ABSTRACT
This systematic review describes herpes zoster (HZ) economic burden in terms of healthcare resource use and cost outcomes in the Latin America and Caribbean (LAC) region. We searched online databases from 1 January 2000 to 20 February 2020 to identify eligible publications. We identified 23 publications that reported direct costs, indirect costs, and resources associated with HZ and its complications. The primary direct medical resources reported in the different studies were visits to doctors, transportation, days in the hospital, nursing, medication schedules, and physical therapy. Direct total costs per patient ranged from $99.99 to $4177.91. The highest cost was found in Brazil. Direct costs are, in average, 81.39% higher than indirect costs. The cost per patient that includes postherpetic neuralgia treatment is 115% higher on average for the directs and 73% for the indirect costs. Brazil reported a higher total cost per patient than Argentina and Mexico, while for indirect costs per patient, Brazil and Argentina had higher costs than Mexico, respectively. A meta-analysis on the number of days due to HZ hospitalization, performed on non-immunosuppressed patients over 65 years of age from three studies, resulted in a cumulative measure of 4.5 days of hospitalization. In the LAC region, the economic burden of HZ and associated complications is high, particularly among high-risk populations and older age groups. Preventative strategies such as vaccination could help avoid or reduce the HZ-associated disease economic burden in the LAC region.
Introduction
Varicella zoster virus (VZV) is a herpes virus of the alphaherpesvirus subfamily and causes varicella (chickenpox) and herpes zoster (HZ). Primary infection with VZV usually occurs in childhood and causes varicella, which is characterized by a vesicular pruritic rash and fever. The disease is usually benign with complications related to skin and soft-tissue bacterial superinfection in children and pneumonia in adults. After the varicella episode, VZV can remain latent in the dorsal root ganglia or in nerve cells, where it may reactivate and cause HZ. Typically, HZ lesions consist of a group of vesicles or blisters, restricted to a small area, usually located on the trunk or face and associated with pain in the affected area.Citation1 Individuals may experience pain or it may be the only occurrence of the disease,Citation2 along with an increase in antibodies against VZV.Citation1,Citation3,Citation4 Clinical and diagnostic confirmation of HZ can be achieved through polymerase chain reaction (PCR) and the direct immunofluorescence assay tests.Citation1,Citation5 Seroconversion, i.e. increased titration of specific antibodies in serum samples taken from acute and convalescent stages, may also be used to confirm HZ diagnosis.Citation3,Citation6
The lifetime risk of developing HZ is estimated to be 0%,Citation7 rising to 50% in individuals older than 85 years.Citation8 The HZ risk increases in individuals with a weakened immune system, who also have a high likelihood of severe disease.Citation1,Citation9 Age is the main risk factor for VZV reactivation, as it is associated with reduced virus-specific cell immunity, with an increased risk of disease observed in the population from 50 to 60 years of age.Citation1–11 Other groups of individuals with an immunocompromised immune system include transplant recipients, patients with autoimmune diseases, individuals under treatment with corticosteroids or chemotherapeutic agents and those diagnosed with cancer or human immunodeficiency virus (HIV).Citation12,Citation13 Other risk factors for HZ include female sex, being Caucasian, traumas, and other comorbidities, such as chronic lung and kidney diseases.Citation14–16 HZ complications occur in about 13–26% of all HZ cases and the most commonly observed complication is postherpetic neuralgia (PHN) (10–18% of HZ cases) which is associated with persistent pain four weeks after the appearance of skin lesions.Citation1,Citation9,Citation17 The risk of developing PHN in individuals increases after 50 to 60 years of age,Citation1 while the severity and length of an episode is proportional to the patient’s age.Citation18 Other less common complications include ocular herpes, acute retinal necrosis, Ramsay Hunt syndrome, neurological impairment, as well as secondary skin and soft tissue bacterial infections.Citation19–21
Several antiviral medicines are available to treat HZ as they reduce the appearance of skin lesions and relieve neuropathic pain.Citation22 Management of complications such as PHN depends upon patient’s characteristics and include long-term treatments with anticonvulsants and tricyclic antidepressants.Citation23 To prevent HZ and PHN, the Zoster Vaccine Live (ZVL) was approved by the US Food and Drug Administration (FDA) in 2006 for adults over 60 years of age and later for adults over 50 years of age in 2011,Citation24 after which the vaccine was approved in several countries including those in the Latin America region.Citation25 In Argentina, the ZVL was authorized by the Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT) in 2013 for individuals >50 years of age.Citation26,Citation27 In 2017 in the United States, a two-dose Recombinant Zoster Vaccine (RZV) became available and recommended by the United States Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) for use in immunocompetent adults >50 years of age.Citation28 Later, in 2021, the ACIP recommendation extended to adults immunodeficient or immunosuppressed aged ≥19 years.Citation29 Patients with prior ZVL are advised to receive the two doses of RZV due to its higher efficacy and length of protection and to prevent both HZ and PHN (particularly in people >70 years of age). While the best time to repeat vaccination is not fully clear, the ACIP recommends administering the first dose of RZV at least eight weeks after receiving ZVL.Citation30
The Latin America and the Caribbean (LAC) region is diverse in terms of socio-economic status. The economic classification of countries in the LAC region has often varied in the past two decades, but most countries were classified as ‘upper middle income countries,’ with only a few classified as either ‘high income countries’ or ‘lower middle income countries’ in 2021.Citation31
To understand the value of preventative strategies such as vaccination in the LAC region, a complete understanding of the burden of disease is required. This will equip decision-makers with information to develop vaccine policies in a timely and relevant manner. The decision to introduce a vaccine in a national immunization program is not only made based on clinical data but is also shaped by health economic parameters. A systematic literature review (SLR) was conducted to understand the epidemiology, clinical and economic burdens of HZ in the LAC region. The epidemiology and clinical burden of HZ in the last 20 years, which imply a consistent increase in the rate of HZ incidence among high-risk populations and elderly individuals, have been reported previously.Citation32 Here, we cover the economic impact of HZ in the LAC region, which includes healthcare resource use and costs associated with HZ and its complications. Audio-slides summarizing this manuscipt are available on https://doi.org/10.6084/m9.figshare.21311445.v1
Methods
A systematic review was performed following the Cochrane Systematic Reviews Manual,Citation33 the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA)Citation34,Citation35 and Meta-analyses of Observational Epidemiology (MOOSE)Citation36 guidelines specifically for reviews of observational studies. The study protocol is registered with the prospective international systematic review registry PROSPERO (CRD42020186586).Citation37
Search sources and strategy
Online databases such as Medline (via PubMed), Latin American and Caribbean Health Sciences Literature (LILACS), Excerpta Medica Database (EMBase), Cumulative Index of Nursing and Allied Health Literature (CINAHL), Cochrane Library, Center for Reviews and Dissemination (CRD) York, and EconLIT were searched to identify published articles relevant to the research objectives. The search strategy to identify published articles on epidemiology, clinical and economic burden of HZ, is shown in the supplementary material (File S1). Searches were time-limited to identify articles published between 1 January 2000 and 20 February 2020.
