ABSTRACT
To inform the public and policy makers, we investigated and compared the risk of cerebral venous sinus thrombosis (CVST) after SARS-Cov-2 vaccination or infection using a national cohort of 2,643,699 individuals aged 17 y and above, alive, and resident in Wales on 1 January 2020 followed up through multiple linked data sources until 28 March 2021. Exposures were first dose of Oxford-ChAdOx1 or Pfizer-BioNTech vaccine or polymerase chain reaction (PCR)-confirmed SARS-Cov-2 infection. The outcome was an incident record of CVST. Hazard ratios (HR) were calculated using multivariable Cox regression, adjusted for confounders. HR from SARS-Cov-2 infection was compared with that for SARS-Cov-2 vaccination. We identified 910,556 (34.4%) records of first SARS-Cov-2 vaccination and 165,862 (6.3%) of SARS-Cov-2 infection. A total of 1,372 CVST events were recorded during the study period, of which 52 (3.8%) and 48 (3.5%) occurred within 28 d after vaccination and infection, respectively. We observed slight non-significant risk of CVST within 28 d of vaccination [aHR: 1.34, 95% CI: 0.95-1.90], which remained after stratifying by vaccine [BNT162b2, aHR: 1.18 (95% CI: 0.63-2.21); ChAdOx1, aHR: 1.40 (95% CI: 0.95-2.05)]. Three times the number of CVST events is observed within 28 d of a positive SARS-Cov-2 test [aHR: 3.02 (95% CI: 2.17-4.21)]. The risk of CVST following SARS-Cov-2 infection is 2.3 times that following SARS-Cov-2 vaccine. This is important information both for those designing COVID-19 vaccination programs and for individuals making their own informed decisions on the risk-benefit of vaccination. This record-linkage approach will be useful in monitoring the safety of future vaccine programs.
Introduction
COVID-19 disease remains a prominent global public health issue. Current evidence shows that vaccinations are the best way to protect people from the impacts of COVID-19 disease, with studies showing a high level of real-world effectiveness and efficacy between 66% and 95% for Pfizer-BioNTech (BNT162b2) and Oxford-ChAdOx1 (ChAdOx1) against symptomatic SARS-CoV2Citation1–4 and a significant reduction in hospitalizations and deaths.Citation5,Citation6 In Wales, the vaccination program started from 7 December 2020, and three medically regulated vaccines: BNT162b2, ChAdOx1, and Moderna (mRNA-1273) have been made available by the National Health Service (NHS) to the individuals.Citation7
However, given the use of vaccinations on healthy populations and the importance of public and professional confidence in immunization,Citation8 vaccine safety is a critical element and a key determinant of uptake and hesitancy. The mass rollout of the COVID-19 vaccination program in early December 2020 was hampered by reports of adverse thromboembolic events (mostly within the first 4 weeks of the vaccine), including cerebral venous sinus thrombosis (CVST), particularly following the ChAdOx1.Citation9–12 This has led to temporary withdrawal or restricted use of the vaccines in some countries.Citation13 In the UK, for example, adults under the age of 40 were offered an alternative to the ChAdOx1 vaccine, if available.Citation14,Citation15
Although a few studies have investigated the possible association between CVST and COVID-19 vaccines or disease, results so far have remained inconsistent. Hence, the actual risk of such adverse events and whether or not post-vaccine or infection cases of CVST are indeed excess events are yet to be established, given that rare events are not usually identified even in large clinical trials. In addition, studies investigating the risk of CVST following COVID-19 disease from the perspective of risk-benefit analysis are limited. This is crucial for the vaccination program, particularly the global campaign to reduce hesitancy and increase uptake.
We compared the risk of CVST from COVID-19 vaccines with the risk from the COVID-19 disease itself in a national cohort using multiple linked electronic health record (EHR) data sources.
Materials and methods
Study setting and participants
The National Health Service in Wales (NHS Wales) provides a comprehensive health service that is free at the point of care for all residents. Our base population for this study included 2.6 million adult residents aged 17 years or above (79.0% of the national population) registered with a SAIL providing general practice (GP) in Wales. Appendices 1 & 2 show a flowchart of the study selection process.
