ABSTRACT
Infectious diseases continue to disproportionately affect low- and middle-income countries (LMICs) and children aged <5 y. Developing vaccines against diseases endemic in LMICs relies mainly on strong public-private collaborations, but several challenges remain. We review the operating model of the GSK Vaccines Institute for Global Health (GVGH), which aims to address these challenges. The model involves i) selection of vaccine targets based on priority ranking for impact on global health; ii) development from design to clinical proof-of-concept; iii) transfer to an industrial partner, for further technical/clinical development, licensing, manufacturing, and distribution. Cost and risks associated with pre-clinical and early clinical development are assumed by GVGH, increasing the probability to make the vaccine more affordable in LMICs. A conjugate vaccine against typhoid fever, Vi-CRM197, has recently obtained WHO prequalification, within a year from licensure in India, demonstrating the success of the GVGH model for development and delivery of global health vaccines.
Introduction
Addressing the burden of neglected diseases through vaccine development
More than 20 y into the twenty-first century, child mortality rates are still soaring, especially in low-income countries, despite tremendous efforts and progress achieved over the last decades. By 2015, the United Nations Millennium Development Goals initiative resulted in a decline of the mortality rate by more than half, among children <5 y of age compared to 1990, but still not reaching the targeted 75% reduction. Starting from 2016, the Sustainable Development Goals (SDGs) program is being implemented and targets an under-5 mortality rate of ≤25/1,000 livebirths by 2030.Citation1 In 2019, 38/1,000 livebirths were reported, a decline of 59% from 1990. Disparities between high-income and low-/middle-income countries (LMICs) still exist, with the highest mortality rates being reported for Sub-Saharan Africa and Central and Southern Asia, together accounting for 80% of the total deaths in children <5 y of age in 2018.Citation2
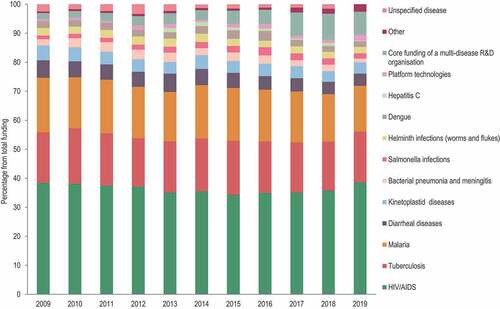
In particular, infectious diseases continue to disproportionately affect LMICs and children <5 y of age.Citation3 Pneumonia, diarrheal disease, and malaria were the leading causes of mortality in this age group in 2018. Nevertheless, most communicable diseases can be controlled by improvements in the access to and quality of water and sanitation, which is not always achieved in low-income countries. Other prevention methods such as vaccination can be effective, but costs and time associated with development and introduction of a vaccine are limiting this approach, especially in poor-resource settings. Vaccine development, starting from proof-of-concept through approval and market placement, is an enterprise involving considerable resources and financial risk and which cannot always be undertaken in LMICs. This is, however, feasible for developed countries, which have the required financial resources and a suitable research and development (R&D) infrastructure but not the commercial drive stemming from an unmet need, because most infectious diseases with high medical and economic burden in LMICs are already controlled in high-income geographies. Therefore, the support of public and private organizations plays a major role in the control of neglected diseases worldwide. Funding of R&D and product development for these diseases have increased over the last five years. However, there is still a disproportionate distribution of financial resources from one disease to another, with the bulk of funds being allocated to HIV, malaria, and tuberculosis ().Citation4,Citation5
Figure 1. Distribution of research and development funding by neglected disease between 2009 and 2019. Data were taken from the G-Finder 2019 and 2020 reports.Citation4,Citation5.
The GSK Vaccines Institute for Global Health (GVGH), formerly functioning within Novartis, was created in 2007 and became operational in 2008 as a science-led organization, with the main purpose of developing effective and affordable vaccines for neglected infectious diseases of impoverished communities.Citation6,Citation7 GVGH is now a subsidiary of GSK and part of its global health organization and focuses on the development of public health priority vaccines in the absence of a strong commercial drive. The institute’s aim is to de-risk early technical and clinical vaccine development to enable sustainable supply of the vaccine in LMICs by a commercializing partner. In 2019, the model introduced by the institute was adopted by GSK as the official model for developing its global health strategy, to further facilitate access to vaccines and other medicinal products in LMICs. We review the GVGH operating model and showcase its performance through its first success story: the development of a typhoid conjugate vaccine (TCV) against typhoid fever (Vi-CRM197), with rapid advance from research to licensure and World Health Organization (WHO) prequalification.
Materials and methods
Search strategy and selection criteria
We performed a text search on PubMed and Google Scholar, using the terms “Novartis Vaccines Institute for Global Health,” “NVGH,” “GSK Vaccines Institute for Global Health,” and “GVGH.” We also performed a gray literature search on 24 August 2021. In addition, we performed a gray literature search to identify press releases related to GVGH and NVGH. Articles/press releases referring to the operating model of the institute and describing any phase in the development of Vi-CRM197 were included in the current review.