To ensure all relevant articles were found, a manual search was performed across reference lists of included publications, databases of national and international congress proceedings and doctoral theses. The websites of major local medical associations, experts and associations related to the field were visited and the authors of relevant papers were contacted about any missing or information or when a clarification was needed.
Greyliterature was searched in the following sources: generic internet and meta-search engines (Google, Google Scholar), regional Ministries of Health (Argentina, Brazil, Colombia, Chile, and Mexico), Pan American Health Organization, Virtual Health Library, and hospital reports. Information was also searched in Global Burden Disease (GBD) of the Institute for Health Metrics and Evaluation (IHME).Citation38
Article selection
Publications included in this review were identified independently through peer review by the research team using predefined eligibility criteria. Discrepancies were solved with the agreement of the entire team. All screening phases of the study used COVIDENCE, an online platform used to process systematic reviews.Citation39,Citation40
Articles were qualified for inclusion in the review if the study population was comprised of individuals ≥15 years of age from the LAC region, regardless of their risk status (>60 years of age, with a transplantation, diagnosed with HIV/acquired immunodeficiency syndrome, cancer, under treatment with corticosteroids/immunosuppressants/chemotherapy). Studies were included regardless of the utilized intervention. Study designs included in this review were economic evaluations and costs or budget impact studies with full text in Spanish, English, or Portuguese, published from 1 January 2000 onwards. Case series involving ≥50 HZ cases or ≥10 HZ complications were also considered relevant for inclusion. Studies were excluded if the population was outside of the scope of the inclusion criteria, if the outcomes were other than those specified as eligible, if they were published outside of the eligible time period, if they were not reporting outcomes in countries of interest, and if they were published in a language other than the above-mentioned ones. Systematic reviews, meta-analysis, narrative reviews, interventional studies, cost-effectiveness or health economic studies, surveys, non-human data, case reports, letters to editor, newspapers, editorials, comments, opinions, molecular studies, pilot studies, protocol and pre-clinical studies, and studies with insufficient methodological details were excluded. Reference lists within systematic review and meta-analyses were screened for additional relevant articles, as deemed necessary by the reviewer.
Data extraction
After determining the final list of eligible publications for review, the research team extracted data based on three pre-defined parameters. These included: study characteristics (type of publication, year published, authors, geographic location, study design including domains for the risk of bias method); participant characteristics (inclusion criteria applied, age, sex, sample size, latent immune-compromising conditions, risk evaluation for HZ); and perspective data of diagnosis and treatment (use of resources for the management of HZ, length of stay in the general ward and intensive care, direct costs – i.e., costs for outpatient visits, costs of laboratory tests, costs related to stays in the general ward and in intensive care, costs of medication schemes, costs of pain and complication management, rehabilitation – and indirect costs such as productivity, caregivers and transportation, among others).
Risk of bias assessment of included studies
To assess the risk of bias in economic studies, we applied the Consolidated Health Economic Evaluations Reporting Standards (CHEERS) checklist.Citation41 Based on this, the following aspects were assessed, where applicable: the population, the perspective of evaluated costs, the timeline of analysis, the clarity of direct and indirect costs, the valuation from evidence, the analytical approach utilized to estimate results (types of decision models), and the methods of cost adjustment based on inflation and discount rates. We utilized the CiCERO tool (draft version) for economic evaluations, as it assesses the most relevant parameters and criteria for such type of studies.Citation42 Example response options include presence, absence, no reporting and non-applicability of criteria, such as analytical perspective, establishing the target population and standardization and adjustment of costs.
The risk of bias assessment of the observational studies was done according to the United States National Heart, Lung, and Blood Institute guidelines checklists, consisting of 14 items to evaluate risk of bias in cohort and cross-sectional studies, and 9 items for case series.Citation43 The studies were rated as “Bad,” “Poor” and “Good” for high risk of bias, uncertain risk of bias, and low risk of bias, respectively.