Study design and period
We conducted a population-based cohort study to examine any associations between ChAdOx1 or BNT162b2 vaccines and CVST events. We also investigated the association between infection with SARS-CoV-2 and CVST events in the same population. All participants were followed for index PCR confirmed COVID-19 positive test or COVID-19 vaccination between 1 January 2020 (baseline) and 28 February 2021 and for incident hospital or GP contact for CVST outcome from 1 January 2020 to 28 March 2021, emigration, death, or occurrence of the outcome, whichever came first.
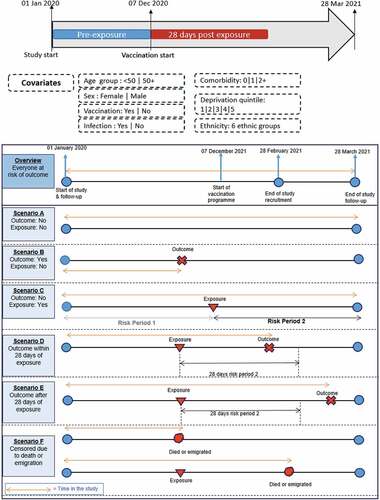
We assumed that all the participants were equally at risk of the outcome at baseline. We used Cox proportional hazard regressionCitation16 to investigate the risk of CVST after vaccination or infection compared to the period before (both vaccination and infection are treated as time-varying covariates). The study period timeline with risk period scenarios is shown in . The rationale for examining risk in the 28 d after vaccination or infection was that cases reported to the regulatory agencies were within 28 d after vaccination.
Data sources
We used anonymized individual-level, population-scale data available in the privacy-protecting trusted research environment (TRE), the Secure Anonymized Information Linkage (SAIL) Databank, hosted by Swansea University,Citation17–19 using the SAIL Databank Con-COV e-cohort.Citation20 The C19_COHORT20, which includes all Welsh residents alive and living in Wales on 1 January 2020, with follow-up through multiple data sources until the end of 2022, was the study population. All relevant data sources were linked to this cohort, namely:
COVID-19 Vaccination Data (CVVD) for vaccination date and vaccine type (start of vaccination was 7th December 2020).
Pathology COVID-19 Daily data (PATD) for the index PCR-positive SARS-CoV-2 infection date, defined as any PCR-positive SARS-CoV-2 test allowing for 90 d between episodes for those with potential repeat infection.
Patient Episode Dataset for Wales (PEDW) for hospital contact date for CVST, allowing for at least 365 d between admissions for those with potential repeat admission.
Wales Longitudinal General Practice (WLGP) for GP contact date for CVST, allowing for at least 365 d between events for those with potential repeat events.
Inclusion and exclusion criteria
We included all persons aged 17 y or above alive and resident in Wales as at 1 January 2020 with at least 12 consecutive months of NHS GP registration prior to and after 1 January 2020. We excluded participants younger than 17 y and those who moved to Wales within 365 d prior to 1 January 2020 (ascertained from GP registration). We also excluded participants (n = 12) with a sample date prior to February 2020 or a vaccination date prior to 7 December 2020.
Exposure definition
We considered only the first dose of the BNT162b2 or ChAdOx1 vaccines or PCR confirmed positive COVID-19 test results. An individual was defined as exposed if they received at least a single dose of vaccine or had a positive COVID-19 result between 1 January 2020 and 28 February 2021. The baseline period (without exposure) was defined as any time between 1 January 2020 and the exposure date or the censored date, if earlier. To reduce the potential bias from double exposures, we derived a covariate called dual exposure to flag when a participant’s exposure to both a COVID-19 vaccine and infection was within 28 d of each other.
Outcomes and covariates
Outcomes were CVST hospitalization or GP contact identified using International Classification of Diseases-10 (ICD-10) codes in PEDW or Read codes in WLGP (codes available in Appendix 3). We used the earliest date of hospital admission or GP contact as the event date, including where there were multiple records for the same participant in both PEDW and WLGP. We defined a CVST event as one occurring during the study period with no repeat occurrence for the same participant in the previous 365 d. For multiple events in the same participant, there must be an interval of at least 365 d.