GVGH – an innovative model for sustainable global health strategies
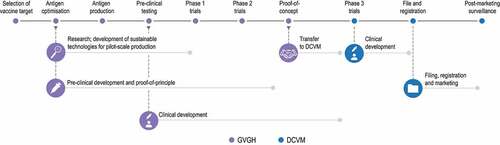
The GVGH operating model is structured around several main steps: selection of the candidate vaccine targets (based on a priority ranking for impact on global health); development of the vaccine (from design to clinical proof-of-concept in the target population); and transfer of the vaccine to an industrial partner vaccine manufacturer which will undertake further technical and clinical development, licensing, manufacturing, and distribution of the vaccine ().
GVGH relies on a rigorous selection process for the identification of its potential projects, with several key criteria being applied. The primary criterion is the possibility to achieve the best impact on public health of impoverished communities with the available resources. With this in mind, pathogens are “ranked’ by disease burden in LMICs and the potential for a vaccine to substantially impact public health. Neglected diseases for which vaccines are not in advanced development elsewhere, or for which a strong commercial driver is not available, are identified as priority targets. The probability of technical success and likelihood of the vaccine being implemented, if safe and effective, are also factored in,Citation8 along with a good fit with the institute’s prioritization for developing and implementing innovative and generic technologies, suitable for a wide range of vaccines. In addition, the development of a targeted vaccine is fully aligned with the parent pharmaceutical company’s overall global health strategy to advance products tailored for ease of implementation in any manufacturing facility throughout the world. In the selection of the initial GVGH projects, a triage approach was used. Thus, the disease burden in LMICs was considered for approximately 60 infectious diseases; from these, non-neglected infectious diseases, those with commercialized vaccines or vaccines in advanced development, and with low burden or with effective treatment were eliminated in the selection process. This led to the identification of around 20 potential vaccines for neglected diseases, and vaccines against Salmonella and Shigella were selected as priority for development.
While recognized as a leading incident cause of health loss in all ages,Citation9 the burden of enteric infections is probably still underestimated, especially in LMICs.Citation9,Citation10 Based on the key selection criteria, enteric bacterial diseases were selected as the initial GVGH priority projects. The leading cause of enteric fever in impoverished communities of South and Southeast Asia is Salmonella enterica subspecies enterica serovar Typhi (S. Typhi). An increase in the incidence of S. Paratyphi A cases has also been observed recently.Citation11 This led to the prioritized development of Vi-CRM197, a TCV, and that of a bivalent typhoid-paratyphoid A conjugate vaccine. GVGH is also pursuing vaccines against invasive nontyphoidal Salmonella (a major cause of morbidity and mortality in Sub-Saharan Africa, especially among malnourished childrenCitation11), Shigella (a major cause of diarrhea in children <5 y of age in LMICsCitation7), and group A Streptococcus (a pathogen responsible for a wide range of diseases, some of which can lead to long-term morbiditiesCitation12). Each of these bacteria is known to carry antibiotic resistance markers, and effective vaccines would also contribute to better antibiotic stewardship.
Once the target vaccine is identified, its initial development is carried out at GVGH. To date, the institute has partnered for the antigen discovery phase, and focused on the optimization of suitable antigens and their formulation into products that can generate optimal safety and immune response. GVGH also designs and implements manufacturing processes for pilot-scale production, including the integration of good documentation/manufacturing practices guaranteed to comply with regulatory requirements for product licensure. Special emphasis is put on sustainable technologies which are applicable to low-income settings due to their flexibility and affordability by design, with robust, non-complex processes, easily scalable and adaptable to commonly available equipment/facilities. These technologies should also allow for the production of vaccines that are easy to deliver, store, and administer.
Phase 1 and phase 2 clinical trials are designed and conducted to demonstrate an acceptable safety profile and adequate immunogenicity of the vaccine in the target population, before transfer to the industrial partner for further clinical development. Over the last decade, GVGH initiated more than 10 phase 1/2 trials in the European Union, the United States, India, Pakistan, the Philippines, and Kenya () and has plans to start several additional first-in-human phase 1 trials in Europe and Africa within the next two years.
Table 1. Phase 1 and 2 clinical trials conducted with GVGH vaccine candidates.
The preferred industrial partner is a previously identified, suitable business entity, which may be a developing country vaccine manufacturer (DCVM), other pharmaceutical company, or GSK. The GVGH model implies that the institute’s resources are directed toward technical feasibility and the early clinical development of the product, while the industrial partner takes full responsibility for phase 3 clinical development, manufacturing, approval, WHO prequalification (when applicable), and marketing of the vaccine. As the cost and risks associated with the pre-clinical and early clinical development are not supported by the DCVM, the probability is higher that the vaccine can be made available at affordable prices in LMICs. The DCVM is therefore expected to commit to sustainable development of the vaccine and to ensure access to the final product across the target population (in countries where the target disease is endemic). The transfer from GVGH may occur under terms for marketing of the vaccine to certain priority countries, as assessed by the institute. The selection of the partner DCVM heavily relies on their track record of vaccine licensing, WHO prequalification, and distribution to poor-resource settings. The model does not exclude having more than one DCVM involved in the manufacturing and marketing of the vaccine.