Analyses and reporting
In this paper, we provide a descriptive overview of healthcare resource use and cost outcomes as of 2019 together with the risk of bias results of the economic studies. In addition to a descriptive overview of the main findings, a meta-analysis on the number of days due to HZ hospitalization was performed on non-immunosuppressed patients over 65 years of age. This meta-analysis used a random effects model due to the heterogeneity of the studies. For studies reporting total direct and indirect costs per patient, an adjustment was made for comparison across countries. First, study-reported costs were converted to the local currency in the country where costs were reported. The exchange rate reported in the original study was used whenever possible, otherwise the exchange rate reported by the World Bank was used.Citation44 Second, the sums in the local currency for each year until 2018 were adjusted based on inflation to allow for comparison through the subsequent conversion to International Dollars ($). Inflation rates were developed from the information provided by the World Bank for all countries where data were available.Citation45 Finally, to make costs across countries comparable, $ was used by implementing the adjustment coefficient published by the World Bank.Citation46
Results
Finding from the systematic review
Overview of included studies
A total of 1,278 studies were identified after duplicates were removed from the initial search of publications. From these, 102 studies were selected for a full-text review based on the review eligibility criteria. Lastly, 23 studies were included for a descriptive analysis of the healthcare resource use and costs (direct or indirect) associated with HZ or its complications ( and ).Citation47–69 None of the included studies mention the vaccination status of participants.
Figure 1. PRISMA flow diagram.
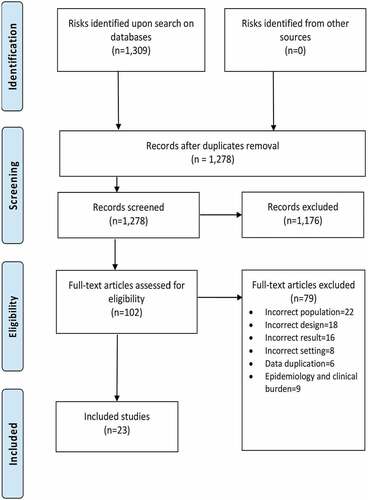
Table 1. Use of resources and costs per patient, as reported in reviewed studies (N = 23). Costs in 2018 international dollars ($).
The 23 studies reported data for Brazil (n = 7),Citation53–58–Citation65 Argentina (n = 5),Citation47,Citation48-Citation50–52, Colombia (n = 4),Citation59–61–Citation64 Mexico (n = 3),Citation63,Citation66,Citation68 Costa Rica (n = 1),Citation62 Nicaragua (n = 1),Citation69 and Latin America [including Brazil, Mexico and Argentina] (n = 1)Citation49 whereas one multi-regional study provided aggregate results for Latin America (n = 1),Citation67 with the same countries included ().
The majority of studies included in this review were case series (n = 11) followed by cohort studies (n = 7), cost-effectiveness evaluations (n = 3), pooled cost-analysis (n = 1) and budget impact analysis (n = 1) (). While all the studies provide data health-care resource use,Citation47–69 direct and indirect medical costs were reported in two studies (Mexico,Citation63 and Latin America including Brazil, Mexico and Argentina)Citation49 and 1 study (Latin America including Brazil, Mexico and Argentina),Citation49 respectively ().
Health care resource use
provides an overview of direct and indirect costs and resource use reported in the studies. Across the studies, the type of direct use of resources comprised doctor visits, transportation, hospitalizations, nursing, medication, and physical therapy. The indirect use of resources included missed days at work for the patient, missed days at work for the relative, impaired labor capacity and the need for caretakers.
From the studies identified within the scope of the epidemiological search,Citation32 the number of patients requiring hospitalization ranged from 4 to 7,042,Citation52,Citation68 with a frequency of hospitalization from 3% to 35.7%.Citation50,Citation57 With regard to treatment pattern, a total of 3,046 patients received systemic antiviral treatment, with acyclovir being the most prescribed treatment (34.7%), followed by valacyclovir (6%), brivudine (0.6%), and famciclovir (0.5%). Patients received non-steroid anti-inflammatory drugs (NSAIDs), anticonvulsants, tricyclic antidepressants, and topical pain-relieving drops for the management of pain. A few patients were even prescribed corticosteroids (71 patients) and antibiotics (14 patients). In addition, the use of topical medication such as drying agents, antivirals, antibiotics, steroids, or analgesics were given to 570 patients ().
Table 2. Hospitalizations due to HZ and treatment options.
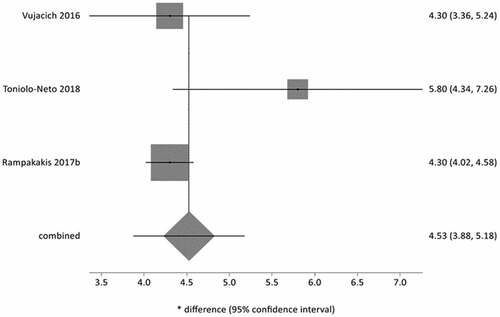
A meta-analysis on the number of days due to HZ hospitalization which was performed on non-immunosuppressed patients over 65 years of age from three studies,Citation52,Citation58,Citation62 resulted in a cumulative measure of 4.5 days of hospitalization (95%CI = 3.9 to 5.2; I2: 49%) ().
Figure 2. Meta-analysis of number of days of hospitalization due to HZ in immunocompetent patients ≥65 years of age.
Cost burden
provides an overview of cost per patient data. Studies reported different perspectives when estimating costs such as social perspective (n = 3),Citation49,Citation59,Citation60 third-party payer perspective (n = 1)Citation64 and the public perspective (n = 1).Citation65 Out-of-pocket expenses were reported in two studies.Citation52,Citation58
Total direct and indirect cost per patient were estimated based on the corresponding categories of reported resource use. In one study, costs were reported per country and as a whole, and differentiated according to the presence or absence of PHN for Argentina, Brazil and Mexico.Citation49 When total costs were compared in 2018 $,Citation49 a higher total cost was reported for HZ patients from Brazil than for patients from Argentina and Mexico. Including patients with and without PHN; this cost is observed to be 2.24 times higher than the cost accrued by patients in Argentina and 1.76 times higher than Mexico. Upon comparison of indirect medical costs, both Brazil and Argentina had higher costs versus Mexico for PHN, non-PHN and the total population. Argentina had a higher cost than Brazil in terms of total indirect medical costs, but Brazil had higher indirect costs for the PHN population ().