Other covariates were age, sex, Welsh index of multiple deprivation (WIMD) quintile version 2019, ethnicity, and common comorbidities identified a priori as risk factors for CVST. Comorbidities were derived from the QCovidCitation21,Citation22 risk groups on 7 December 2020 based on the conditions on UK shielding patient list or clinical and other risk groups eligible for COVID-19 vaccination.Citation23 We described the total number of comorbidities (0 vs 1 vs ≥2 comorbidities). A full list of comorbidities considered is available in Appendix 4.
Statistical analysis
Main analysis
Continuous variables were expressed as medians and interquartile ranges (IQR). Categorical variables were summarized as n (%) in each category. Wilcoxon rank sum test, Pearson’s Chi-squared test, and Fisher’s exact test were applied where appropriate. Univariable and multivariable Cox proportional hazard regressions were used to explore the covariates, with outcome periods introduced as time varying covariates.
We analyzed the observed incident CVST in the post-exposure period compared to those from the pre-exposure period. The pre-exposure period was the number of days from the start of the study (1 January 2020) to the exposure date. The post-exposure period was the 28-d period following vaccination or infection. Hence, the same participant could contribute both exposed and unexposed time under observation to the study.
Variables with p < .25 in univariable analysis were included in the model building of multivariable Cox regression. A stepwise selection strategy was used to remove variables that did not significantly affect the final model. The unadjusted model included no covariates other than the outcome period (i.e. before, 1–28 d). The hazard ratio (HR) and 95% confidence intervals (95% CI) were calculated. Where at least one stratum of a variable did not have enough number of CVST events to estimate hazard ratios, that variable was excluded from the analysis.
Missing data (in deprivation quintiles and ethnicity) were classified as ‘unknown,’ while missing data for body mass index (BMI) were classified as “not obese” because we assumed, based on local knowledge, that participants were more likely to have a BMI record if they were truly obese.
The Extended Cox proportional hazards (PH) assumption was checked using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. There was no evidence of violation of the PH assumption.
All analyses were carried out with R programming software (version 4.0.5).
Supplementary analyses
We conducted some pre-specified analyses; we stratified the analyses by sex and age (<50 y and ≥50 y). To further investigate subgroup effects, we also stratified the analyses by the vaccine received by participants (BNT162b2and ChAdOx1).
Sensitivity analyses
We conducted sensitivity analyses, restricting the analyses to the period after vaccination or after COVID-19 positive test in order to compare the relative risk of outcome for the exposed periods (i.e. 28 d after vaccination or COVID-19 infection) compared to the unexposed baseline period (i.e. time to event, exposure, death, or migration, whichever came first). The estimate of the effect measure is within participants and, by implication, intrinsically controls for all covariates that remain constant during the study period.
We also repeated the original analysis with a longer follow-up period (56 d) to ascertain whether rates were higher or not after the study cutoff period of 28 d and compared the first 28 d with the second 28 d of follow-up.
Role of the funding source
The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the report.
Results
This study included 2,643,699 adults in Wales (79.0% of the national population) aged 17 y or above, followed from 1 January 2020 to 28 March 2021. During this period, 34.0% received at least one dose of a COVID-19 vaccination, while 6.3% had a positive COVID-19 test result. Of all participants who received a COVID-19 vaccine, 53.0% had ChAdOx1.
The median (IQR) age in years of vaccinated participants was 69 (56–76) and 41 (29–53) for the unvaccinated; approximately half the participants were female; 82% were of White ethnicity, 5.5% and 6.7% of the participants’ deprivation quintile and ethnicity were unknown. Nearly 7.0% had at least one comorbidity, with asthma, mental illness, and diabetes as the top three (Appendix 5).
A total of 1,372 people experienced a CVST event (excluding 5 on the start date of the study), of which 52 (3.8%) and 48 (3.5%) occurred in the 28 d after vaccination or infection, respectively. Appendix 6 shows the weekly incidence and 7-d rolling average of CVST events during the study period.
A total of 49,802 (0.02%) of the participants died during the study period, of which 0.2% and 0.3% were vaccinated or infected, respectively. provides a full breakdown of baseline characteristics of study participants.
