?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Vaccination of children with special health status has become one of the most urgent issues in China. We aim to evaluate vaccination coverage and safety as well as its associated factors among children with special health status in China during 2016‒2020. We conducted a retrospective cohort review of all children with special health status recorded in the Electronic Immunization Registries System in Chongqing, China, between 2016 and 2020. Univariate and multivariate logistic regression analyses were used to analyze the influence factors. Among the 2,175 children with special health status enrolled in the study, the overall vaccination coverage rate was lower than that among the general population, and the incidence of adverse event in them following immunization was very rare. Children with congenital heart disease were better vaccinated (aOR = 1.508–6.331), while most of the jaundice children had missed vaccination (aOR = 0.441‒0.556). The purchase of vaccine compensation insurance was associated with higher completion rate of basic immunization for Bacillus Calmette-G vaccine (aOR = 1.706, 95% CI: 1.249‒2.329) and rotavirus vaccine (aOR = 1.346, 95% CI: 1.061‒1.708). Children with special health status can be safely vaccinated. However, the vaccination coverage in these huge and vulnerable group is too low to protect them from vaccine-preventable diseases through immunization. More researches and interventions should be conducted to ensure a higher vaccination rate among the children with special health status.
Introduction
With the help of Expanded Programme on Immunization (EPI), the vaccination rate of EPI vaccines among healthy children in China has reached 99%.Citation1 However, the immune status of children with special health status, such as preterm infants, and children with immune-compromising medical conditions or other physiological and pathological conditions, is still unknown.Citation1 These children are deemed as special groups with increased risk of vaccine-preventable diseases. Previous studies have shown that to decide whether to vaccinate children with special health status is a difficult task for vaccination workers.Citation2 According to the 2011–2020 Global Vaccine Action Plan, immunization is recommended to all people because it is one of the most successful and cost-effective public health interventions.Citation1–4 The annual incidence of premature infants is 7% in China according to the National Health and Wellness Committee, which means there are a huge number of children with special health status.Citation2,Citation5 Despite demonstrations of effectiveness and safety, vaccine uptake in these special groups is lower than expected even in developed countries with well-established vaccination strategies in place.Citation4,Citation6,Citation7 Due to ambiguous instructions on vaccination for children with these special health statuses in China and the fear of vaccine safety-related issues, many special health statuses are mistakenly considered as contraindications for vaccination, which may lead to a low vaccination rate in these groups.Citation2,Citation8,Citation9 As a result, vaccination of children with special health status has become one of the most urgent issues in China.
In order to improve the vaccination coverage in children with special status and establish herd immunity, a number of expert consensuses and recommendations have been issued in China in recent 5 years, and they have become practical guidelines for vaccinators. Since then, some vaccinators have gradually begun to vaccinate children with special health status. Nonetheless, many still suspect that special health status is a predisposition to Adverse Events Following Immunization (AEFI).Citation3,Citation10 It is likely that children with special health status do not complete their immunization schedule.Citation10 Consequently, a further national instruction on Chinese immunization procedures has been published in 2021, which points out that certain diseases, such as premature birth, infectious diseases and jaundice, should not be regarded as contraindications or precautions for vaccination.Citation11 However, just as many other countries which do not monitor or report vaccination coverage in groups at risk, the national scale data at individual level are still unavailable in the Chinese national immunization recording system presently.Citation4,Citation12,Citation13 That is to say, little is known about the extent of age-appropriate immunizations and the actual immunization safety in this sub-population. An evidence-based evaluation of vaccines to ease the mental pressure of parents and vaccine providers is imperative.
Chongqing is a municipality located in the southwest part of China with a population of 33 million. In the last ten years, rapid progress has been made to develop a municipal level Electronic Immunization Registries System (EIRS) in Chongqing. Vaccinators are required to input electronic information of children and vaccine into EIRS. Based on the data from the National AEFI Information System (CNAEFIS), death monitoring system (DMS), vaccine compensation insurance purchase system (VCIPS) and EIRS in Chongqing Municipality, it is possible to comprehensively analyze the data of vaccination coverage and safety for children with special health status. Starting from 2016, some vaccination clinics began to try to provide vaccination services for children with special health status, and any untoward medical occurrence which follows immunization will be recorded in CNAEFIS, even if these events does not necessarily have a causal relationship with the usage of the vaccine. Given the importance of vaccination for this vulnerable group, we evaluate the vaccination status and safety among children with special health status and determine the factors affecting the vaccination status from 2016 to 2020 in Chongqing, China.