Risk of bias among included studies
Quality assessment of economic studies (n = 8) is provided in . Most economic studies were of adequate quality. However, of the eight studies with economic study designs,Citation49–52–Citation58–65 only one studyCitation60 used the discount rate, while none of the studies adjusted their cost estimates based on inflation rates. Four studies did not report any comparator, and three studies did not report any estimation of direct or indirect costs.
Table 3. Quality evaluation for use of resources and cost studies (n = 8).
The risk of bias assessment of case series and cohort studies reporting resource usage has been reported elsewhere and is shown in Tables S1 and S2, respectively. Seventy-five percent of the cohort studiesCitation49,Citation56,Citation61,Citation67 and 91% of the case series/epidemiological surveillance studiesCitation47–48-50–51-53–55-57–66-68–Citation69 were assessed as having low risk of bias.
Country-specific burden data from department of health databases
An internet-based search for information was conducted on HZ disease burden on the websites of Departments of Health of Brazil, Chile, Colombia, and Mexico. It should be noted that it was not possible to access data for Colombia and Panama due to restricted access to the Colombia Department of Health data,Citation70 and no availability of free access to the Panama Department of Health data. Cost data was only found for Brazil and has been reported.
Mexico
Two different health systems exist in Mexico, namely the Mexican Institute of Social Security (IMSS) which provides health-care services for individuals with formal employment and the ‘Secretaría de Salud’ serving individuals without formal employment. The IMSS reported an average in-hospital stay of 4.8 days due to HZ, and an average of 443 discharges were observed per year (2010–2017) among patients ≥15 years of age.Citation71 In contrast, the ‘Secretaría de Salud’ reported an average in-hospital stay of 3.8 days with 131 annual average hospitalizations (2018–2019) due to HZ.Citation71 Please refer to Fig S1 for an average number of hospital discharges per international classification of diseases, tenth revision (ICD-10) category in Mexico from 2010 to 2017.
Brazil
Health information published by the Information Department of the Unified Health System (DATASUS) was utilized.Citation72 The data published by DATASUS on hospital morbidity come from the Hospital Information System of the SUS (SIH/SUS), administered by the Department of Health, through the Healthcare Secretariat and the State and Municipal Health Secretariats. Hospital units that were part of the SUS sent their corresponding information on admissions to the municipal or state managers through the Authorization for Hospitalization (AIH). This information was then consolidated by DATASUS into a database which includes information related to most hospital admissions in Brazil; primary care coverage by this database is 75–80% of the general population.Citation73 Total cost is available in the hospital morbidity database (defined as the cost for approved AIHs in the period to be considered as the approved cost of production), also comprising hospital costs and professional service costs. Information on costs (total cost, hospital costs, and professional service costs) and on use of resources (days and mean length of stay in hospital) was obtained, and costs per hospitalization and per day of hospitalization were estimated.
The total cost of hospitalization for varicella and HZ in patients ≥65 years of age, in the 2010–2019 period, expressed in current Brazilian real (R$) per year, was R$ 17,683,123 (total annual cost ranged from R$ 1,284,498 in 2012 to R$ 2,041,051 in 2017). The total cost of hospital services in the period was R$ 15,470,797 (annual cost of hospital services ranged from R$ 1,116,799 in 2012 to R$ 1,796,096 in 2017) and a total cost of professional services was R$ 2,212,142 (annual cost of professional services ranged from R$ 167,699 in 2012 to R$ 251,233 in 2013). Between 2010 and 2019, the average total cost per hospital stay was R$ 1,064 (ranging from R$ 888 in 2012 to R$ 1,203 in 2017), while the average cost of hospital services per hospital stay was R$ 931 (ranging from R$ 772 in 2012 to R$ 1,058 in 2017). The average cost of professional services per hospital stay was R$ 133 (ranging from R$ 116 in 2012 to R$ 152 in 2014). The average total cost per day of hospitalization was R$ 148 (range from R$ 132 in 2012 to R$ 167 in 2014), with an average cost of hospital services per day of R$ 129 (range from R$ 115 in 2012 to R$ 146 in 2014) and an average cost of professional services per day of R$ 19 (range from R$ 17 in 2012, 2018 and 2019 to R$ 21 in 2014) (Table S3).
Additionally, R$ was converted to international $ for comparison with data from different countries (). The total cost of hospitalizations per VZV in patients ≥65 years of age for each year ranged from $791,948 in 2019 to $1,324,051 in 2010. The total cost of hospital services ranged from $695,392 in 2012 to $1,152,290 in 2010, while the total cost of professional services ranged from $94,457 in 2019 to $171,762 in 2010. Between 2010 and 2019, total cost per hospital stay ranged from $455 in 2019 to $674 in 2013. The costs for hospital services per hospital stay were $401 in 2019 to $588 in 2013, and the costs for professional services per hospital stay were $54 in 2019 to $87 in 2010. Lastly, with respect to costs per day of hospitalization, the total cost per day ranged from $62 in 2019 to $100 in 2010, while the cost of hospital services per day ranged from $55 in 2019 to $87 in 2010 and the cost of professional services per day of hospitalization ranged from $7 in 2019 to $13 in 2010 and 2011. It could be observed that the hospitalization costs were lower in 2019 that in 2010, this might be due to two main factors: 1) the number of patients hospitalized in 2010 was the highest for the period and 2) the Real currency devaluated against the US dollar.