Table 1. Summary statistics of the study subjects.
Where possible, multivariable models were adjusted for age, sex, ethnicity, deprivation, comorbidity, and exposure to both SARS-CoV-2 virus and vaccination within 28 d of each other.
Risk of CVST after COVID-19 vaccination
Compared to the period before vaccination, we found no significant association between CVST event and any COVID-19 vaccination (1–28 d’ adjusted hazard ratio (aHR) [1.34; 95% CI: 0.95-1.90]). Similar results were found after stratifying the type of vaccine received [BNT162b2 1–28 d], aHR [1.18; 95% CI: 0.63-2.21]; ChAdOx1 1–28 d, aHR [1.40; 95 CI: 0.95-2.05] – . Full details of the univariable and multivariable analyses for all covariates are provided in .
Figure 2. Cox regression estimates of CVST events following SARS-CoV-2 vaccination: all participants.
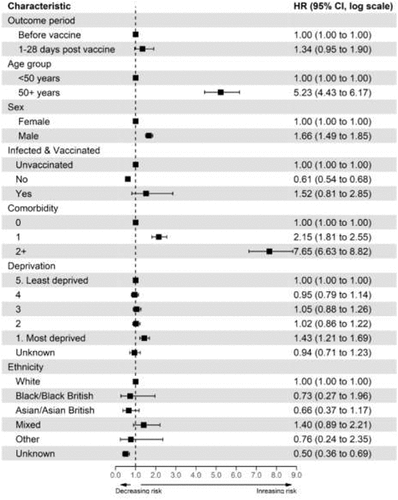
Table 2. Cox regression estimates of CVST events following SARS-CoV-2 vaccination by vaccine type.
When stratified by sex, we found no association between CVST and any COVID-19 vaccination ([1–28 d aHR for males (1.47; 95% CI: 0.95-2.28)]; [1–28 d for females, aHR (1.16; 95% CI: 0.66-2.06]), [Appendix 7]. Compared to the unvaccinated cohort, exposure to both COVID-19 vaccination and infection within 28 d of each other was not associated with an increased risk of CVST event (aHR 1.52; 95% CI: 0.81-2.85) [].
Risk of CVST after Covid-19 infection
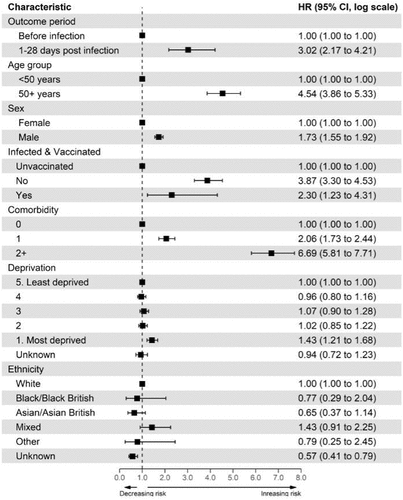
We found an increased risk of CVST event after COVID-19 infection (1–28 d, aHR [3.02; 95% CI: 2.17-4.21]). Full details of the univariate and multivariate analysis for all covariates are provided in . The risk remained significant after stratifying by age and sex, with males [vs females aHR 3.33; 95% CI: 2.24-4.97] and those over 50 y old [vs <50 y aHR 3.15; 95% CI: 2.22-4.48] having the greater risk (Appendix 8 & 9).
There was an increased risk of CVST in the participants from the most deprived quintile [1–28 d aHR 1.43; 95% CI: 1.21-1.68] compared to the least deprived quintile. A positive linear association between the number of comorbidities and risk of CVST was found even after adjusting for all potential confounders [compared to no comorbidity: single comorbidity aHR 2.06; 95% CI 1.73 to 2.44 & two or more comorbidities aHR 6.69; 95% CI 5.81 to 7.71].
Discussion
We undertook a national cohort study investigating the occurrence of CVST in SARS-CoV-2 positive or SARS-CoV-2 vaccinated adults. We found a slightly elevated but non-significant risk of CVST in the first 28 d following vaccination when compared to the period before vaccination, even after adjusting for age, sex, comorbidity, deprivation, and ethnicity, a finding that remained similar even after stratifying with the type of vaccine received. In contrast, the risk was significantly elevated following a positive PCR confirmed SARS-CoV-2 infection, compared to the period before the infection.
