-L1 can enhance the tumoricidal activity of CIK cells.Citation5 In patients with non-small-cell lung cancer (NSCLC) and renal cell carcinoma (RCC), this combination therapy has shown better benefits.Citation16,Citation17 There are also study reports that trastuzumab and CIK cell therapy have a synergistic effect in the treatment of HER2-positive tumors.Citation18 In addition, pembrolizumab can also provide more favorable conditions for CIK cell therapy by inhibiting the immune escape mechanism of tumors.
We report a patient who achieved long-term survival after targeted therapy and immunotherapy. This patient used the dual-antibody strategy of trastuzumab plus pembrolizumab as the first-line therapy after diagnosis of postoperative metastases, after two cycles, pembrolizumab was discontinued due to immune-related adverse events, and CIK cell immunotherapy was simultaneously infused 9 times. The patient survived 36.6 months after the diagnosis of postoperative metastases. The patient in our study was positive for HER2 (3+) and PD-L1 (<5%) and presented a good disease response after two cycles of pembrolizumab plus trastuzumab (the CEA decreased from more than 1000 ng/ml to 41.73 ng/ml). We believe that the dual-antibody therapy played an important role in the patient’s disease response. Simultaneously, the patient also accepted CIK cell immunotherapy. At the same time, CIK cell immunotherapy struggles to show effects in the short term, so we believe that it has played an important role in the long-term survival of the patient. Furthermore, CD3+ T cell infiltration in baseline tumor biopsy is a potential predictive biomarker for combination therapy, and due to the long storage time of the patient’s tissue, we did not perform IHC staining for further clarification.
Moreover, our patient could not tolerate chemotherapy due to low platelet counts, so he chose chemo-free therapy. At present, there are few studies on chemo-free therapy. In nonfirst-line therapy of patients with advanced GC, the single-use dual-antibody strategy of margetuximab plus pembrolizumab also achieved better benefits (median OS: 12.48 months).Citation14 Another study used the pembrolizumab plus trastuzumab dual-antibody strategy, but unfortunately, the study was terminated unexpectedly for unknown reasons (NCT02318901). Interestingly, the methods of another study are similar to ours.Citation11 The study did not use chemotherapy in the initial induction cycle, but the results showed that there was no significant difference in survival benefit between patients who received or did not receive the initial induction.
Besides, the expression status of PD-L1 may be a biomarker. Some studies have found that a higher expression of PD-L1 is associated with better benefits.Citation19,Citation20 However, there are also studies showing that the expression status of PD-L1 is unrelated to patient survival and response.Citation11,Citation12 Therefore, identifying possible biomarkers to determine the population with the greatest benefit is necessary.
This case indicates that the dual-antibody strategy of pembrolizumab and trastuzumab may increase the survival rates of patients with HER2-positive, PD-L1-positive advanced or metastatic gastric or GEJ cancer when used as first-line treatment, which may represent a new chemo-free approach for patients. We also added CIK cell immunotherapy; its mechanism is not clear, but it has been confirmed to have curative effects on patients with advanced gastric or GEJ cancer. Thus, the combination of targeted therapy and immunotherapy can provide a reference for patients who cannot receive chemotherapy, and there are many options for immunotherapy. In addition, the expression status of PD-L1 and HER2 may be biomarkers related to prognosis, and determining the population with the greatest benefit is still an urgent problem to be solved. In summary, we report the successful management of a patient with advanced metastatic GEJ cancer who was treated with trastuzumab and pembrolizumab or CIK cell immunotherapy; this combination of anti-HER2-targeted therapy and immunotherapy may provide additional survival benefits for patients with advanced or metastatic HER2-positive GC. However, because we only reported one patient’s case, its reliability remains to be verified, its mechanism may be clarified in in vitro studies in the future, and further clinical trials should be carried out.
Ethics statement
The patient provided his written informed consent. Written informed consent was obtained from him for the publication of any potentially identifiable images or data included in this article.