All GVGH projects are co-funded by external public or private organizations, such as the Wellcome Trust, the Bill & Melinda Gates Foundation, Sclavo Vaccine Association (through grants from Monte dei Paschi di Siena and Regione Toscana), the European Commission (through The European & Developing Countries Clinical Trials Partnership and other Horizon 2020 programmes) and CARB-X. All funding partners are allowed input and full access to results throughout the entire vaccine development process. Sharing decision-making and costs between GVGH and these organizations creates a more sustainable framework for GVGH activities and ensures that GVGH focuses on vaccine development in areas that are also a priority for the external organizations. GVGH is committed to make all results available to the research community and to the public at large, but also to patent any discoveries that could ensure a greater business sustainability for its economic partners.
In addition, GVGH is dedicated to offering educational opportunities through fully funded MSc, PhD, and post-doctoral programs on all aspects of vaccine development and public health, in partnership with European universities. Several positions in the MSc program have so far been awarded to medical doctors from LMICs (Sri Lanka, Nigeria, Pakistan, Kenya, and Tanzania). Capacity building is also embedded into the conduct of phase 2 studies in endemic settings and by supporting the DCVM during the final licensure phase and commercial launch.
A story of success: from discovery research to the licensure of a conjugate vaccine against typhoid fever
The selection of a vaccine against typhoid fever as the first GVGH project was validated by the findings of the WHO Product Development for Vaccines Advisory Committee (PDVAC)Citation13 as well as other identified global health needs. Through its GLASS initiative, the WHO has included fluoroquinolone-resistant Salmonella spp. as Priority 2 (High) on the “Global Priority List of Antimicrobial Resistant Bacteria”Citation14 and has recently stressed the need to address S. Typhi in the pediatric population. Typhoid and paratyphoid A fevers remain a global health concern, with 14 · 3 million cases, 9 · 8 million disability-adjusted life years, and around 136,000 deaths estimated in 2017.10 S. Typhi alone was responsible for >75% of the global enteric fever cases in 2017, with children being especially susceptible to infection and bearing most of the disease burden. The highest incidence of typhoid fever is observed in South and Southeast Asia, and Africa, where large outbreaks continue to occur.Citation15,Citation16 In India, where the disease is endemic, estimates of incidence reach 377/100,000 person-years, with a peak in children 2–4 y of age.Citation17 By the late 1990ʹs, resistance of S. Typhi to several antibiotics was well documented, and treatment recommendations include azithromycin or cefixime for mild cases and ceftriaxone in intravenous therapy.Citation18 Resistance to antibiotics is increasing,Citation19 including multidrug resistance against first-line therapies, and extensively drug resistant (XDR) typhoid outbreaks have been reported.Citation12 Recently, an effectiveness trial has shown vaccination with a TCV to be 97% effective against culture-confirmed XDR S. Typhi,Citation20 thus confirming that vaccination can prevent infection with multidrug resistant strains and thus, significantly reduce the use of antibiotics and the emergence of further antibiotic-resistant Salmonella strains.
Due to the low incidence of typhoid fever in high-income countries, efforts for the development of an efficacious vaccine, which can be used in all age groups stalled. Starting in 2008, the WHO recommended programmatic vaccination for the prevention of typhoid fever in endemic countries. The use of unconjugated Vi polysaccharide (ViPS), live attenuated Ty21a vaccine, and more recently, of TCVs is recommended.Citation16,Citation21 As initially typhoid vaccines (such as ViPS and Ty21a) were developed primarily for high-income populations (travel market), their target product profile was not aligned with programmatic considerations of a vaccine for pediatric use in LMICs. Misaligned attributes included, among others, a limited fit within the childhood immunization schedule, lack of ease of administration, number of doses, duration of protection, moderate protective efficacy and cold chain requirements. Additionally, they were never licensed for use in children <2 y of age.Citation16,Citation22 These issues were overcome by the development of TCVs, with efforts starting in the late 1980s.Citation23 After the first Vi-TCV vaccine efficacy trial showing an estimated efficacy of 91.5% for a nontoxic recombinant Pseudomonas aeruginosa exotoxin A conjugate in children 2–5 y of age,Citation24 a Vi polysaccharide tetanus toxoid-conjugated vaccine (Typbar-TCV) received WHO prequalification in 2017.Citation25 Other vaccines (e.g., TCVs and ViPS) are available in India only. Additional TCV candidates are in different phases of their preclinical and clinical development.Citation21 In 2018, the WHO recommended the use of TCVs in children >6 months of age.Citation21 TCVs developed for pediatric public sector use in typhoid endemic countries can also be marketed in the private sector. In addition, they could be efficiently used as high-income country traveler’s vaccines, for outbreak control in non-endemic countries (in view of recent reports on XDR S. Typhi infections not only in travelers from endemic to non-endemic countriesCitation26 but also in residents who do not travel internationallyCitation27), with appropriate regulatory approval. These additional market segments will contribute to the sustainable vaccine value of TCVs.