Methods
Study design and setting
A retrospective study of medical records from 2016 was conducted. For each child, the collected data included the demographic characteristics (e.g., sex, birth weight, gestational age, birth order, and premature or not), the immunization status data, safety data (AEFI), survival status, and vaccine compensation insurance purchases. All data were fully anonymized, and the study was approved by the Ethics Institutional Review Board of Chongqing Municipal Center for Disease Control and Prevention (Project No: CQCDCLS (2021)006).
The data on immunization status were obtained from EIRS in Chongqing. It is a population-based lifespan immunization information system for all residents in Chongqing. Since 2013, all the people immunized in Chongqing were directly recorded in EIRS. The health status of newborns was recorded in EIRS within one month after birth, while the vaccination status was accurately recorded at the time of vaccination. To ensure that all children eligible for vaccination are recorded in the system, all children’s vaccination records must be checked before they are allowed to enroll in kindergartens and primary schools since 2005. Therefore, the record in EIRS can be considered to comprise all people who have been immunized since the establishment of EIRS in Chongqing.
The safety data in this study were acquired from CNAEFIS, a national post-marketing vaccine safety monitoring system based on passive reporting.Citation14 In this system, an AEFI case is defined as a reaction or event following vaccination that is suspected to be related to the vaccine administration. The AEFI surveillance covers all vaccines marketed in mainland China, and more than 90% of vaccination clinics in Chongqing report AEFIs to CNAEFIS. In order to analyze factors that influence vaccination status, the information on vaccine compensation insurance purchases and deaths data were collected from the vaccine compensation insurance purchase system and death monitoring system, respectively. Through the unique national identification number of each child, all the data of the same person were matched. After the data matching, the national identification number of all children were deleted in the database, and hence the personal information could not be identified after data collection.
Study population
Starting from 2016, vaccination clinics in Chongqing municipality began to provide vaccination services to children with special health status. Therefore, the target population in this study consisted of all the children with a special health status in EIRS with the following inclusion criteria: (1) born between 1 January 2016 and 31 December 2020; and (2) the health status recorded in the system indicating that more observation was required after vaccination. All the profiles of eligible cases in EIRS were obtained on 1 October 2021 to ensure that all the children in the study had reached the age for measles vaccination (8 months after birth)- one of the most important indicators of vaccine coverage in China. As a comparison, the vaccination rate of the children without special health status in EIRS was obtained, too.
Data source and variables
According to the Chinese National Immunization Programme schedule, the routine vaccination coverage indicators refer to the percentage of children who have received vaccines recommended in the Expanded Programme on Immunization (EPI), which includes one dose of Bacillus Calmette-G vaccine (BCG) after birth; three doses of hepatitis B vaccine (HepB) at birth, 1 month, and 6 months of age; three doses of polio vaccine (two doses of inactivated poliovirus vaccine and one dose of live oral poliovirus vaccine) at 2 months, 3 months, and 4 months of age; three doses of diphtheria-tetanus-pertussis-containing (DTP) vaccine at 3 months, 4 months, and 5 months of age; one dose of measles-containing vaccine (MCV) at 8 months of age; two dose of Group A meningococcal polysaccharide vaccine (MPSV-A) at 6 months and 9 months of age; and one dose of Japanese encephalitis vaccine (JEV) at 8 months of age.Citation15 If EPI vaccines are replaced by equivalent non-EPI vaccines, children are also considered to be fully vaccinated for those vaccines. For the Children over 12 months of age, if they have received BCG before 4 months and the rest EPI vaccines before 12 months of age, they will be classified as up to date (UTD). Considering that the children with special health status were vulnerable to many other diseases, the completion of non-EPI vaccines is also taken into account according to the vaccine instructions, which includes the following immunizations before 12 months of age: 3 doses of Haemophilus influenza type-b vaccine (Hib), 3 doses of rotavirus vaccine (ROV), 1 dose of varicella vaccine (VarV), 2 doses of enterovirus 71 (EV71), at least 1 dose of influenza vaccine (InfV), and 3 doses of pneumococcal conjugate vaccine (PCV13-valent).