Our sensitivity analysis which restricted the data to only vaccine recipients also found no significant association between CVST outcome and ChAdOx1 or BNT162b2 vaccination. Instead, the hazard ratios were considerably lower and indicated that CVST outcomes were less likely after the vaccine than before it, a finding that is reassuring for the ongoing campaign to increase vaccine uptake globally.
The hazard ratios for COVID-19 infection were significantly higher than those for either vaccine or both vaccines combined. We found that a participant was about 10 times more likely to develop a CVST outcome in the first 28 d post-infection compared to the first 28 d post-vaccine – a result that is consistent with a recent English study.Citation24 The risk, however, drops to at about three times, after adjusting for age, sex, comorbidity, deprivation, ethnicity, and whether or not participants were exposed to both the infection and vaccination. Being male or over the age of 50 y was associated with greater risks of CVST in the first 28 d after a positive test. There appeared to be no association in the under 50s, potentially due to the very small number of CVST events. As expected, exposure to both the vaccine and infection within 28 d of each appeared to increase the risk of CVST compared to those with no exposure at all.
Our findings are broadly consistent with other similar studies. Recent studies from the UK and US have found that SARS-CoV-2 was associated with a markedly greater risk of CVST than the vaccines that protect against it.Citation24–26 However, studies that have examined the possible association between thromboembolic events including CVST and SARS-CoV-2 vaccines have reported mixed results so far. Like our study, some have found no increased risk of thromboembolism, including CVST from vaccines against COVID-19 disease or insufficient CVST events to draw reliable conclusions.Citation27,Citation28 However, others have identified slightly elevated risks, particularly with the ChAdOx1 vaccine.Citation29,Citation30
Furthermore, recent systematic reviews and meta-analyses Citation31–33 found that rare cases of thrombosis and thrombocytopenia syndrome (TTS) are frequently associated with CVST. Palaiodimou et al. found that half of the individuals who developed TTS post a vector-based vaccination with ChAdOx1 or Janssen Ad26.COV2.S vaccine presented with CVST. Citation34 Our study did not find a statistically significant elevated risk of CVST after vaccination. However, it is important to note that whereas these systematic reviews and meta-analyses found an association with TTS, and particularly with vector-based vaccines, these studies examined a more specific case definition of TTS with CVST as compared to ours, which was for CVST more broadly (see Appendix 3). The results of other studies should be considered alongside ours in order to provide a more complete picture of the balance of risk and benefit.
There are still very few population-based studies investigating and comparing the association between COVID-19 vaccines and CVST probably due to the extreme rarity of CVST. While our findings appear to be consistent with findings from a few other studies, our method differs from these. Unlike others, we used Cox regression for the period before individuals either had their first dose of vaccine or diagnosed with the disease as the reference period to compare with the first 28 d after they have had either the vaccine or disease and controlled for baseline variables. This method, however, is similar to the self-controlled case series methods where variables that are constant over the observation period are implicitly controlled for. This was confirmed by one of our sensitivity analyses where data was restricted only to those who had the vaccine and the results were similar.
As the roll out of COVID-19 vaccination program across the world continues to be met with concerns about possible associations between vaccines (particularly the ChAdOx1) and various blood clotting disorders including CVST, our finding suggests that the risk of CVST caused by COVID-19 vaccines is significantly outweighed by the risk of CVST caused by infection with the SARS-CoV-2 virus, based on risk-benefit analysis, at least. This is of great public health importance and strengthens the overarching preventative approach adopted globally to control the spread of COVID-19.
Strengths and limitations of this study
The main strength of this study was its individual-level, population-scale national coverage. Using the SAIL Databank and COVID-19 e-Cohort allowed us to follow all individuals whether or not they were vaccinated or tested for SARS-CoV-2 regardless of test location, symptom severity, access to health care, or vaccine priority group, thus leading to extremely low attrition rate, recall bias, and increasing the representativeness, data completeness, timeliness, external validity, and generalizability of our findings.