Pre-clinical and early clinical development of Vi-CRM197
The main immunogenic component of S. Typhi is the Vi capsular polysaccharide and anti-Vi levels have been shown to correlate with protection against the pathogen.Citation28 However, S. Typhi is a highly pathogenic organism, and large-scale manufacture poses an occupational safety hazard, which can raise additional difficulties for DCVMs. The Vi polysaccharide is also produced by the nonpathogenic Citrobacter freundii. Based on this observation, a C. freundii strain that stably expresses Vi on its surface, was identified as a suitable polysaccharide source for Vi-CRM197 as it leads to high yields of Vi under industrial-scale fermentation conditions and was observed to elicit high anti-Vi antibody levels in animal models.Citation29,Citation30 The selection of CRM197 (a well-characterized variant of diphtheria toxin which does not require detoxification and is used in many licensed conjugate vaccinesCitation31,Citation32) as the carrier protein allowed for an affordable conjugate, which can be obtained in a reproducible manner. A previously applied conjugation technology was optimized for higher yields, and improved analytics were developed to facilitate the production of Vi-CRM197.Citation33 These activities were carried out by GVGH.
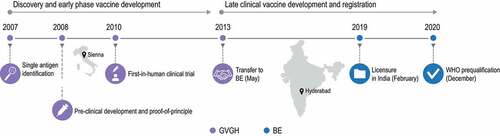
When used in clinical trials, the vaccine was well tolerated and showed higher anti-Vi antibody levels than those induced by a licensed ViPS vaccine in adults.Citation34 In two large age de-escalation studies conducted in South and Southeast Asian countries, the vaccine induced strong immune responses, had an acceptable clinical safety profile, and did not impact the immunogenicity of vaccines co-administered under the Expanded Program on Immunization.Citation35 Therefore, not only an efficient manufacturing process was established for Vi-CRM197, but this was done in a relatively short period of time: the first human trial was started within a year from project start, and clinical trials were started in endemic countries only one year after ().
Figure 3. Milestones of the GVGH operating model as applied to the development of the typhoid conjugate vaccine.
However, this first construct performed in part as a T-cell independent vaccine as no incremental effect in antibody levels was observed following a second vaccination,Citation34 and no evidence of induced immunological memory was seen.Citation35 Antibody persistence was similar to that reported for the unconjugated Vi.Citation34 These limitations identified in clinical trials led to the further refinement of the vaccine candidate to improve antibody responses. Based on data from non-clinical studies indicating a link between the Vi-conjugate chain length and immune response,Citation36,Citation37 the levels of non-covalently associated Vi polysaccharide were reduced and a more appropriately sized polysaccharide chain length was eventually produced, purified, and used for conjugation.
This represents an added value of the GVGH model, as early testing in the target population allows identification of potential issues that can be addressed before further development by the commercial partner. GVGH’s continued work to improve the vaccine prior to, during, and after technology transfer, and to support the DCVM provides opportunities to de-risk development, ensuring advancement to licensure, public health recommendations, and deployment.
Technology transfer from GVGH to manufacturer
According to the GVGH model, the manufacturing partner can be any pharmaceutical company (including GSK). For Vi-CRM197, an Indian business partner was considered due to the high burden of typhoid fever in this country. In addition, India has one of the most competitive pharmaceutical sectors worldwide, due in large part to low-production costs, the presence of well-trained workforce, and manufacturing sites functioning in compliance with international standards,Citation38 which would facilitate national production and distribution of the vaccine.
Biological E Limited (BE), a Hyderabad-based pharmaceutical company founded in 1953, is the first private sector biological products company in India and the first pharmaceutical company in Southern India. BE develops, manufactures, and supplies vaccines and therapeutics to central and state governmental hospitals, public sector undertakings, and the Indian Armed Forces. The company is also active in the international market in over 100 countries and has a long history of working with global pharmaceutical companies, through in-licensing and development agreements, and in the United States Food and Drug Administration and the European Medicines Agency environments. BE has a current portfolio of nine WHO prequalified vaccinesCitation25 and has experience in the development of conjugate, inactivated, and live attenuated vaccines.
GVGH transferred all technology pertaining to Vi-CRM197 under a licensing agreement with BE in May 2013. The technology transfer was undertaken by multiple short secondments of staff between the organizations.
Further development by the manufacturer
BE undertook manufacturing process optimization and scale-up, pre-clinical studies, and clinical trials in India as part of the late development of Vi-CRM197. The vaccine is produced at a recently built multipurpose facility within the Hyderabad manufacturing site, in the Special Economic Zone at Genome Valley in Kolthur Village.
