 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of this post hoc analysis was to describe the immunogenicity of the 20-valent pneumococcal conjugate vaccine (PCV20) in adults with chronic medical conditions or smoking that place them at increased risk of developing pneumococcal disease. Data from 2 phase 3, randomized, active-controlled, double-blind studies in pneumococcal vaccine-naive adults were analyzed. Study 1: adults ≥18 years were enrolled in 1 of 3 age-based cohorts (18‒49, 50‒59, and ≥60 years) and randomized (1:1, adults ≥60 years; 3:1, younger cohorts) to receive 1 dose of PCV20 or 13-valent PCV (PCV13). Participants ≥60 years who received PCV13 were administered 23-valent polysaccharide vaccine 1 month later. Study 2: adults 18‒49 years were randomized (2:2:2:1) to receive 1 dose of PCV20 from 1 of 3 lots or PCV13. Opsonophagocytic activity (OPA) titers were measured in sera collected before and 1 month after vaccination. We investigated immune responses of PCV20 among participants 18‒64 and 18‒49 years of age with ≥1 medical condition or other factor (smoking) that increases the risk of serious pneumococcal disease. Of 4369 participants overall (PCV20, n = 2975; PCV13, n = 1394), 1329 participants (30%) had ≥1 risk factor; most commonly smoking, diabetes, and chronic pulmonary disease. Among participants with risk factors, substantial increases in OPA geometric mean titers were observed across the 20 vaccine serotypes from before vaccination to 1 month after PCV20. Robust immune responses to all 20 vaccine serotypes 1 month after PCV20 were observed in adults with increased risk of serious pneumococcal disease.
Clinical trial registration
NCT03760146, NCT03828617.
Introduction
Invasive pneumococcal disease (IPD) and noninvasive diseases caused by Streptococcus pneumoniae are significant global public health burdens.Citation1,Citation2 The introduction of pneumococcal conjugate vaccines (PCVs) into immunization programs has significantly reduced the pneumococcal disease burden worldwide across age groups.Citation1,Citation3 The 20-valent pneumococcal conjugate vaccine (PCV20) was developed to expand protection against pneumococcal disease beyond that of the 13-valent PCV (PCV13) and includes polysaccharide conjugates for seven additional serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F). These additional serotypes cause a substantial proportion of pneumococcal disease and have been associated with antibiotic resistance and severe disease.Citation4–10 PCV20 is anticipated to expand protection against pneumococcal disease in adults. Based on safety and immunogenicity in adults, PCV20 was approved for adults ≥18 years of age in the United States in June 2021.Citation11–13
Adults ≥65 years of age are at increased risk of pneumococcal disease, as are adults 18 to 64 years of age with certain comorbidities or who are immunocompromised.Citation14,Citation15 One study found that the risk of IPD in nonimmunocompromised adults with a comorbid condition (e.g., diabetes, chronic obstructive pulmonary disease) or being a cigarette smoker was twice that for the general population, and the risk increased with each additional condition.Citation15 Another study reported that IPD rates in at-risk persons are 4 times higher than for those without risk factors.Citation16 Thus, the immune response to vaccination against pneumococcal disease in these groups is important to understand.
Data pooled from 2 phase 3 studies with PCV20 in adults ≥18 years of age with no prior pneumococcal vaccinationCitation12,Citation13 show robust immune responses to all 20 vaccine serotypes 1 month after vaccination across age groups.Citation17 These phase 3 studies included participants with stable chronic medical conditions (e.g., asthma, diabetes mellitus, chronic heart or lung disease) or who smoked, which put them at increased risk of developing pneumococcal disease. The current post hoc analysis sought to describe the immunogenicity of PCV20 in these populations. Data from both studies were combined to create a larger subset of adults 18–64 years of age to evaluate immune responses in at-risk adults who were likely to be considered for recommendations based on risk, rather than age-based pneumococcal immunization programs in adults ≥65 years of age. Such recommendations have been adopted for PCV20 by the Advisory Committee on Immunization Practices in the United States.Citation18 As consideration has been given to age-based recommendations in individuals ≥50 years of age as a way to achieve higher uptake among adults,Citation18 and this may be revisited in the future, the results in a younger, smaller subset 18–49 years of age of at-risk adults are also described.