The size of our study enabled us to estimate the pre-exposure risks with good precision, adjust for possible confounders, and stratify by type of vaccine (BNT162b2 vs ChAdOx1). Our analysis was designed to be specific to the timing of event occurrence and restricting the analysis to only those exposed to the vaccine only, for example, yielded similar results.
Our study also has several limitations. First, as with any linked data, we cannot completely rule out bias from linkage errors such as missed-matches and false-matches. However, the accuracy of linkage in SAIL has been demonstrated to be around 99.85% when NHS numbers are used as in this case.Citation17 These are the records with the highest match rates. Another limitation is the unavailability of universal PCR COVID-19 testing, particularly at the start of the pandemic. This might have resulted in a selection or misclassification bias for symptomatic or asymptomatic and mild or severe cases. Indeed, the majority of participants with SARS-CoV-2 were hospital inpatients. Similarly, selective vaccination based on priority groupsCitation27 meant that the majority of vaccinated participants were already vulnerable participants (i.e. the elderly, more prone to CVST events). Hence, it is possible that some participants were exposed to both the infection (but undiagnosed) and the vaccination. Therefore, we cannot rule out that all the participants we classified as having only the vaccine did not also have COVID-19, and vice versa. To somewhat mitigate this, our sensitivity analysis was restricted to only those who were exposed to either a vaccine or infection, which has a major advantage of reducing within-person potential confounding for all fixed characteristics. Moreover, to account for double exposure, we adjusted for having both exposures within 28 d of each other. The main results were robust to sensitivity analyses, except for the association between CVST and COVID-19, which reduced significantly in the sensitivity analysis, potentially due to the small number of CVST events.
Other limitations of the study included restricting the analysis to the first dose of the vaccine only and a short exposure window. Follow-up after infection or vaccination was limited to 28 d only, which might not yet account for all diagnoses of CVST after COVID-19 infection or vaccination. This might have resulted in an imbalance of incidence of CVST between the pre-exposure and post-exposure periods. Further analysis with a much longer follow-up period may be relevant in the future.
In addition, the lack of key hematological laboratory data, particularly information regarding anti-platelet factor 4 (PF4) antibodies that have been associated with VITT,Citation12 meant that we could not ascertain whether or not the mechanism of CVST after infection with SARS-Cov-2 was similar to that observed after vaccination. Furthermore, our results might be limited by our broader definition of CVST. Finally, we had some missing data on body mass index (BMI), which we grouped as “not obese” (BMI <35). This is unlikely to affect our findings under the assumption that, more often than not in NHS clinical care, obese patients are highly likely to have their BMI information on EHR. In addition, our sensitivity analysis without the BMI variable showed no significant difference.
Conclusion
Our study provides evidence that the risk of CVST from SARS-CoV-2 infection is substantially greater than the risk from SARS-CoV-2 vaccination. A non-significant elevated risk of CVST was observed in the short-term period after vaccination compared to the period before vaccination, regardless of the type of vaccine. This requires further investigation and ongoing surveillance, perhaps by combining results from different jurisdictions in a meta analysis.
The rarity of CVST in all populations means that larger sample sizes of exposed participants and complementary study designs are required to confirm and interpret results. Further investigations including other relevant blood clot disorders, relevant hematological data, and a longer time period after vaccination and infection may consolidate our findings.
Authors’ contributions
CW, DT & CO led the conception and design of this work with input from JS & SC. CO performed the analysis and JL created the underlying QCOVID dataset. CO drafted the first iteration of the manuscript, proofread by RM. All authors critically reviewed the manuscript, provided important intellectual input, approved the final version, and agreed to be accountable for their contributions.