Further clinical testing in phase 1 (CTRI2018/03/012558), 2/3 (CTRI2018/11/016419), and 3 (CTRI2019/07/020451) trials were conducted by BE to evaluate the final formulation and to comply with regulatory requirements, which at the time specified that any vaccine needed to undergo full clinical development in India to obtain licensure. In the phase 1 trial, the safety and immunogenicity of a single intramuscular dose of Vi-CRM197 were tested in healthy adults 18–45 y of age, while study CTRI2019/07/020451 was a single-arm trial evaluating the safety and tolerability of a single dose of Vi-CRM197, administered as an intramuscular injection to infants ≥6 months to adults ≤45 y of age. Study CTRI2018/11/016419 was a single-blind randomized controlled trial in healthy infants, children, and adults (≥6 months to 64 y old) evaluating the immunogenicity and safety of one dose of Vi-CRM197 as compared with the Typbar-TCV. In this trial, non-inferiority of Vi-CRM197 to the licensed vaccine was demonstrated with respect to seroconversion (anti-Vi immunoglobulin G [IgG] concentration ≥2 μg/mL) rates measured 42 d post-vaccination, and a high percentage of participants (95 · 6%) were shown to have anti-Vi IgG concentrations ≥4 · 3 μg/mL,Citation39 a threshold considered as indicative of sustained long-term protection.Citation28 In addition, Vi-CRM197 vaccination was well tolerated and raised no safety concerns.Citation39 A phase 3, non-randomized, active controlled trial (CTRI2020/03/023712) is also ongoing to assess persistence of anti-Vi IgG antibodies at 12, 24, and 36 months post-primary dose and to evaluate the immune response to a single booster dose of Vi-CRM197 administered at 36 months post-primary dose to individuals who had participated in the CTRI2018/11/016419 study.
In 2020, the authorization to license and market Vi-CRM197 under the commercial name TYPHIBEV was granted to BE by the Indian healthcare authorities. The vaccine is indicated as a single-dose injectable administration in individuals from 6 months up to 45 y of age. As of November 2020, the Advisory Committee on Vaccines and Immunization Practices of the Indian Academy of Pediatrics also recommends the routine administration of a TCV to children 6–9 months of age.Citation40 Prequalification of single and multidose vaccine vials of TYPHIBEV by the WHO was obtained in December 2020,Citation25,Citation37 which constitutes an important step for future inclusion in Gavi’s investment strategy to enable access in LMICs.Citation41
Going further: prospects and challenges
At the time the GVGH was founded, the gap in vaccine translational researchCitation42 (often referred to as “the first valley of death”) had become obvious. Strong public-private collaborations funded mainly by nonprofit organizations expedited the early clinical development of numerous vaccines, only to reveal a “second valley of death” in the late development stage and at-scale implementation.Citation43,Citation44 It was the institute’s bespoke mission to contribute to the covering of these gaps. The development of Vi-CRM197 is a success story, showing how in-house technical and clinical development expertise can translate a good scientific concept into a manufacturable vaccine, and how key regulatory and production experience of a rigorously chosen DCVM can facilitate its accessibility to the target population, thus addressing a global need. It took only 13 months from project initiation in 2008 to reach the milestone of first-in-human clinical trial. Since then, GVGH carried out early development and remained involved in late development conducted by the DCVM post-technological transfer. Once the vaccine was licensed in India, BE rapidly achieved WHO prequalification. This has resulted in a life-saving vaccine, now made available in a typhoid endemic country via Gavi.
Certainly, the global emergency created by the COVID-19 pandemic has led to changes in the approach of both pharmaceutical companies and regulatory authorities in the clinical development and approval process of candidate vaccines. The unprecedented funds made globally available for the development of COVID-19 vaccines have made expedited timelines possible and has shown that a new vaccine can be marketed in less than 2 y from its conception. GVGH is therefore evaluating from the COVID-19 experience which ways could accelerate vaccine development, even in the absence of a pandemic threat. Increasing operational efficiency or running some clinical trials in parallel rather than sequentially could significantly shorten vaccine development time. In addition, the success of the GVGH operating model demonstrated by the Vi-CRM197 licensure is already triggering more collaboration and financial support from the private and public sectors.
Going forward, the pillars of the GVGH model are science-led, sustainable vaccine development, with focus on impact. The institute will continue to address the considerable burden of infectious diseases in LMICs by focusing on diseases for which a vaccine can be developed with state-of-the-art expertise and technologies. While other organizations, such as the International Vaccine Institute in South Korea or the Hilleman Laboratories in India, have similar missions and aims, we believe that GVGH’s strength lies in its placement within a powerful R&D site, which enables direct access to platform technologies and experience in all aspects of vaccine development. GVGH aims to reproduce the success of Vi-CRM197, with several vaccine candidates currently in the pipeline, expected to reach proof-of-concept trials between 2024 and 2026. These include vaccines against S. Paratyphi A (the second leading cause of invasive Salmonella infections in Asia) and S. Enteritidis and S. Typhimurium (causing non-typhoidal Salmonella invasive disease in Sub-Saharan Africa). The development of a vaccine against Shigella sonnei has now expanded to a four-component vaccine to prevent shigellosis caused by S. sonnei and S. flexneri. A Group A Streptococcus vaccine is also in development, to prevent the sequelae of untreated streptococcal superficial infections (pharyngitis and impetigo), including rheumatic fever and rheumatic heart disease. However, the institute’s activity has not been without challenges and several projects had to be discontinued, such as a meningococcal vaccine based on generalized modules for membrane antigens (GMMA), an inactivated rotavirus vaccine (as other competitive alternatives became available) or a conjugate vaccine against invasive non-typhoidal salmonellosis (for which the GMMA technology is now considered a more suitable vaccine platform in LMICs).