Materials and methods
Data from 2 phase 3, randomized, active-controlled, double-blind studies in adults with no history of pneumococcal vaccination were included in this post hoc analysis (ClinicalTrials.gov NCT03760146, NCT03828617).Citation12,Citation13 The study designs of the phase 3 trials have been described previously.Citation12,Citation13 In Study 1,Citation12 adults ≥18 years of age were enrolled in 1 of 3 cohorts based on age (18‒49, 50‒59, and ≥60 years of age) and randomized (adults ≥60 years of age were randomized 1:1, and both younger age cohorts 3:1) to receive 1 dose of PCV20 or PCV13 at study entry. Participants ≥60 years of age who received PCV13 at study entry were administered 23-valent polysaccharide vaccine (PPSV23) or saline 1 month later. In Study 2,Citation13 adults 18‒49 years of age were randomized (2:2:2:1) to receive a single dose of PCV20 from 1 of 3 lots or PCV13. In both studies, adults on immunosuppressive therapy or with immunocompromising conditions were not eligible for participation. Opsonophagocytic activity (OPA) titers were measured in sera collected before and 1 month after vaccination to evaluate immune responses. Both studies were conducted in accordance with all ethical, legal, and regulatory requirements.Citation12,Citation13
A post hoc analysis was conducted from both studies to understand the immune responses of PCV20 and control vaccines (PCV13 and PPSV23) among participants 18–64 years of age with ≥1 medical condition or other factor (smoking) that increases the risk of serious pneumococcal disease. Participants 18–64 years of age from Study 1 were combined with the Study 2 population 18–49 years of age. Both studies enrolled participants from sites in the United States and were similar in study design, inclusion and exclusion criteria, and immunogenicity assessments.Citation12,Citation13
OPA geometric mean titers (GMTs) and geometric mean ratios (GMRs) and corresponding 95% CIs were calculated based on linear regression models, including terms for study, vaccine group, sex, age at vaccination in years (continuous), and baseline OPA titers. Percentages of participants with a ≥ 4-fold rise in OPA titers from before to 1 month after vaccination were summarized by descriptive summary statistics. All analyses were performed on the evaluable immunogenicity populations defined in the study protocols, which included participants without major protocol deviations who received vaccination(s) as randomized and had ≥1 valid OPA titer from a blood sample collected within a prespecified window 1 month after vaccination. Data from both studies were combined to create subset age groups of 18–64 and 18–49 years of age to evaluate immune responses in at-risk adults who would not be covered by age-based pneumococcal immunization programs targeting adults ≥65 or ≥50 years of age, respectively.
Results
Overall there were 4369 participants 18–64 years of age (PCV20, n = 2975; PCV13, n = 1394). Demographic characteristics among participants with risk factors were generally similar between the PCV13 and PCV20 groups (). The median age was somewhat lower in the PCV20 group compared with the PCV13 group, consistent with the higher PCV20:PCV13 randomization ratios in the younger participants in Study 2 (6:1) compared to Study 1 (3:1). Overall, 1329 of 4369 participants (30%) had ≥1 risk factor and were included in this analysis. Smoking, diabetes, and chronic pulmonary disease were the most common risk factors in the pooled sample (Table S1).
Table 1. Demographics of participants with ≥1 risk factor*: pooled, evaluable immunogenicity population.