Availability of data and materials
The data used in this study are available in the SAIL Databank at Swansea University, Swansea, UK, but as restrictions apply they are only available to bona fide researchers. All proposals to use SAIL data are subject to review by an independent Information Governance Review Panel (IGRP). Before any data can be accessed, approval must be given by the IGRP. The IGRP gives careful consideration to each project to ensure proper and appropriate use of SAIL data. When access has been granted, it is gained through a privacy protecting safe haven and remote access system referred to as the SAIL Gateway. SAIL has established an application process to be followed by anyone who would like to access data via SAIL at https://www.saildatabank.com/application-process
Supplemental Material
Download MS Word (474.5 KB)Acknowledgement
Authors of this output would like to thank all PHW staff involved in routine SARS-CoV-2 diagnosis and surveillance, the DHCW WIS, Data Warehouse and IS teams, the WIS Data Quality Group, and all those carrying out data entry for vaccinations into WIS and associated QA work.
We wish to acknowledge the collaborative partnership that enabled acquisition and access to the de-identified data, which contributed to this analysis. The collaboration was led by the Swansea University Health Data Research UK team under the direction of the Welsh Government Technical Advisory Cell (TAC) and includes the following groups and organizations: the Secure Anonymized Information Linkage (SAIL) Databank, Administrative Data Research (ADR) Wales, NHS Wales Informatics Service (NWIS), Public Health Wales, NHS Shared Services Partnership, and the Welsh Ambulance Service Trust (WAST). All research conducted has been completed under the permission and approval of the SAIL independent Information Governance Review Panel (IGRP) project number 0911. Special thanks also go to Rachel Merrick (RM) – UK Field Epidemiology Training Programme fellow and Dr Gemma Hobson Public Health Specialty Registrar – who meticulously proofread the manuscript and made useful contributions and to Jana Zitha (Epidemiologist, PHW) who helped with putting together the relevant ICD-10 codes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2127572
Additional information
Funding
References
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):1–11. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE. Safety and efficacy of the ChAdox1 nCov-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdox1 nCov-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91. doi:10.1016/S0140-6736(21)00432-3.
- Bernal JL, Andrews N, Gower C, Stowe J, Robertson C, Tessier E, Simmons, R, Cottrell, S, Roberts, R, O'Doherty, M, et al. Early effectiveness of COVID19 vaccination with BNT162b2 mRNA vaccine and ChAdox1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv. 2021. doi:10.1101/2021.03.01.21252652.
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A. Interim findings from first dose mass COVID-19 vaccination rollout and COVID-19 hospitalisations in Scotland: national prospective cohort study of 5.4 million people. Lancet. 2021;397(10285):1646–57. doi:10.1016/S0140-6736(21)00677-2.
- Covid-19 vaccines guidance. [accessed 2022 March 29th]. https://gov.wales/covid-19-vaccines.
- World Health organisation (2021), Adverse Events Following Immunization (AEFI). [ accessed 2022 Mar 29th]. https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi.
- Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt A-H, Skattør TH, Tjønnfjord GE, et al. Thrombosis and thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021 Jun 3;384(22):2124–30. doi:10.1056/NEJMoa2104882. Epub 2021 Apr 9. PMID: 33835768; PMCID: PMC8112568.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021 Jun 3;384(22):2092–101. doi:10.1056/NEJMoa2104840. Epub 2021 Apr 9. PMID: 33835769; PMCID: PMC8095372.
- See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–56. doi:10.1001/jama.2021.7517. PMID: 33929487.
- Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021;384(23):2202–11. doi:10.1056/NEJMoa2105385. PMID: 33861525.
- Wise J. Covid-19: European countries suspend use of Oxford-ChAdox1 vaccine after reports of blood clots. BMJ. 2021;372:n699. doi:10.1136/bmj.n699.
- GOV.UK. JCVI statement on use of the ChAdox1 COVID-19 vaccine. 2021 Apr 7. [cited 2022 Feb 22]. Available from: https://www.gov.uk/government/publications/use-of-the-ChAdOx1-covid-19-vaccine-jcvi-statement/jcvi-statement-on-use-of-the-ChAdOx1-covid-19-vaccine-7-april-2021.
- GOV.UK. JCVI advises on COVID-19 vaccine for people aged under 40, 2021 May 7th. [cited 2022 Feb 22]. Available from: https://www.gov.uk/government/news/jcvi-advises-on-covid-19-vaccine-for-people-aged-under-40.
- Cox DR. Regression models and life tables (with discussion). J Roy Stat Soc B. 1972;34:187–220.