Table 4. Brazil. Costs of hospitalizations caused by varicella and HZ in patients ≥65 years of age in the public health system (SUS). Values in International dollars ($)a per year.
Please refer to Table S4 and Fig S2 for data on hospitalizations due to varicella and HZ in patients ≥65 years of age in Brazil from 2010 to 2019.
Chile
The open-access data on hospital discharges and deaths was accessed from the official Statistics and Health Information Department’ (DEIS) in Chile. Hospital discharge data was collected from 2010 to 2018.Citation74 Uncomplicated HZ (B029) had an average hospital discharge of 246 per year during 2010 and 2018. The rest of the ICD-10 categories ranged from 8.00 to 53.00 discharges on average per year (Table S5). Average annual in-hospital stays per ICD-10 category from the period of 2010 to 2018 show that encephalitis caused by HZ (B020) required the highest number of in-hospital days for the period of analysis (14.5 days on average) with the rest varying from 3.61 to 8.91 days. The average length of in-hospital stays as per the ICD-10 category from 2010 to 2018 for uncomplicated HZ is 6.37 days (Table S6).
Discussion
This study summarizes the available economic evidence associated with HZ resource use over the past 20 years in the LAC region. A review of 23 published studies and the country’s Department of Health websites provided the data for direct and indirect costs related to HZ in the general and at-risk populations in the region. Overall, the review shows that there is a significant economic burden of HZ and PHN in the LAC region which underscores the importance of HZ vaccination in high-risk patients, especially for elderly adults who have a higher HZ risk and are more likely to suffer from chronic zoster-associated pain.
Among the 23 studies which reported direct costs, indirect costs and resource use associated with HZ and its complications, country-level results were presented for Brazil, Argentina, Colombia, Mexico, Costa Rica, and Nicaragua. In addition to the regional studies, a multi-country study was also conducted which included Argentina, Brazil and Mexico. Of the 23 studies, five studies reported the use of direct medical resources, four studies reported the use of indirect resources and only two studies reported a monetary value of utilized resources.Citation49,Citation63 We found that direct costs were related to medical consultations, transfers, hospitalizations, nursing consultations, physical rehabilitation, and medication schemes. The main source of indirect resource use identified in this review was workdays lost by patient and family members, the need for caregivers and the deterioration of work capacity. Both Brazil and Argentina had higher indirect costs versus Mexico for PHN, non-PHN and the total population. Patients in Argentina accrued a higher indirect medical cost than Brazil, but Brazil had higher indirect costs for the PHN population.
Information on resource use and costs was also available from the ministerial databases of Mexico, Brazil and Chile. Data from these databases show important time trends in the burden of the disease. Hospitalizations remained stable over time in Brazil, Mexico, and Chile, and both in Mexico and in Chile the main cause of hospitalization was uncomplicated HZ. Similarly, the in-hospital length of stay remained stable in these three countries, and the main reasons for admission were uncomplicated HZ in Mexico, and HZ encephalitis in Chile. In Mexico, the mean stay in hospital was 4.8 days, relative to 443 discharges per year on average for HZ in patients over 15 hospitalized in public health-care facilities (between 2010 and 2017), and 3.8 days in the Secretaría de Salud (SS) facilities (from an average 131 annual hospitalizations for HZ between 2018 and 2019).Citation71 This means that Mexico had a lower mean stay in hospital versus Brazil. The difference in in-hospital stay between Mexico (average stay of 3.8–4.8 days among patients ≥15 years of age) and Brazil (average stay of 7.2 days among patients ≥65 years of age) can be explained by the fact that hospitalizations reported for Brazil correspond to patients ≥65 years of age, with 41% of hospitalizations being reported among patients ≥80 years of age while in Mexico, hospitalizations correspond to patients ≥15 years of age, with 54–56% of the hospitalizations pertaining to the group aged 15–64. Therefore, either an older age in patients might be associated with more severe cases, or cases may be more severe, or there may be a different proportion of disease complications regardless of age, and thus, a larger need to remain hospitalized in Brazil. Only Brazil reported the cost of hospitalizations associated with VZV from its ministerial database. The reported average total cost per hospitalization among patients ≥65 years of age was R$ 1,064 and the average total cost per day of hospitalization for this group was R$ 148 (annual values expressed in Brazilian reals; exchange rates to $ ranged from 1.39 in 2010 to 2.25 in 2019).Citation72 Hospitalization costs were lower in 2019 than in 2010 owing to the number of hospitalized patients in 2010 which was the highest during the 2010–2019 period and a devaluation in R$ during this time period.Citation75 Despite an absence of large population-based cohort studies in the LAC region, the costs from Brazil are comparable to the results described in previously published literature.Citation49
While this is one of the most updated systematic reviews of the economic burden of HZ in the LAC region, several limitations warrant further discussion. For some of the studies included in our analysis, the design was not always meeting the highest epidemiological standards, for example, outcomes were not always clearly defined, or length of follow-up properly reported, however for the majority of them, the evaluator deemed the quality of the evidence as good, and all studies provided valuable information from the public health perspective. Due to the lack of HZ-related costs from countries other than Brazil, a comparison between countries was not feasible. None of the countries included in this review adjusted their health-care costs based on inflation rates to allow for comparison through the subsequent conversion to international dollars. To this end, it should also be noted here that fluctuations in foreign exchange rates could render difficult the comparisons. This highlights the need for more evidence-based studies of cost and resource utilization in countries of the LAC region. Additionally, there was a considerable amount of heterogeneity in the populations included in the studies such as different age groups, at-risk populations versus general population and studies with small and large number of participants. Due to this heterogeneity, results described in this study may not be generalizable to other settings. The results described in this study might suffer from underreporting due to the lack of active surveillance systems and mandatory reporting in the region. An issue inherent in systematic review is publication bias, or the tendency to report only clinically significant findings, however we mitigated this risk by looking for outcomes of interest into gray literature such as ministry of health websites. Further research on the economic burden of HZ in the LAC region is needed to better understand HZ related impact on the patients, society, and health-care systems. In future studies, it would be helpful to report separately costs incurred by VZV and costs incurred by HZ, and to report the HZ vaccination status of participants. Further research may also provide relevant insights to health-care policymakers in the region.