Statistical analysis
Statistical analysis was performed using the statistical software SPSS 26.0 (International Business Machines Corporation, Armonk, USA). Immunization status and safety data were presented as numbers and percentages in children with/without special health status first. Then, depending on the traditional classification of vaccination contraindication, children were classified into 7 groups with different special health statuses: history of infectious diseases (HID), jaundice, congenital heart disease (CHD), premature birth, surgical diseases (SD), immunological disease (ID) and chronical disease (CD). Among them, the HID, jaundice, CHD and premature birth used to be considered contraindications for a long time, which caused a high incidence of measles in these groups in a child hospital of Chongqing in 2014, and hence they are analyzed separately in our study; SD refers to children with congenital malformations, intestinal obstruction and other surgical diseases; ID refers to children who have autoimmune diseases, asthma, allergic diseases, or are in immunosuppression state due to medication; and CD refers to children with chronic diseases that require long-term medication but are not accompanied by abnormal immune functions.Citation16
The vaccine coverage rates among groups of different health statuses were also compared. Demographics and vaccination coverage rate for each vaccine were stratified according to health status groups. Proportion of the groups was compared by Chi-square tests. Univariate and multivariate logistic regression were used to compare the proportional differences in factors associated with vaccination coverage. Significance tests were two-tailed, and P values <0.05 were considered statistically significant.
Results
Characteristics of the population
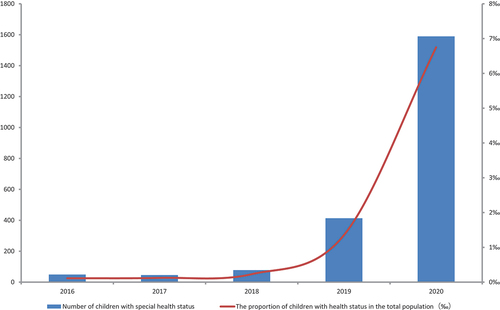
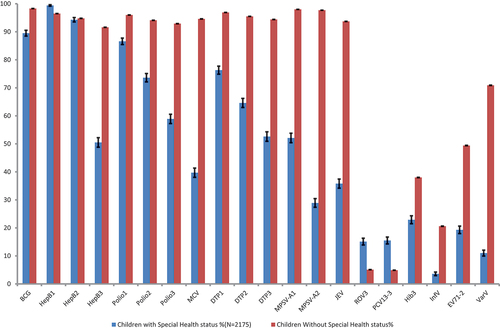
From 1 January 2016 to 31 December 2020, 2,175 children with special health status and 1,694,875 children without special health status were recorded and verified in EIRS. The number and proportion of children with special health status recorded in EIRS are increasing year by year (). The vaccination coverage rates for each vaccine for children with and without special health status are shown in . Considering that the target population of this study is only a sample of the national population, the 95% confidence intervals of vaccination coverage rates were also calculated. Except for the 1st and 2nd dose of hepatitis B vaccine, the coverage rate of all vaccines among children with special health status are lower.
Figure 2. Vaccination coverage rates for the individual vaccines in children with and without special health status.
2,175 children with special health status recorded in EIRS represent people who meet the criteria for vaccination according to the immunization guidelines and are willing to be vaccinated at least once, and a total 14,068 doses of vaccines have been administered. Children with different special health statuses do show hesitancy toward certain vaccines. Therefore, the vaccination rates between different subgroups in this cohort were compared to identify the key factors for vaccine hesitancy. The distribution of the special health status among them is shown in , and the characteristics of these children are reported in . There were 245 children with a history of infectious diseases (11.3%), 633 with jaundice or jaundice history (29.1%), 81 with a history of surgery (3.7%), 91 with immunological disease (4.2%), 777 with congenital heart disease (35.7%), 125 with premature birth (5.7%) and 223 with chronical disease (10.3%). 54.2% of the children were male, 75.2% had a normal birth weight, 66.6% were the first born of their mother, and 61.9% did not purchase the vaccine compensation insurance. Only 3 (0.1%) of them reported a history of AEFI, corresponding to a rate of 21.3 per 100,000 doses, all of which were common reactions. As the disease progresses, 0.1% of the children died. The vaccine compensation insurance was the only factor that showed statistically significant difference in groups of different special health status.
Figure 3. The group distribution of the children’s special health status.