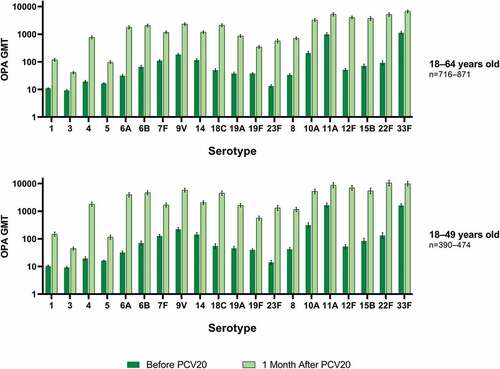
Among PCV20 recipients with risk factors, substantial increases in OPA GMTs were observed across the 20 vaccine serotypes from before vaccination to 1 month after PCV20 ().
Figure 1. Pneumococcal OPA GMTs with 2-sided 95% CIs for the PCV20 serotypes before vaccination and 1 month after PCV20 in participants with ≥1 risk factor*: evaluable immunogenicity population.
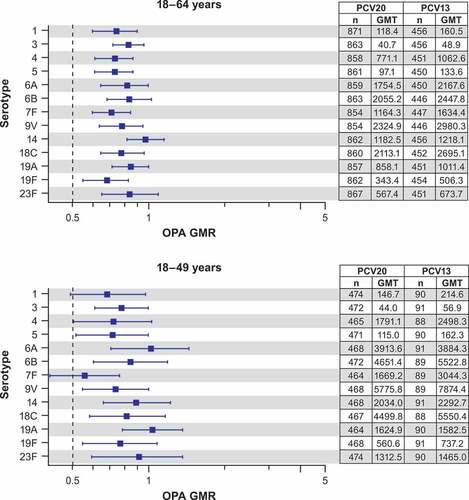
Among adults 18–64 years of age with risk factors, the observed OPA GMRs of PCV20 to PCV13 1 month after vaccination for each of the 13 serotypes had a lower bound of the 95% CI of >0.5 and therefore would have met noninferiority applying a 2-fold noninferiority criterion typically used for the OPA immune responses (). Among the subset of adults 18–49 years old with risk factors, the observed OPA GMRs of PCV20 to PCV13 for each of the matched serotypes also had lower bound of the 95% CI of >0.5, except serotype 1 and serotype 7F with OPA GMRs of 0.68 (95% CI: 0.48, 0.97) for serotype 1 and 0.55 (95% CI: 0.40, 0.76) for serotype 7F (). Wider CIs were observed with this subset of participants 18–49 years of age due to overall smaller sample size, particularly in the PCV13 group (only approximately 90 participants), which can exhibit variability and allow a relatively small number of participants to have a disproportionate impact on the results. The observed point estimates for those two serotypes were both >0.5. When the analysis among adults 18–64 years of age was restricted, defining risk factors by chronic medical conditions only (i.e., excluding smoking only as a risk factor [population in the row labeled “any selected medical history“ in Table S1]), the OPA GMR findings were similar to the population that included smoking for the 13 matched serotypes. The OPA GMTs for the seven additional serotypes were substantially higher than in the PCV13 group (Table S2).
Figure 2. Pneumococcal OPA GMRs (PCV20/PCV13) with 2-sided 95% CIs for the PCV13 serotypes 1 month after vaccination in participants with ≥1 risk factor*: evaluable immunogenicity population.
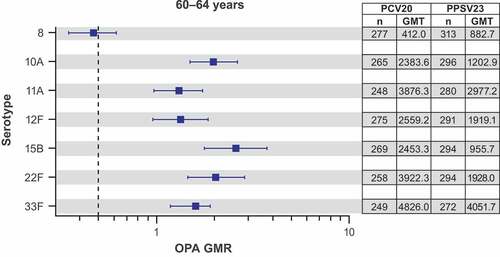
In adults with risk factors 60–64 years of age who received PCV20 or PPSV23 (administered 1 month after PCV13 in the control group) in Study 1, the observed OPA GMTs of PCV20 were generally higher than those of PPSV23, with GMRs >1.0, for the 7 additional serotypes 1 month after vaccination for all serotypes, except serotype 8 with an OPA GMR of 0.47 (95% CI: 0.35, 0.62) ().