Conclusion
This review demonstrates that although evidence is limited, HZ and its sequelae suggests a substantial economic burden in the LAC region, which is expected to rise as the population ages and the number of HZ cases increase. The results support the need for preventative strategies such as vaccination and improved disease management to avoid or reduce the HZ-associated economic disease burden in the LAC region.
Author’s contributions
AB, AC, JNG and JG participated to the conception/design of the review; AB, AC, TA, CP, MS, JG and DB and FA participated to the collection/assembling of the data; AB, AC, TA, CP, MS, and DB performed/supervised the analysis; AB, AC, TA, CP, MS, and DB participated to the interpretation of the data in the application of the methodology. All authors agreed to the publication of the present work.
Data availability
All relevant data are within the manuscript.
Supplemental Material
Download MS Word (155.6 KB)Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Amrita Ostawal provided medical writing support.
Disclosure statement
AB, AC, TA, CP, MS, DB reports grants from GSK during the conduct of the study.
JG and JNG are employees and hold shares in GSK.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2131167.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bardach A, Cafferata ML, Klein K, Cormick G, Gibbons L, Ruvinsky S. Incidence and use of resources for chickenpox and herpes zoster in Latin America and the Caribbean—a systematic review and meta-analysis. Pediatr Infect Dis J. 2012;31(12):1263–13. doi:10.1097/INF.0b013e31826ff3a5.
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1(1):15016. doi:10.1038/nrdp.2015.16.
- Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of Varicella–zoster virus. N Engl J Med. 2000;342(9):635–45. doi:10.1056/NEJM200003023420906.
- Schmutzhard J, Merete Riedel H, Zweygberg Wirgart B, Grillner L. Detection of herpes simplex virus type 1, herpes simplex virus type 2 and varicella-zoster virus in skin lesions. Comparison of real-time PCR, nested PCR and virus isolation. J Clin Virol. 2004;29(2):120–26. doi:10.1016/S1386-6532(03)00113-6.
- Bennett J, Dolin R, Blaser M. Mandell, Douglas and Bennett's - principles and practice of infectious diseases. Philadelphia (PA): Elsevier; 2019.
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–30. doi:10.1212/WNL.0b013e3182a3516e.
- Cohen JI. Herpes zoster. New Engl J Med. 2013;369(3):255–63. doi:10.1056/NEJMcp1302674.
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–49. doi:10.4065/82.11.1341.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833.
- Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32(10):1481–86. doi:10.1086/320169.
- Buchbinder SP, Katz MH, Hessol NA, Liu JY, O’Malley PM, Underwood R, Holmberg SD. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166(5):1153–56. doi:10.1093/infdis/166.5.1153.
- Chen SY, Suaya JA, Li Q, Galindo CM, Misurski D, Burstin S, Levin MJ. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–34. doi:10.1007/s15010-013-0550-8.
- Grabar S, Tattevin P, Selinger-Leneman H, de La Blanchardiere A, de Truchis P, Rabaud C, Rey D, Daneluzzi V, Ferret S, Lascaux AS, et al. Incidence of herpes zoster in HIV-infected adults in the combined antiretroviral therapy era: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2015;60(8):1269–77. doi:10.1093/cid/ciu1161.
- Levin MJ, Anderson JP, Seage GR 3rd, Williams PL. Short-term and long-term effects of highly active antiretroviral therapy on the incidence of herpes zoster in HIV-infected children. J Acquir Immune Defic Syndr. 2009;50(2):182–91. doi:10.1097/QAI.0b013e31819550a4.
- Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995;171(3):701–04. doi:10.1093/infdis/171.3.701.
- Watson PN, Evans RJ. Postherpetic neuralgia. A review. Arch Neurol. 1986;43(8):836–40. doi:10.1001/archneur.1986.00520080074027.
- Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982;61(5):310–16. doi:10.1097/00005792-198209000-00003.
- Elliott KJ. Other neurological complications of herpes zoster and their management. Ann Neurol. 1994;35(S1):S57–61. doi:10.1002/ana.410350717.
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2):S3–12. doi:10.1016/j.ophtha.2007.10.009.
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg. 1990;102(2):177–79. doi:10.1177/019459989010200215.
- Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22(2):341–47. doi:10.1093/clinids/22.2.341.
- Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS, Woolf CJ. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164. doi:10.1371/journal.pmed.0020164.
- CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528.
- Willis ED, Woodward M, Brown E, Popmihajlov Z, Saddier P, Annunziato PW, Halsey NA, Gershon AA. Herpes zoster vaccine live: a 10 year review of post-marketing safety experience. Vaccine. 2017;35(52):7231–39. doi:10.1016/j.vaccine.2017.11.013.
- Administración Nacional de Medicamentos AyTMA. Disposición ANMAT N° 1850-2013. 2013 [accessed 2021 Mar 12]. http://www.anmat.gov.ar/boletin_anmat/marzo_2013/Dispo_1850-13.pdf.