GVGH has also launched the GVGH Innovation Academy, an initiative meant to advance and promote breakthrough innovation in global health, by creating the opportunity for collaborations to explore new technologies in different therapeutic areas. The Innovation Academy’s main aim is to strengthen this success by developing other products based on new technologies (i.e., mRNA, nanoparticles, monoclonal antibodies, adjuvants) in a manner that makes them readily deployable to LMICs.
The sustainability of the GVGH model heavily relies on attracting an influx of funding from external funders and key partner organizations to ensure end-to-end product viability. Global investments in new products targeting neglected diseases in LMICs reached a record high in 2018, maintained also throughout 2019.Citation5 The upward trend in global funding for Salmonella infections continued, with the most important contribution to the yearly increase coming from the Bill & Melinda Gates Foundation and the Wellcome Trust, although public funding remained the main source of funds. Among diarrheal diseases, Shigella was the only one for which an increase in funding was still observed over the last years, with support going toward the development of conjugate vaccines.Citation5 These trends in funding validate GVGH’s selection of targeted diseases, with the institute receiving expanded public and private support for their projects.
The final step in the GVGH model is to ensure access to the vaccine for the targeted population, and the DCVM plays the key role.Citation45 Through an intelligent selection of targeted diseases, innovative technologies, and strategic partnerships, the involvement of a qualified DCVM can also be achieved early in the development timelines (prior to proof-of-concept), thus further accelerating the timelines for vaccine manufacturing and licensure. In addition, experiences of the COVID-19 pandemic may be the basis for the streamlining of new regulatory and clinical approaches to facilitate interventions targeting neglected diseases in LMICs; the Coalition for Epidemic Preparedness Innovations (CEPI) is focusing on compressing vaccine development timelines to 100 d instead of years.Citation46
With a major milestone achieved, a first vaccine made accessible in low-income settings, the GVGH demonstrated the success of its model for the development and delivery of global health vaccines. We envision that investing in safe, effective, and affordable vaccines will continue to have world-wide impact on human health for years to come. GVGH is uniquely placed to develop vaccines for global health: a dedicated global health vaccine unit, within a pharmaceutical company. Over the coming years, GVGH will continue to grow its mission to bring new products and state-of-the-art technologies to developing countries, addressing global health challenges.
Authors contribution
All authors attest they meet the ICMJE criteria for authorship. All authors contributed to the drafting on the manuscript, revised it critically for important intellectual content and gave final approval to submit for publication.
Acknowledgments
The authors thank Vikram Paradka (Biological E Limited) for careful review of the manuscript. The authors also thank Modis for editorial assistance and manuscript coordination, on behalf of GSK. Petronela M. Petrar provided medical writing support and Carlos Marin P. coordinated the manuscript development and provided editorial support.
Disclosure statement
RR and FBS are and LBM and AP were employees of the GSK group of companies. All authors except LBM hold shares in the GSK group of companies. LBM is currently an employee of USP (Rockville, MD USA), is inventor on patents owned by the GSK group of companies and reports grants from the Wellcome Trust, CARB-X, Bill & Melinda Gates Foundation, and the European Union, outside the submitted work.
Additional information
Funding
References
- United Nations General Assembly. Transforming our world: the 2030 agenda for sustainable development; 2015 [accessed 2021 Jul 29. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E.
- Levels & trends in child mortality. Report 2019. Estimates developed by the UN Inter-agency Group for Child Mortality Estimation; 2019 [accessed 2021 Jul 26]. http://childmortality.org/wp-content/uploads/2019/10/UN-IGME-Child-Mortality-Report-2019.pdf.
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):1–9. doi:10.1016/s0140-6736(16)31593-8.
- Chapman N, Doubell A, Barnsley P, Goldstein M, Oversteegen L, Chowdhary V, Rugarabamu G, Ong M, Borri J, Hynen A, Policy Cures Research, et al. G-Finder 2019. Uneven progress 2019. http://s3-ap-southeast-2.amazonaws.com/policy-cures-website-assets/app/uploads/2020/02/11150341/G-Finder2019.pdf.
- Chapman N, Doubell A, Tuttle A, Barnsley P, Goldstein M, Oversteegen L, Chowdhary V, Borri J, Hynen A, Kearney M. Policy Cures Research. G-Finder 2020. Neglected disease research and development: Where to now? 2020. http://policy-cures-website-assets.s3.ap-southeast-2.amazonaws.com/wp-content/uploads/2021/04/15055816/G-FINDER-2020_Final-Report.pdf.