Figure 3. Pneumococcal OPA GMRs (PCV20/PPSV23) with 2-sided 95% CIs for the 7 additional serotypes 1 month after vaccination in participants 60–64 years old* with ≥1 risk factor†: evaluable immunogenicity population.
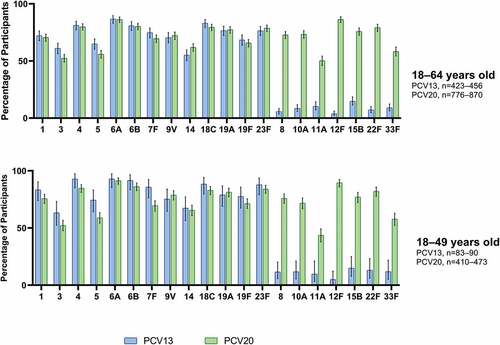
Robust immune responses to all vaccine serotypes, as measured by the percentage of participants with ≥4-fold rises in OPA titers from before to 1 month after PCV20, were observed in the subpopulation with risk factors across both age groups (). The percentage of participants with ≥4-fold rises in OPA titers from before to 1 month after PCV20 was generally slightly lower (<10%) than after PCV13 for the 13 matched serotypes. In the 18–49-year age group, the observed responses to serotypes 3, 5, and 7F were somewhat greater than 10% (11–16%) lower in the PCV20 group than in the PCV13 group, however, a ≥4-fold rise was observed in more than 50% of participants after PCV20. The responses to the seven additional serotypes after PCV20 were well above those in the PCV13 group.
Figure 4. Percentage (with 95% CIs) of participants with a ≥4-fold rise in OPA titers from before to 1 month after PCV13 or PCV20 for the PCV20 serotypes in participants with ≥1 risk factor*: evaluable immunogenicity population.
Discussion
PCV20 was recently approved for adults ≥18 years of age and recommended as a single vaccination in all adults with no or unknown history of prior pneumococcal vaccination, ≥65 years of age, and in those 19–64 years of age with increased risk of pneumococcal disease in the United States.Citation18,Citation19 This analysis shows that PCV20 elicits robust immune responses to all 20 vaccine serotypes 1 month after vaccination in at-risk adults with underlying medical conditions or current smokers based on post hoc analysis from 2 phase 3 clinical studies.
Observed OPA GMRs in this post hoc analysis showed that after vaccination of this population of adults 18–64 years with PCV20 were comparable with PCV13 for all 13 matched serotypes. Observed OPA GMRs to PCV20 were higher than PPSV23 for 6 of 7 additional serotypes; that is, for all serotypes except serotype 8. This reflects the relative findings of PCV20 to the controls found in the overall pivotal study population.Citation12 Among the subpopulation of adults 18–49 years with chronic medical conditions and smoking, the responses to PCV20 were also generally comparable to PCV13, although the lower bound of the 95% confidence interval of the OPA GMRs for serotypes 1 and 7F was below 0.5, the statistical comparisons were limited by small sample size, especially of the PCV13 group. Serotype 8 missed the statistical noninferiority criterion for at-risk adults 18–64 years of age (also observed in the pivotal study comparison).Citation12 The other immunogenicity data such as percentage with ≥4-fold rises in OPA titers and increases in geometric mean titers from before to 1 month after PCV20 showed that robust responses were elicited to all vaccine serotypes in adults 18–64 years of age with risk factors.
Multiple factors may influence the immune response to vaccination, including underlying medical conditions, such as diabetes or smoking, which were common among the risk factors in these study populations.Citation20–23 The exact nature of this influence is not well established, but various immune mechanisms (specific and nonspecific) are likely to be involved. These immune mechanisms may contribute to the increased burden of pneumococcal disease among persons with certain chronic conditions compared with those without; those with ≥2 conditions approach rates observed among persons ≥65 years of age or with high-risk conditions.Citation16 Vaccination against pneumococcal disease is therefore especially important in these at-risk populations.Citation21
The Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA) study showed robust, persistent efficacy of PCV13 against vaccine-serotype community-acquired pneumonia in at-risk older adults.Citation24 Similarly, a real-world study of PCV13 effectiveness in Louisville, Kentucky, found that adjusting for at-risk status did not change vaccine effectiveness against vaccine-type community-acquired pneumonia requiring hospitalization.Citation25 An additional real-world study in Southern California reported that PCV13 in at-risk persons had a modestly lower effectiveness against all-cause lower respiratory tract infection and pneumonia compared with those with normal risk.Citation26 Given that there is a likely higher background incidence of pneumonia in those at risk, PCV20, like PCV13,Citation24–26 is expected to be effective at preventing IPD and pneumonia in this population.