Acknowledgment
We are very grateful for this patient’s willingness to share this case.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–5. doi:10.3322/caac.21660.
- Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84. doi:10.1007/s10120-014-0402-y.
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi:10.1016/S0140-6736(10)61121-X.
- FDA Approves Merck’s KEYTRUDA® (pembrolizumab). Combined with trastuzumab and chemotherapy as first-line treatment in locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma.
- Dai C, Lin F, Geng R, Ge X, Tang W, Chang J, Wu Z, Liu X, Lin Y, Zhang Z, et al. Implication of combined PD-L1/PD-1 blockade with cytokine-induced killer cells as a synergistic immunotherapy for gastrointestinal cancer. Oncotarget. 2016;7(9):10332–44. doi:10.18632/oncotarget.7243.
- Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang Y-J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (London, England). 2019;39(1):10. doi:10.1186/s40880-019-0349-9.
- Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQM, Juco J, Lunceford J, Saraf S, Perini RF, O’Donnell PH. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212–20. doi:10.1016/S1470-2045(17)30007-4.
- Hofmann M, Stoss O, Gaiser T, Kneitz H, Heinmoller P, Gutjahr T, Kaufmann M, Henkel T, Ruschoff J. Central HER2 IHC and FISH analysis in a trastuzumab (Herceptin) phase II monotherapy study: assessment of test sensitivity and impact of chromosome 17 polysomy. J Clin Pathol. 2008;61(1):89–94. doi:10.1136/jcp.2006.043562.
- FDA Approves Merck’s KEYTRUDA® (pembrolizumab) for previously treated patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction cancer whose tumors express PD-L1 (CPS greater than or equal to 1). 2017.
- Wang S, Wang X, Zhou X, Lyerly HK, Morse MA, Ren J. DC-CIK as a widely applicable cancer immunotherapy. Expert Opin Biol Ther. 2020;20(6):601–07. doi:10.1080/14712598.2020.1728250.
- Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31. doi:10.1016/S1470-2045(20)30169-8.
- Rha SY, Lee C-K, Kim HS, Kang B, Jung M, Bae WK, Koo, D-H, Shin, S-J, Jeung, H-C, Zang, DY , et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J Clin Oncol. 2022;38(15_suppl):3081–. doi:10.1200/JCO.2020.38.15_suppl.3081.
- Chung HC, Bang Y-J, S Fuchs C, S-K Qin, Satoh T, Shitara K, Tabernero J, Van Cutsem E, Alsina M, Cao ZA, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol (London, England). 2021;17(5):491–501. doi:10.2217/fon-2020-0737.
- Catenacci DVT, Kang Y-K, Park H, Uronis HE, Lee K-W, Mch N, Enzinger PC, Park SH, Gold PJ, Lacy J, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–76. doi:10.1016/S1470-2045(20)30326-0.
- Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11(1):83. doi:10.1186/1479-5876-11-83.
- Han Y, Mu D, Liu T, Zhang H, Zhang J, Li S, Wang R, Du W, Hui Z, Zhang X, et al. Autologous cytokine-induced killer (CIK) cells enhance the clinical response to PD-1 blocking antibodies in patients with advanced non-small cell lung cancer: a preliminary study. Thoracic Cancer. 2020;12(2):145–52. doi:10.1111/1759-7714.13731.
- Dehno MN, Li Y, Weiher H, Schmidt-Wolf IGH. Increase in efficacy of checkpoint inhibition by cytokine-induced-killer cells as a combination immunotherapy for renal cancer. Int J Mol Sci. 2020;21(9):21. doi:10.3390/ijms21093078.
- Cappuzzello E, Tosi A, Zanovello P, Sommaggio R, Rosato A. Retargeting cytokine-induced killer cell activity by CD16 engagement with clinical-grade antibodies. Oncoimmunology. 2016;5(8):e1199311. doi:10.1080/2162402X.2016.1199311.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi:10.1001/jamaoncol.2018.0013.
- Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80. doi:10.1001/jamaoncol.2020.3370.