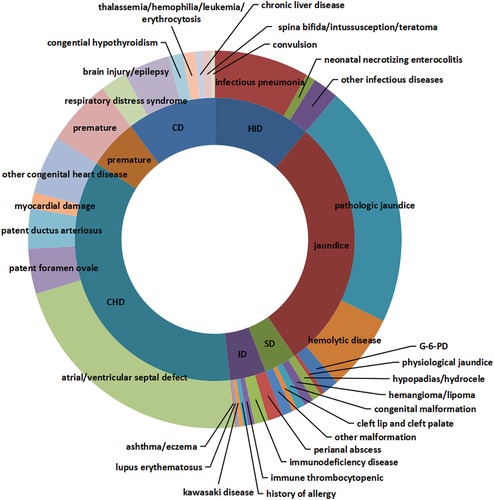
Table 1. The characteristics of the study population (n = 2,175).
Vaccination status
Vaccination coverage
shows the coverage rate of each vaccine in children with different special health statuses. In the seven different subgroups, HepB3 coverage was lower in the HID, jaundice, and premature groups, polio 1,2,3 coverage were all lower in the ID group, and MCV coverage was lower in the jaundice, premature, and CD groups. The coverage of DTP1,2,3, MPSV-A1,2 and JEV were all lower in the jaundice group. This study also calculated the completion of basic immunization of non-EPI vaccine. Compared with other subgroups, CHD group had the highest vaccination rate of all vaccines except for BCG and HepB, and children with jaundice or a history of jaundice were less likely to be vaccinated with HepB3, MCV, DTP1,2,3, MPSV-A, JEV, and all of the non-EPI vaccines.
Table 2. Vaccination coverage of vaccines in the study population (n = 2,175).
Vaccination up-to-date status
There were 1824 children older than 12 months. As shown in , compared with the HID group, these children with a history of jaundice were significantly (P<0.05) less likely to be UTD for all the EPI vaccines except for BCG. Compared with the HID group, the BCG UTD rate was lower in the SD (OR = 0.501, 95% CI: 0.281–0.894), CHD(OR = 0.553, 95% CI: 0.382–0.801)) and CD (OR = 0.624, 95% CI: 0.398–0.980) groups, the HepB UTD rate was lower in the jaundice group (OR = 0.482, 95% CI: 0.345–0.675), the Polio vaccine UTD rate was lower in the in the jaundice group (OR = 0.591, 95% CI: 0.415–0.841), the MCV UTD rate was lower in the jaundice (OR = 0.539, 95% CI: 0.385–0.756) and CD groups (OR = 0.634, 95% CI: 0.424–0.949), the DTP UTD rate was lower in the in the jaundice group (OR = 0.590, 95% CI: 0.422–0.825), and the JEV UTD rate was lower in the premature birth (OR = 0.474, 95% CI: 0.336–0.668) and CD groups (OR = 0.501, 95% CI: 0.331–0.759). Overall, the jaundice (OR = 0.533, 95% CI: 0.372–0.765), the SD (OR = 0.533, 95% CI: 0.288–0.983), and the CD groups (OR = 0.541, 95% CI: 0.348–0.842) were less likely to be up to date.
Table 3. Vaccination up-to-date status in the study population (n = 1,824).
Influencing factors of vaccination
Several variables were correlated with the coverage of each vaccine and the demographic and health status in the univariate analysis. All variables with P < .20 in the univariate analysis were input into an initial logistic multivariable regression model followed by a backward selection procedure that retained variables with P < .05 to create a final multivariable model. In the multivariate analysis, four factors – birth weight, parity, vaccine compensation insurance, and health status – remained independently associated with vaccination status after controlling covariates (). Most of the children with jaundice missed vaccination (aOR = 0.441 ‒0.556), while most of children with CHD were immunized (aOR = 1.508 ‒6.331). The SD was associated with a higher completion rate of basic immunization of PCV13 (aOR = 2.669, 95% CI: 1.431–4.980) and InfV (aOR = 16.279, 95% CI: 1.872–141.581). Premature birth was associated with a lower completion rate of basic immunization of EV71 (aOR = 0.378, 95% CI: 0.184–0.777) and JEV (aOR = 0.551, 95% CI: 0.342–0.899). The status of chronic diseases was associated with a lower completion rate of basic immunization of JEV (aOR = 0.623, 95% CI: 0.422–0.0.921) and a higher completion rate of basic immunization of Hib (aOR = 1.772, 95% CI: 1.146–2.738).