- Podda A. Aims and role of Novartis Vaccines Institute for Global Health (NVGH). Procedia Vaccinol. 2010;2(2):124–27. doi:10.1016/j.provac.2010.07.003.
- Saul A, Rappuoli R. The Novartis Vaccines Institute for Global Health: a new initiative for developing vaccines for neglected diseases in developing countries. J Infect Dev Ctries. 2009;2(2):154–55. doi:10.3855/t2.2.154.
- Saul A, O’Brien KL. Prioritizing vaccines for developing world diseases. Vaccine. 2017;35(Suppl 1):A16–19. doi:10.1016/j.vaccine.2016.10.087.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. doi:10.1016/s0140-6736(18)32279-7.
- Troeger C, Colombara DV, Rao PC, Khalil IA, Brown A, Brewer TG, Guerrant RL, Houpt ER, Kotloff KL, Misra K, et al. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health. 2018;6(3):e255–e269. doi:10.1016/s2214-109x(18)30045-7.
- GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19(4):369–81. doi:10.1016/s1473-3099(18)30685-6.
- Basnyat B, Qamar FN, Rupali P, Ahmed T, Parry CM. Enteric fever. BMJ. 2021;372:n437. doi:10.1136/bmj.n437.
- Prudden HJ, Hasso-Agopsowicz M, Black RE, Troeger C, Reiner RC, Breiman RF, Jit M, Kang G, Lamberti L, Lanata CF, et al. Meeting Report: WHO workshop on modelling global mortality and aetiology estimates of enteric pathogens in children under five. Cape Town, 28-29th November 2018. Vaccine. 2020;38(31):4792–800. doi:10.1016/j.vaccine.2020.01.054.
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. doi:10.1016/s1473-3099(17)30753-3.
- Appiah GD, Chung A, Bentsi-Enchill AD, Kim S, Crump JA, Mogasale V, Pellegrino R, Slayton RB, Mintz ED. Typhoid outbreaks, 1989-2018: implications for prevention and control. Am J Trop Med Hyg. 2020;102(6):1296–305. doi:10.4269/ajtmh.19-0624.
- World Health Organization. Typhoid vaccines: WHO position paper. Week Epidemiol Rec. 2008;83(6):49–59.
- John J, Van Aart CJ, Grassly NC. The burden of typhoid and paratyphoid in India: systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10(4):e0004616. doi:10.1371/journal.pntd.0004616.
- Mukhopadhyay B, Sur D, Gupta SS, Ganguly NK. Typhoid fever: control & challenges in India. Indian J Med Res. 2019;150(5):437–47. doi:10.4103/ijmr.IJMR_411_18.
- Browne AJ, Kashef Hamadani BH, Kumaran EAP, Rao P, Longbottom J, Harriss E, Moore CE, Dunachie S, Basnyat B, Baker S, et al. Drug-resistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis. BMC Med. 2020;18(1):1. doi:10.1186/s12916-019-1443-1.
- Yousafzai MT, Karim S, Qureshi S, Kazi M, Memon H, Junejo A, Khawaja Z, Ur Rehman N, Ansari MS, Ali R, et al. Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study. Lancet Glob Health. 2021;9(8):e1154–e1162. doi:10.1016/s2214-109x(21)00255-2.
- World Health Organization. Typhoid vaccines: WHO position paper, March 2018 - recommendations. Vaccine. 2019;37(2):214–16. doi:10.1016/j.vaccine.2018.04.022.
- Pulickal AS, Pollard AJ. Vi polysaccharide-protein conjugate vaccine for the prevention of typhoid fever in children: hope or hype? Expert Rev Vaccines. 2007;6(3):293–95. doi:10.1586/14760584.6.3.293.
- Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987;166(5):1510–24. doi:10.1084/jem.166.5.1510.
- Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344(17):1263–69. doi:10.1056/nejm200104263441701.
- World Health Organization. WHO prequalified vaccines. [accessed 2021 Oct 29]. http://extranet.who.int/pqweb/vaccines/prequalified-vaccines.
- Akram J, Khan AS, Khan HA, Gilani SA, Akram SJ, Ahmad FJ, Mehboob R. Extensively Drug-Resistant (XDR) typhoid: evolution, prevention, and its management. Biomed Res Int. 2020;2020:6432580. doi:10.1155/2020/6432580.
- Centers for Disease Control and Prevention. HAN archive - 00439: extensively drug-resistant Salmonella Typhi infections among U.S. residents without international travel; 2021 Feb 12 [accessed 2021 Aug 24]. http://emergency.cdc.gov/han/2021/han00439.asp.
- Szu SC, Klugman KP, Hunt S. Re-examination of immune response and estimation of anti-Vi IgG protective threshold against typhoid fever-based on the efficacy trial of Vi conjugate in young children. Vaccine. 2014;32(20):2359–63. doi:10.1016/j.vaccine.2014.02.050.