With the pooled data from 2 phase 3 studies, the large number of participants, including participants 18–49 years of age with risk factors, is a key strength of this analysis. Although this analysis did not assess safety specifically in at-risk individuals, no safety concerns were identified in the phase 3 studies, and the PCV20 safety profile was comparable with PCV13.Citation12,Citation13 We do not anticipate any safety issues in the at-risk population. The large pivotal study (Study 1) showed that the safety and immunogenicity of PCV20 is comparable with PCV13, thus we expect that PCV20 will perform similarly to PCV13 in nonimmunocompromised at-risk adults.Citation12 Limitations of the study include the post hoc nature of the analysis and that the at-risk population was still relatively small despite the large number of participants in the PCV20 group who were 18–49 years of age.
In summary, the data support the use of PCV20 to help prevent pneumococcal disease in adults with increased risk. Such individuals may experience a greater potential benefit with vaccination compared with the general population.
Data sharing statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Supplemental Material
Download Zip (126.4 KB)Disclosure statement
CS, YP, LM, TB, KUJ, WCG, DAS, BG, LJ, and WW are employees of Pfizer and may hold stock and/or stock options. VS reports no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2126253
Additional information
Funding
References
- World Health Organization. Pneumococcal vaccines WHO position paper—2012. Wkly Epidemiol Rec. 2012;87(14):1–7.
- Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18(suppl 5):7–14. doi:10.1111/j.1469-0691.2012.03937.x.
- Vadlamudi NK, Chen A, Marra F. Impact of 13-valent pneumococcal conjugate vaccine among adults: a systematic review and meta-analysis. Clin Infect Dis. 2018;69(1):34–49. doi:10.1093/cid/ciy872.
- Perdrizet J, Wasserman M, Farkouh RA, Chilson E. Estimated pneumococcal disease and economic burden for current and future vaccine serotypes in United States in children under five years of age. Paper presented at: International Symposium on Pneumococci and Pneumococcal Diseases; 2020 Jun 19–23; Toronto, Canada. Digital Library. https://cslide.ctimeetingtech.com/isppd20/attendee/confcal/session/list?q=Perdrizet.
- van der Linden M, Imohl M, Perniciaro S. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS One. 2019;14(8):e0220453. doi:10.1371/journal.pone.0220453.
- de Miguel S, Domenech M, Gonzalez-Camacho F, Sempere J, Vicioso D, Sanz JC, Garcia Comas L, Ardanuy C, Fenoll A, Yuste J. Nationwide trends of invasive pneumococcal disease in Spain (2009-2019) in children and adults during the pneumococcal conjugate vaccine era. Clin Infect Dis. 2020;73(11): e3778–e3787. doi:10.1093/cid/ciaa1483.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Impact of 13-valent pneumococcal conjugate vaccine used in children on invasive pneumococcal disease in children and adults in the United States: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74. doi:10.1080/21645515.2015.1118593.
- Metcalf BJ, Gertz RE Jr., Gladstone RA, Walker H, Sherwood LK, Jackson D, Li Z, Law C, Hawkins PA, Chochua S, et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect. 2016;22(1):60.e69–60.e29. doi: 10.1016/j.cmi.2015.08.027.
- Tomczyk S, Lynfield R, Schaffner W, Reingold A, Miller L, Petit S, Holtzman C, Zansky SM, Thomas A, Baumbach J, et al. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2016;62(9):1119–25. doi:10.1093/cid/ciw067.