Table 4. Adjusted odds (CI) of vaccination factors among study populationa.
Multivariate analysis showed that parity was associated with BCG vaccination status (aOR = 0.433, 95% CI: 0.243‒0.771). The higher birth weight was associated with a lower completion rate of basic immunization of PCV13 (aOR = 0.635, 95% CI: 0.473‒0.853) and ROV (aOR = 0.697, 95% CI: 0.529‒0.919). The purchase of vaccine compensation insurance was associated with a higher completion rate of basic immunization of BCG (aOR = 1.706, 95% CI: 1.249‒2.329) and ROV (aOR = 1.346, 95% CI: 1.061‒1.708).
Discussion
The correlation between chronic illness and immunization status in China is inconclusive. Most earlier studies are limited to specific subpopulations or vaccines.Citation17 This study characterizes the general immunization status of children with special health status in Chongqing, China, based on data at individual level. At the present moment, there is no such data available at national scale, and our study on the municipal scale represents the most comprehensive study of such kind in China, which provides an evidence-based assurance to ease the concerns on vaccination risks.
Children with special health status comprise a large population in China. For instance, the cumulative prevalence of asthma among children under 14 years old in cities was 3.02% nationwide in 2010, and the majority of them can be safely vaccinated according to the state-recommended schedule.Citation11,Citation17 However, although twenty-three percent of parents reported that their adolescent had asthma,Citation5,Citation18 but only 2 children with asthma were recorded in our study. That is to say, only a small portion of children with special health status were vaccinated and recorded in EIRS accordingly. As a result, although the vaccination rate among children with special health status is increasing year by year as shown in , it is still not high from enough to protect these vulnerable groups through the immunization. Because it is difficult for the vaccinator to reach this population, how to benefit more children with special health status with vaccines has become a problem that must be considered in next step.
Because an individual would be registered as vaccinated as long as one vaccine dose was administered, the actual vaccination status among children with special health status is even more pessimistic. Different from the study in USA which indicates that children with special needs have a higher vaccination coverage than children without, our study shows a significantly lower level of vaccination coverage among the children with special health status than that among the general population in Chongqing, China.Citation6 A low vaccination coverage may increase the risk of morbidity and mortality from vaccine-preventable diseases among those children.Citation19–21 Fortunately, having benefited from a large number of expert consensuses and recommendations in recent 5 years, the incidence of AEFI is very rare; the reported rate of AEFI among children with special health status is only 21.3 per 100,000 doses which is lower than that among the general population (46.5 per 100,000).Citation22 Considering that many vaccinators cannot fully comprehend the expert consensuses issued previously, we advocate a well-organized multi-intervention vaccination campaign for children with special health status and their parents, in which closer monitoring of vaccination status and optimizing practice recommendations for the immunization are two key components. We believe that this campaign will be effective, as several other studies have shown a significant increase in vaccine uptake as a result of similar approaches.Citation23,Citation24
In accordance with the study, it is observed that children with CHD are better vaccinated against major vaccine-preventable diseases (VPDs), which is probably because most researches in China are devoted to promoting vaccination in this subpopulation. However, the findings suggest that the jaundice group is at higher risk of VPDs because of the low vaccination rate. This group includes four kinds of diseases: pathologic jaundice, G-6-PD, physiological jaundice and hemolytic disease of newborn. Children with a history of jaundice are less likely to be vaccinated with HepB3, MCV, DTP1,2,3, MPSV-A, JEV and all of the non-EPI vaccine, and less likely to be UTD for all the EPI vaccines except for BCG. According to the multivariate analysis, most of the jaundice children have missed vaccination (aOR = 0.441 ‒0.556). According to the expert consensus, children with physiological jaundice and G-6-PD can actually be vaccinated safely, and only the children with pathological jaundice need to be further evaluated for vaccination by identifying the cause of the condition.Citation25 The main causes of pathological jaundice are infection, breastfeeding, hemolysis, and metabolism, and most of them are usually not contraindications for vaccination. This study also shows that the ID group has an immunization rate equal to the HID group, which indicates that the ID children can be vaccinated safely.