- Food and Drug Administration. May 25, 2006 approval letter - Zostavax. STN 125123/0, approval of biologics license application (BLA) for zoster vaccine, Live, (Oka/Merck). 2006 [accessed 2021 Mar 12]. http://wayback.archive-it.org/7993/20170723093336/https:/www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm132873.htm.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–08. doi:10.15585/mmwr.mm6703a5.
- Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Dooling KL. Use of Recombinant Zoster Vaccine in immunocompromised adults aged ≥19 years: recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi:10.15585/mmwr.mm7103a2.
- CDC. Clinical considerations for use of Recombinant Zoster Vaccine (RZV, Shingrix) in immunocompromised adults aged ≥19 years. 2022 [accessed 2022 Jul 27]. https://www.cdc.gov/shingles/vaccination/immunocompromised-adults.html.
- The World Bank. The world by income and region. 2022 [accessed 2022 Aug 29]. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html.
- Bardach AE, Palermo C, Alconada T, Sandoval M, Balan DJ, Nieto Guevara J, Gómez J, Ciapponi A, Andrei G. Herpes zoster epidemiology in Latin America: a systematic review and meta-analysis. PLoS One. 2021;16(8):e0255877. doi:10.1371/journal.pone.0255877.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.0 (updated August 2019). 2021 [accessed 2021 Mar 12]. https://training.cochrane.org/handbook.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama. 2000;283:2008–12.
- Agustín Ciapponi AB, Comandé D, Zambosco TA, Palermo MC, Balan D, Sandoval MM. Burden of herpes zoster in Latin America: a systematic review and meta-analysis. PROSPERO 2020 CRD42020186586. 2020 [accessed 2021 Mar 12]. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=186586.
- The Institute for Health Metrics and Evaluation (IHME). GBD compare- Viz Hub. 2017 [accessed 2021 Feb 12]. https://vizhub.healthdata.org/gbd-compare/.
- Babineau J. Product review: covidence (systematic review software). Journal of the Canadian Health Libraries Association / Journal de l’Association des Bibliothèques de la Santé du Canada. 2014;35(2):68–71. doi:10.5596/c14-016.
- Veritas Health Innovation . Covidence systematic review software. Melbourne (Australia). 2021. https://www.covidence.org/.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated health economic evaluation reporting standards (Cheers)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–50. doi:10.1016/j.jval.2013.02.002.
- Mandrik O, Severens JL, Bardach A, Ghabri S, Hamel C, Mathes T, Vale L, Wisløff T, Goldhaber-Fiebert JD. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR good practices task force report. Value Health. 2021;24(4):463–72. doi:10.1016/j.jval.2021.01.002.
- National Institutes of Health. Study quality assessment tools. 2019 [accessed 2021 May 26]. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- World Bank. Official exchange rate (LCU per US$, period average). 2020 [accessed 2021 Mar 12]. https://data.worldbank.org/indicator/PA.NUS.FCRF.
- World Bank. Inflation, consumer prices (annual %). 2020 [accessed 2021 Mar 12]. https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG.
- World Bank. PPP conversion factor, GDP (LCU per international $). 2020 [accessed 2021 Mar 12]. https://data.worldbank.org/indicator/PA.NUS.PPP.
- Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, Torre AC, Kowalczuk AM, Galimberti RL, Ferreyro BL. Clinical characteristics and outcomes in a population with disseminated herpes zoster: a retrospective cohort study. Actas Dermo-Sifiliográficas (English Edition). 2017;108(2):145–52. doi:10.1016/j.adengl.2016.12.019.
- Corti M, Villafañe MF, Vittar N, Banco MC, Priarone M, Mammana L, Gilardi L. Meningoencephalitis due to varicella zoster virus in aids patients. Report of eleven cases and review of the literature. Revista Do Instituto de Medicina Tropical de São Paulo. 2015;57(6):505–08. doi:10.1590/S0036-46652015000600007.
- Rampakakis E, Pollock C, Vujacich C, Toniolo Neto J, Ortiz Covarrubias A, Monsanto H, Johnson KD. Economic burden of herpes zoster (“culebrilla”) in Latin America. Int J Infect Dis. 2017;58:22–26.
- Rozenek M, Romani A, Aronson S, Ramilo MD, Abellán V, Pérez MA, Cámera L. [Herpes zoster in elderly adults in a community hospital in Buenos Aires. June 2013-May 2014]. Medicina (B Aires). 2017;77:24–30.
- Vujacich C, Poggi E, Cecchini D, Luchetti P, Stamboulian D. Herpes zoster: epidemiología y clínica [Clinical and epidemiological aspects of Herpes zoster]. Medicina (BAires). 2008;68:125–28.
- Vujacich C, de Wouters L, Margari AM, Gordóvil M, Rampakakis E, Psaradellis E, Sampalis JS, Johnson K, Montes JL, Monsanto HA, et al. Dolor, calidad de vida relacionada con la salud y utilización de servicios médicos asociados al herpes zoster en Argentina. Actualizaciones En SIDA E Infectología. 2016;24:53–63.
- Alvarez FK, de Siqueira SR, Okada M, Teixeira MJ, de Siqueira JT. Evaluation of the sensation in patients with trigeminal post-herpetic neuralgia. J Oral Pathol Med. 2007;36(6):347–50. doi:10.1111/j.1600-0714.2006.00489.x.
- Andrade FMX, Bezerra FM, Santos MSD, Araujo M. Perfil clínico e achados oftalmológicos no herpes zoster Oftálmico. Rev Bras Oftalmol. 2019;78(3):170–74. doi:10.5935/0034-7280.20190122.
- Antoniolli L, Rodrigues C, Borges R, Goldani LZ. Epidemiology and clinical characteristics of herpes zoster in a tertiary care hospital in Brazil. Braz J Infect Dis. 2019;23(2):143–45. doi:10.1016/j.bjid.2019.03.001.