- Food and Drug Administration. BLA approval and BLA accelerated approval letter. [accessed 2021 June 18]. https://www.fda.gov/media/150021/download.
- Essink B, Sabharwal C, Cannon K, Frenck R, Lal H, Xu X, Sundaraiyer V, Peng Y, Moyer L, Pride M, et al. Pivotal phase 3 randomized clinical trial of the safety, tolerability, and immunogenicity of 20-valent pneumococcal conjugate vaccine in adults 18 years and older. Clin Infect Dis. 2022;75(3):390–98. doi:10.1093/cid/ciab990.
- Klein NP, Peyrani P, Yacisin K, Caldwell N, Xu X, Scully IL, Scott DA, Jansen KU, Gruber WC, Watson W. A phase 3, randomized, double-blind study to evaluate the immunogenicity and safety of 3 lots of 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults 18 through 49 years of age. Vaccine. 2021;39(38):5428–35. doi:10.1016/j.vaccine.2021.07.004.
- Grant LR, Slack MPE, Yan Q, Trzcinski K, Barratt J, Sobczyk E, Appleby J, Cane A, Jodar L, Isturiz RE, et al. The epidemiologic and biologic basis for classifying older age as a high-risk, immunocompromising condition for pneumococcal vaccine policy. Expert Rev Vaccines. 2021;20(6):691–705. doi:10.1080/14760584.2021.1921579.
- Baxter R, Yee A, Aukes L, Snow V, Fireman B, Atkinson B, Klein NP. Risk of underlying chronic medical conditions for invasive pneumococcal disease in adults. Vaccine. 2016;34(36):4293–97. doi:10.1016/j.vaccine.2016.07.003.
- Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16(1):182. doi:10.1186/s12913-016-1432-4.
- Sabharwal C, Suaya J, Sundaraiyer V, Peng Y, Moyer L. Immunogenicity of a 20-valent pneumococcal conjugate vaccine across age groups in adults 18 years and older. Paper presented at: European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2021; 2021 Jul 9–12; Virtual congress (online).
- Kobayashi M, Farrar JL, Gierke R, Britton A, Childs L, Leidner AJ, Campos-Outcalt D, Morgan RL, Long SS, Talbot HK, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–17. doi:10.15585/mmwr.mm7104a1.
- PREVNAR 20- pneumococcal 20-valent conjugate vaccine injection, suspension Wyeth pharmaceutical division of Wyeth Holdings LLC. https://labeling.pfizer.com/ShowLabeling.aspx?id=15428; 2021.
- Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084–00018. doi:10.1128/cmr.00084-18.
- Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–89. doi:10.1136/thoraxjnl-2015-206780.
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–16. doi:10.1001/archinte.164.20.2206.
- Fukuda H, Onizuka H, Nishimura N, Kiyohara K. Risk factors for pneumococcal disease in persons with chronic medical conditions: results from the LIFE study. Int J Infect Dis. 2022;116:216–22. doi:10.1016/j.ijid.2021.12.365.
- Suaya JA, Jiang Q, Scott DA, Gruber WC, Webber C, Schmoele-Thoma B, Hall-Murray CK, Jodar L, Isturiz RE. Post hoc analysis of the efficacy of the 13-valent pneumococcal conjugate vaccine against vaccine-type community-acquired pneumonia in at-risk older adults. Vaccine. 2018;36(11):1477–83. doi:10.1016/j.vaccine.2018.01.049.
- McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018;67(10):1498–506. doi:10.1093/cid/ciy312.
- Lewnard JA, Bruxvoort KJ, Fischer H, Hong VX, Grant LR, Jodar L, Cane A, Gessner BD, Tartof SY. Effectiveness of 13-valent pneumococcal conjugate vaccine against medically-attended lower respiratory tract infection and pneumonia among older adults. Clin Infect Dis. 2022;75(5): 832–41. doi:10.1093/cid/ciab1051.