- Rondini S, Micoli F, Lanzilao L, Hale C, Saul AJ, Martin LB. Evaluation of the immunogenicity and biological activity of the Citrobacter freundii Vi-CRM197 conjugate as a vaccine for Salmonella enterica serovar Typhi. Clin Vaccine Immunol. 2011;18(3):460–68. doi:10.1128/cvi.00387-10.
- Rondini S, Micoli F, Lanzilao L, Pisoni I, Di Cioccio V, Saul AJ, Martin LB. Characterization of Citrobacter sp. line 328 as a source of Vi for a Vi-CRM197 glycoconjugate vaccine against Salmonella Typhi. J Infect Dev Ctries. 2012;6(11):763–73. doi:10.3855/jidc.2495.
- Micoli F, Adamo R, Costantino P. Protein carriers for glycoconjugate vaccines: history, selection criteria, characterization and new trends. Molecules. 2018;23(6):1451. doi:10.3390/molecules23061451.
- Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother. 2013;9(12):2505–23. doi:10.4161/hv.26109.
- Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, Rappuoli R, Szu S, Saul A, Martin LB. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011;29(4):712–20. doi:10.1016/j.vaccine.2010.11.022.
- van Damme P, Kafeja F, Anemona A, Basile V, Hilbert AK, De Coster I, Rondini S, Micoli F, Qasim Khan RM, Marchetti E, et al. Safety, immunogenicity and dose ranging of a new Vi-CRM197 conjugate vaccine against typhoid fever: randomized clinical testing in healthy adults. PLoS One. 2011;6(9):e25398. doi:10.1371/journal.pone.0025398.
- Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, Juvekar S, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and Southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis. 2014;14(2):119–29. doi:10.1016/s1473-3099(13)70241-x.
- Arcuri M, Di Benedetto R, Cunningham AF, Saul A, MacLennan CA, Micoli F. The influence of conjugation variables on the design and immunogenicity of a glycoconjugate vaccine against Salmonella Typhi. PLoS One. 2017;12(12):e0189100. doi:10.1371/journal.pone.0189100.
- Micoli F, Bjarnarson SP, Arcuri M, Aradottir Pind AA, Magnusdottir GJ, Necchi F, Di Benedetto R, Carducci M, Schiavo F, Giannelli C, et al. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM(197) conjugate vaccine. Proc Natl Acad Sci USA. 2020;117(39):24443–49. doi:10.1073/pnas.2005857117.
- Festa G, Kolte A, Carli MR, Rossi M. Envisioning the challenges of the pharmaceutical sector in the Indian health-care industry: a scenario analysis. J Bus Ind Marketing. 2022;37(8):1662–74. doi:10.1108/JBIM-07-2020-0365.
- Siddalingaiah N, Tuluva S, Turaga K. A multicentre single blind randomised controlled phase-II/III study to evaluate immunogenicity and safety of single intramuscular dose of Biological E’s Vi-capsular polysaccharide-CRM197 conjugate typhoid vaccine in healthy infants, children and adults in comparison with a licensed comparator, IAP ResRCHcon 2020 Abstracts. Indian J Pediatr. 2020;87(11):974–88. doi:10.1007/s12098-020-03504-8.
- Kasi SG, Shivananda S, Marathe S, Chatterjee K, Agarwalla S, Dhir SK, Verma S, Shah AK, Srirampur S, Kalyani S, et al. Indian Academy of Pediatrics (IAP) Advisory Committee on Vaccines and Immunization Practices (ACVIP): recommended immunization schedule (2020-21) and update on immunization for children aged 0 through 18 years. Indian Pediatr. 2021;58(1):44–53. doi:10.1007/s13312-021-2096-7.
- Coalition against typhoid, typhoid vaccine acceleration consortium. TYPHIBEV: Questions & Answers; 2021 [accessed 2021 Jul 29]. http://www.coalitionagainsttyphoid.org/wp-content/uploads/2021/06/TYPHIBEV-QA_May-2021.pdf.
- Butler D. Translational research: crossing the valley of death. Nature. 2008;453(7197):840–42. doi:10.1038/453840a.
- Kaslow DC, Black S, Bloom DE, Datla M, Salisbury D, Rappuoli R. Vaccine candidates for poor nations are going to waste. Nature. 2018;564(7736):337–39. doi:10.1038/d41586-018-07758-3.
- O’Brien KL, Binka F, Marsh K, Abramson JS. Mind the gap: jumping from vaccine licensure to routine use. Lancet. 2016;387(10031):1887–89. doi:10.1016/s0140-6736(16)30394-4.
- Excler JL, Privor-Dumm L, Kim JH. Supply and delivery of vaccines for global health. Curr Opin Immunol. 2021;71:13–20. doi:10.1016/j.coi.2021.03.009.
- Samarasekera U. CEPI prepares for future pandemics and epidemics. Lancet Infect Dis. 2021;21(5):608. doi:10.1016/s1473-3099(21)00216-4.