Emphasis should be put on the administration of live attenuated vaccines (BCG and MCV). The UTD rate of the BCG vaccine is lower in the SD, CHD, and CD groups. This is partly due to the early contraction of the disease, which delays BCG vaccination. However, such an unnecessary delay exposes these children to risk of tuberculosis for a long time. The MCV coverage rate is only 39.7%, particularly low in the jaundice group, but the CHD group is more likely to be immunized. For the non-EPI vaccines, the coverage rate of PCV13 and ROV are significantly higher than that among the general population, but Hib, InfV, EV71 and VarV are much lower as shown in . Same as the pneumococcal vaccination, the InfV is also strongly recommended by the advisory Committee on Immunization practices (ACIP) in the United States, the Belgian National Immunization Technical Advisory Groups (NITAG), and other public health authorities.Citation26–28
In addition to the health status, we have also analyzed the factors associated with insufficient vaccination coverage and found that the birth weight, parity and vaccine compensation insurance are significant determinants of immunization of certain vaccines. A higher birth weight is associated with a lower completion rate of basic immunization of PCV13 (aOR = 0.635, 95% CI: 0.473‒0.853) and ROV (aOR = 0.697, 95% CI: 0.529‒0.919). This can be explained by the fact that the early treatment of these diseases has caused delaying or skipping of these vaccines. In accordance with a prior study[18], we have observed that the purchase of vaccine compensation insurance is associated with a higher completion rate of basic immunization of BCG (aOR = 1.706, 95% CI: 1.249‒2.329) and ROV (aOR = 1.346, 95% CI: 1.061‒1.708). Owning a vaccine compensation insurance promotes the use of live attenuated vaccines in early life, which should be actively recommended for these children.Citation29
The strength of this study is that it assesses the vaccination coverage of recommended vaccines in a large and diverse group of children at risk. Vaccination data at individual level in at-risk groups are scarce and often not monitored.Citation4,Citation13,Citation30 Available studies are often limited to a particular vaccine in a certain risk group. Second, the data are collected from the official information system of Chongqing, China, which ensures that all the vaccination data are recorded, and guaranteed the accuracy and authenticity of the data. The inclusion of the non-EPI vaccine, the data of the vaccine compensation insurance, and the data on the occurrence of AEFI and death also make the analysis more comprehensive.
However, there are some limitations to this study. First, we did not consider the factors associated with vaccinators. Vaccinators may cause the children with special health status to miss vaccination, possibly because the vaccinators are unaware of vaccination recommendations for this population and hence are overly concerned about potential risks associated with vaccines.Citation31–33 Reasons for under-vaccination of special groups include the fear of adverse outcomes or illness caused by vaccines, the inconvenience (and in some scenarios, the cost) of vaccination, and the unawareness of the need for vaccination or national recommendations. The results in this paper are a comprehensive reflection of these reasons, but more surveys need to be done next to identify which one of them is more influential.
Conclusions
Our study provides an early insight into the actual vaccination status among children with special health status in China, and the result shows that this sub-population can be safely vaccinated. However, the vaccination coverage in these huge and vulnerable group is too low to protect them from vaccine-preventable diseases through immunization. It is discovered that children with jaundice are significantly less likely to be vaccinated than children with congenital heart disease. Interventions to address this issue should focus on the purchase of vaccine compensation insurance, which is found to promote the use of live attenuated vaccines in early life. More attentions should be paid to the neglected immunization problem among the children with special health status. We also strongly encourage further research on strategies to improve vaccination for these children in order to improve the uptake of vaccine.
Author contributions
Conceptualization, Rongsheng Luan and Qing Wang; study design, Binyue Xu and Qing Wang; data analysis, Binyue Xu, Yi Zhang, Chao Zhou; data collection, Binyue Xu; data curation, Binyue Xu; writing – original draft preparation, Binyue Xu; writing – review and editing, Yi Zhang, and Qing Wang; visualization, Binyue Xu; supervision, Binyue Xu; project administration, Binyue Xu; funding acquisition, Binyue Xu. All authors involved in the discussion of results and have read and agreed to the published version of the manuscript.
Availability of data and materials
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to participant’s privacy concerns.
Ethics approval and consent to participate
This is a retrospective study of medical records with all data were fully anonymized. And the study is certified by the Ethics Institutional Review Board of Chongqing Municipal Center for Disease Control and Prevention (Project No: CQCDCLS (2021)006).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Immunization coverage. 2021 [accessed 2021 July 15]. https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage.