- Jones KH, Ford DV, Thompson S, Lyons RA. A profile of the SAIL databank on the UK secure research platform. Int J Popul Data Sci. 2019;4(2):1134. doi:10.23889/ijpds.v4i2.1134.
- Lyons RA, Jones KH, John G, Brooks CJ, Verplancke JP, Ford DV, Brown G, Leake K. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. 2009;9(1):3. doi:10.1186/1472-6947-9-3.
- Ford DV, Jones KH, Verplancke JP, Lyons RA, John G, Brown G, Brooks CJ, Thompson S, Bodger O, Couch T, et al. The SAIL databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009;9(1):157. doi:10.1186/1472-6963-9-157.
- Lyons J, Akbari A, Torabi F, Davies GI, North L, Griffiths R, Bailey R, Hollinghusrt J, Fry R, Turner SL, et al. Understanding and responding to COVID-19 in Wales: protocol for a privacy-protecting data platform for enhanced epidemiology and evaluation of interventions. BMJ Open. 2020;10(10):e043010. doi:10.1136/bmjopen-2020-043010.
- Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, Hayward A, Hemingway H, Horby P, Mehta N. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi:10.1136/bmj.m3731.
- COVID-19: the green book, chapter 14a. [accessed 2021 Oct 10th]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1037340/Greenbook-chapter-14a-30Nov21.pdf.
- COVID-19 vaccination first phase priority groups. [Accessed 2020 May 5th]. https://www.gov.uk/government/publications/covid-19-vaccination-care-home-and-healthcare-settings-posters/covid-19-vaccination-first-phase-priority-groups.
- Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi:10.1136/bmj.n1931.
- Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. 2021;39:101061. doi:10.1016/j.eclinm.2021.101061.
- Torjesen I. Covid-19: risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. BMJ. 2021;373:n1005. doi:10.1136/bmj.n1005.
- Uaprasert N, Panrong K, Rojnuckarin P, Chiasakul T. Thromboembolic and hemorrhagic risks after vaccination against SARS-CoV-2: a systematic review and meta-analysis of randomized controlled trials. Thrombosis J. 2021;19(86). doi:10.1186/s12959-021-00340-4.
- Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, McCowan C, Agrawal U, Shah SA, Ritchie LD. First-dose ChAdox1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–97. doi:10.1038/s41591-021-01408-4.
- Pottegard A, Lund LC, Karlstad S, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PLD, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdox1-S in Denmark and Norway: population based cohort study BMJ. BMJ (Clin Res Ed). 2021;373:1114. doi:10.1136/bmj.n1114.
- EMA. COVID-19 vaccine ChAdox1: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. 2021. March. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots.
- Jaiswal V, Nepal G, Dijamco P, Ishak A, Dagar M, Sarfraz Z, Shama N, Sarfraz A, Lnu K, Mitra S, et al. Cerebral venous sinus thrombosis following COVID-19 vaccination: a systematic review. J Prim Care Community Health. 2022;13:21501319221074450. doi:10.1177/21501319221074450.
- Palaiodimou L, Stefanou MI, Katsanos AH, Aguiar de Sousa D, Coutinho JM, Lagiou P, Michopoulos I, Naska A, Giannopoulos S, Vadikolias K, et al. Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: a systematic review and meta-analysis. Neurology. 2021;97(21):e2136–e2147. doi:10.1212/WNL.0000000000012896.
- Hafeez MU, Ikram M, Shafiq Z, Sarfraz A, Sarfraz Z, Jaiswal V, Sarfraz M, Chérrez-Ojeda I. COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): a systematic review and post hoc analysis. Clin Appl Thromb Hemost. 2021;27:10760296211048815. doi:10.1177/10760296211048815.
- Palaiodimou L, Stefanou MI, de Sousa DA, Coutinho JM, Papadopoulou M, Papaevangelou V, Vassilakopoulos TI, Tsiodras S, Filippou DK, Tsivgoulis G. Cerebral venous sinus thrombosis in the setting of COVID-19 vaccination: a systematic review and meta-analysis. J Neurol. 2022;269(7):3413–19. doi:10.1007/s00415-022-11101-2.