- Borba EF, Ribeiro AC, Martin P, Costa LP, Guedes LK, Bonfá E. Incidence, risk factors, and outcome of herpes zoster in systemic lupus erythematosus. J Clin Rheumatol. 2010;16(3):119–22. doi:10.1097/RHU.0b013e3181d52ed7.
- Gormezano NW, Silva CA, Otsuzi CI, Barros DL, da Silva MA, Sallum AM, Pasoto S, Pereira RM, Bonfa E. Higher prevalence and distinct features of herpes zoster infection in children than adults with systemic lupus erythematosus. Pediatr Infect Dis J. 2015;34(8):905–07. doi:10.1097/INF.0000000000000756.
- Toniolo-Neto J, Psaradellis E, Karellis A, Rampakakis E, Rockett TY, Sampalis JS, Johnson KD, Monsanto HA, Acosta CJ. Measuring herpes zoster disease burden in São Paulo, Brazil: a clinico-epidemiological single-center study. Clinics. 2018;73:e243. doi:10.6061/clinics/2018/e243.
- Ordoñez Molina JE, Orozco Giraldo JJ, Gutierrez-Ardila MV. Budget impact of pregabalin for the treatment of neuropathic pain in Colombia. Value Health. 2013;16(3):A114. doi:10.1016/j.jval.2013.03.546.
- Ordoñez Molina JE, Orozco Giraldo JJ, Gutierrez-Ardila MV. Cost-effectiveness analysis of pregabalin for the treatment of neuropathic pain in Colombia. Value Health. 2013;16(3):A118. doi:10.1016/j.jval.2013.03.568.
- Rampakakis E, Monsanto HA, Stutz M, Psaradellis E, Mejia G, Carillo AE, Zapata-Cardenas A, Molina DC, Molina de Salazar DI, Pradilla Vesga OE, et al. Burden of illness of herpes zoster in Colombia: an observational study. Value Health Reg Issues. 2019;19:S43.
- Rampakakis E, Alpizar C, Karellis A, Sampalis JS, Johnson K, Monsanto HA, Acosta CJ. Measuring the burden of herpes zoster disease in Costa Rica. Acta Méd Costarric. 2017;59:146–52.
- Ortiz-Covarrubias A. Measurement of the burden, resources use and health costs associated with herpes zoster and post-herpetic neuralgia in Mexico. Master study. Value Health. 2015;18(7):A870. doi:10.1016/j.jval.2015.09.009.
- Acosta A, Rueda M, Castañeda-Cardona C, Gil-Rojas Y, Rosselli D. Cost-effectiveness of Lidocaine medicated patch compared with pregabalin and gabapentin for the treatment of postherpetic neuralgia and diabetic polyneuropathy in Colombia. Value Health. 2017;20(9):A867–8. doi:10.1016/j.jval.2017.08.2531.
- Piedade A, Kashiura D, Engel T, Rodrigues C. Cost-effectiveness analysis of 5% Lidocaine medicated plaster monotherapy versus pregabalin or gabapentin in the treatment of post herpetic neuralgia and diabetic polyneuropathy under the perspective of Brazilian public healthcare system. Value Health. 2017;20:A221.
- González KG, Alonzo-Romero PL, Campos AG. Herpes zoster oftálmico. Evaluación de complicaciones y secuelas oculares y su relación con diversos tratamientos. Dermatol Rev Mex. 2013;56:392–98.
- Kawai K, Rampakakis E, Tsai TF, Cheong HJ, Dhitavat J, Covarrubias AO, Yang L, Cashat-Cruz M, Monsanto H, Johnson K, et al. Predictors of postherpetic neuralgia in patients with herpes zoster: a pooled analysis of prospective cohort studies from North and Latin America and Asia. Int J Infect Dis. 2015;34:126–31.
- Vázquez M, Cravioto P, Galván F, Guarneros D, Pastor VH. Varicella and herpes zoster: challenges for public health. Salud Publica Mex. 2017;59:650–56.
- Mendoza R, Francisca I, Talavera B, Claudia J. Comportamiento clínico y terapeutico del herpes zoster en el centro nacional de dermatología Doctor Francisco José Gómez Urcuyo enero 2002 - Diciembre 2006. 2007 [accessed 2022 Jul 27]. https://pesquisa.bvsalud.org/portal/resource/en/lil-592990.
- Sispro. Misiterio de Salud Colombia. 2020 [accessed 2021 Mar 12]. https://www.sispro.gov.co/Pages/Home.aspx.
- DGIS. Egresos Hospitalarios, Urgencias y Defunciones. Cubos dinámicos. Secretaría de Salud DGIS Mexico. 2020 [accessed 2020 Jul 16]. http://www.dgis.salud.gob.mx/contenidos/basesdedatos/bdc_egresoshosp_gobmx.html.
- Datasus. Informações de Saúde (TABNET). 2020 [accessed 2020 Jul 16]. http://www2.datasus.gov.br/DATASUS/index.php?area=02.
- Ministério da Saúde. Secretaria de Atenção Primária à Saúde (SAPS). 2021 [accessed 2021 Jul 1]. https://egestorab.saude.gov.br/paginas/acessoPublico/relatorios/relHistoricoCoberturaAB.xhtml.
- Departamento de Estadísticas e Información de Salud. Ministerio de Salud. Gobierno de Chile. 2020 [accessed 2020 July 16].
- XE. US Dollar to Brazilian real exchange rate chart. [accessed 2021 Jul 1]. https://www.xe.com/currencycharts/?from=USD&to=BRL&view=10Y.