- Guo X, Qiu J, Sun X. Application of expert consensus to guide the vaccination of children with special health status. Chin J Prev Med. 2021;55:284–10. doi:10.3760/cma.j.cn112150-20201013-01275.
- Gu JY, Wang NR, Zhang LJ, Shen L. Study on influencing factors of planned immunization in children with special health status in Chongqing. J Mod Med Health. 2021:1989–93. doi:10.3969/j.issn.1009-5519.2021.12.005.
- Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: protecting the vulnerable. Vaccine. 2016;34(52):6681–90. doi:10.1016/j.vaccine.2016.11.015. PMID: 27876197.
- Ji C. The expert consensus on vaccination for children with special health status: preterm infants and vaccination. Chin J Pract Pediatr. 2018;33:737–38. doi:10.19538/j.ek2018100601.
- O’Connor KS, Bramlett MD. Vaccination coverage by special health care needs status in young children. Pediatr. 2008;121(4):e768–774. doi:10.1542/peds.2007-0305. PMID: 18381504.
- Gold M, Goodwin H, Botham S, Burgess M, Nash M, Kempe A. Re-vaccination of 421 children with a past history of an adverse vaccine reaction in a special immunisation service. Arch Dis Child. 2000;83(2):128–31. doi:10.1136/adc.83.2.128. PMID: 10906018.
- Li F, Hang TW, Liu DW, Zijian F. Survey on the knowledge of vaccination contraindication among expanded program of immunization staff in Zhengzhou municipal [J]. Chin J Vaccines Immun. 2009;15(6):498–500. PMID: 20518323.
- Succi RC, Farhat CK. Vaccination in special situations. J Pediatr (Rio J). 2006;82(3 Suppl):S91–100. doi:10.2223/JPED.1474. PMID: 16683052.
- Shen R, Wang AL, Pan XP, Qiao YP, Wang Q, Wang XY, Qu SL, ZHANG T. Levels of vaccination coverage among HIVexposed children in China: a retrospective study. Infect Dis Poverty. 2021;10(1):18. doi:10.1186/s40249-021-00797-5. PMID: 33648599.
- National Health Commission of the People′s Republic of China. Immunization schedules and instructions for vaccines of the national immunization program (2021 version). Chin J Viral Dis. 2021;11(4):241–45. doi:10.16505/j.2095-0136.2021.0021.
- Wu WD, Cao LS, Zheng JS, Cao L, Cui J, Xiao QY. Immunization information system status in China, 2017. Vaccine. 2019;37(43):6268–70. doi:10.1016/j.vaccine.2019.08.070. PMID: 31526621.
- Kanitz EE, Wu LA, Giambi C, Strikas RA, Levy-Bruhl D, Stefanoff P, Mereckiene J, Appelgren E, D’Ancona F. VENICE (vaccine European new integrated collaboration effort) national gatekeepers, contact points. Variation in adult vaccination policies across Europe: an overview from VENICE network on vaccine recommendations, funding and coverage. Vaccine. 2012;30(35):5222–28. doi:10.1016/j.vaccine.2012.06.012. PMID: 22721901.
- Liu DW, Wu WD, Li KL, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015 Jul 31;33(32):4041–46. doi:10.1016/j.vaccine.2015.04.060. Epub 2015 Apr 28. PMID: 25936727.
- National Health and Family Planning Commission of the People′s Republic of China. Immunization schedules and instructions for vaccines of the national immunization program (2016 version). Chin J Viral Dis. 2016;7(2):81–86. doi:10.16505/j.2095-0136.2017.02.001.
- Jiang L. Epidemiological analysis on hospitalized child patients with measles in a child hospital in Chongqing in 2014[J]. Nursing Pract Res. 2017;14:79–81.
- Xu Y, Ji C, Liu Y, Li M, Yao D, Wang X, Du J, Chen J. Vaccination recommendations, immunization status and safety of vaccination for premature infants in Zhejiang, China. Expert Rev Vaccines. 2020;19(10):973–81. doi:10.1080/14760584.2020.1831917. PMID: 33001703.
- Seib K, Underwood NL, Gargano LM, Sales JM, Morfaw C, Weiss P, Murray D, Vogt TM, DiClemente RJ, Hughes JM. Preexisting chronic health conditions and health insurance status associated with vaccine receipt among adolescents. J Adolesc Health. 2016;58(2):148–53. doi:10.1016/j.jadohealth.2015.10.009. PMID: 26683985.
- Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, Mutasa K, Ruff A, Ward B, ZVITAMBO Study Group. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30(1):45–51. doi:10.1097/INF.0b013e3181ecbf7e. PMID: 21173675.
- Slogrove AL, Goetghebuer T, Cotton MF, Singer J, Bettinger JA. Pattern of infectious morbidity in HIV-exposed uninfected infants and children. Front Immunol. 2016;7:164. doi:10.3389/fimmu.2016.00164. PMID: 27199989.
- Thorne C, Idele P, Chamla D, Romano S, Luo C, Newell ML. Morbidity and mortality in HIV-exposed uninfected children. Future Virol. 2015;10(9):1077–100. doi:10.2217/fvl.15.70.
- Zhang LN, Li KL, Wen D, Yan L, Fan CX, Yu WZ, Cao L, Cao LS, Yin ZD. Surveillance of adverse events following immunization in China,2019. Chin J Vaccines Immun. 2021;27(04):438–45. doi:10.19914/j.CJVI.2021075.
- Li A, Chan YH, Liew MF, Pandey R, Phua J. Improving influenza vaccination coverage among patients with COPD: a pilot project. Int J Chron Obstruct Pulmon Dis. 2019;14:2527–33. doi:10.2147/COPD.S222524. PMID: 31814718.
- Mazzoni SE, Brewer SE, Pyrzanowski JL, Durfee MJ, Dickinson LM, Barnard JG, Dempsey AF, O’Leary ST. Effect of a multi-modal intervention on immunization rates in obstetrics and gynecology clinics. Am J Obstet Gynecol. 2016 May;214(5):617. doi:10.1016/j.ajog.2015.11.018. Epub 2015 Nov 25. PMID: 26627727.
- Kong XX. Expert consensus on immunization in children with special health state (Ⅺ): infantile jaundice and immunization. Chin J Pract Pediatr. 2019;34(2):87–88. doi:10.19538/j.ek2019020605.
- Belgian National Immunization Technical Advisory Groups. Vaccination. Brussels (Belgium): Federal public service Public Health Belgium; 2021 [accessed 2021 April 25]. https://www.health.belgium.be/en/search?keyword-search=vaccination.
- Chinese Center for Disease Control and Prevention. Technical guide for influenza vaccination in China. 2021 [accessed 2021 September 16]. https://www.chinacdc.cn/yyrdgz/202109/t20210916_244639.html.
- Welsh Government. The national influenza immunisation programme 2021 to 2022 (WHC/2021/004). 2021 [accessed 2021 February 19]. https://gov.wales/national-influenza-immunisation-programme-2021-2022-whc2021004-html.
- Blewett LA, Davidson G, Bramlett MD, Rodin H, Messonnier ML. The impact of gaps in health insurance coverage on immunization status for young children. Health Serv Res. 2008;43(5 Pt 1):1619–36. doi:10.1111/j.1475-6773.2008.00864.x. PMID: 18522671.
- Boey L, Bosmans E, Ferreira LB, Heyvaert N, Nelen M, Smans L, Tuerlinckx H, Roelants M, Claes K, Derdelinckx I, et al. Vaccination coverage of recommended vaccines and determinants of vaccination in at-risk groups. Hum Vaccin Immunother. 2020;16(9):2136–43. doi:10.1080/21645515.2020.1763739. PMID: 32614656.
- Succi RCM, Krauss MR, Harris DR, Machado DM, de Moraes-Pinto MI, Mussi-Pinhata MM, Pavia Ruz N, Pierre RB, Kolevic Roca LA, Joao E, et al. Immunity after childhood vaccinations in perinatally HIV-exposed children with and without HIV infection in Latin America. Pediatr Infect Dis J. 2018;37(4):304–09. doi:10.1097/INF.0000000000001831. PMID: 29140938.
- Antle BJ, Wells LM, Goldie RS, DeMatteo D, King SM. Challenges of parenting for families living with HIV/AIDS. Soc Work. 2001 Apr;46(2):159–69. doi:10.1093/sw/46.2.159. PMID: 11329645.
- Sensarma P, Bhandari S, Kutty VR. Barriers to immunization among children of HIV-infected mothers in Kolkata, India: a qualitative study. Asia Pac J Publ Health. 2015 Mar;27(2):NP1362–71. doi:10.1177/1010539513486177. PMID: 23666833.