ABSTRACT
Hexavalent (HV) vaccination is a priority for newborn protection and in Italy is included in the National Immunization Plan with a three doses cycle at 61, 121 and 301 days of age. A retrospective clinical study has been conducted to evaluate real life clinical practice of HV vaccination in the fourth most populous Italian Region. Data on the completion of the HV cycle, on the interchangeability between the two HV adopted in 2016–2017 (DTaP3-IPV-HB/Hib) and 2018–2019 (DTaP5-IPV-HB-Hib) and on the use above the established age, were collected in five Sicilian Local Health Authorities. Data showed an average 91.5% completion of the vaccination cycle at 24 months of age. The average age of administration was significantly higher in children who switched between the two hexavalent vaccines compared to those who completed the vaccination cycle with the same product (p-value <.01). Interchangeability with one or two doses of HV was also documented in 17.8% (2018) and 16% (2019) of vaccinated infants. Co-administration with other vaccines included in the Sicilian Vaccination Schedule was 85% with anti-pneumococcal vaccination and 65% with anti-rotavirus vaccination. Children vaccinated above recommended age (from 15 to >36 months) significantly after the introduction of mandatory vaccination in Italy (p-value <.001). This retrospective analysis will contribute to manage potential disruptions due to missed routine immunization opportunities, as the pandemic has caused, with strategies such as catch up above recommended age as well as interchangeability. Data could also help to demonstrate the need to optimize vaccine sessions through co-administration, that strongly contribute to increase vaccination coverage rates and respect of timing of vaccination schedules.
Introduction
The Hexavalent (HV) vaccination is a priority for prevention among newborns.Citation1 HV vaccines avoids six potentially deadly infectious diseases including diphtheria, tetanus, pertussis, Haemophilus influenzae type b, poliomyelitis, and hepatitis B. Currently in Italy, HV is a mandatory vaccination for school attendance together with Measles, Mumps, Rubella and Varicella (MMRV) vaccinations, and three hexavalent vaccines are licensed.Citation2
HV vaccination is included in the 2017–2019 Italian National Immunization Plan (NIP) for the primary immunization dose and for two booster doses during neonatal age (according to the schedule 2, 4 and 11–13 months or 61, 121 and 301 days of age), respectively).Citation3 The HV vaccination is completed usually within 1 year of age together with other pediatric vaccinations including rotavirus, pneumococcus conjugate vaccine and Men B.Citation4
The HV, being a combined vaccine, provides with several advantages. First for children health protection (reduced number of injections and full protection), parents’ convenience (one child visit instead of multiple visits); second for healthcare systems (reaching adequate/optimal vaccinations coverage rates, infectious disease control, healthcare system sustainability itself) and healthcare professionals (safety-related aspects reducing accidental needle stick and better management at the vaccination centers).Citation5–7
Three different hexavalent vaccines are authorized in Italy.Citation8–10 Generally, the same HV vaccine is used to complete the primary cycle.Citation11 However, given the health priority to protect the newborn, if a specific vaccine is not available at the time vaccination dose is due, or if a previous vaccine administered is not known, the international guidelines, supported by the Italian Ministry of Health, acknowledge that a different HV vaccine can be administered to ensure infant vaccination timing and protection is completed as needed.Citation12,Citation13
In Italy HV, as any other vaccine available in the country, is accessible to the Regions, according to a three-year public tender.
Consequently, over time, the HV in use can change and therefore there can be a transition phases between two HV vaccine available leading to a possible HV mixed schedule completion. Real world evidence is relevant on this topic and, as today, literature on HV vaccine mixed schedule is scarce.
For this reason, in Sicily and other Italian Regions, different HV vaccines could be used during the HV vaccine transition phase so to complete the HV schedule once vaccination started with a different HV vaccine. This strategy should avoid any vaccination disruptions (missed or delayed HV vaccinations) potentially affecting overall infants’ healthcare protection.Citation14
In addition to the clinical and public health advantages of combined vaccines, vaccine co-administration is also another critical aspect to complete pediatric vaccinations timely, as needed. According to their respective Summary of Product (SumPC) the three HV vaccines can be co-administered with other pediatric vaccines including rotavirus vaccines (RV1 or RV5), pneumococcal conjugate vaccines (PCV7, PCV10 or PCV13), meningococcal vaccines (Men B, Men C or Men ACWY) or MMRV (1st dose).Citation15–23
Multiple antigens may be administered at the same site using a combined vaccine during the same medical visit through vaccines co-administration practice at different injection sites. Both strategies minimize the required number of vaccine visits to achieve full coverage, improve timeliness of vaccination and allow for a programmatic fit across different vaccine regimens used in the respective country. These features are crucial to increase protection of infants, especially in the first months of life when they are most vulnerable to vaccine-preventable diseases.Citation24
In Sicily, since the beginning of the pediatric conjugate vaccination against pneumococcus in 2004, co-administration of HV and pneumococcal vaccines is largely practiced, as it occurs in many other Regions in Italy and as also recommended by the NIP.Citation3
In addition to clinical data, real life experiences on HV administration practice in different LHAs are critical to further inform national and regional clinical practices contributing to implement/optimize current and future pediatric immunization programs. Data on children receiving at least one dose of HV above 15 months of age were collected in order to understand which is the real burden of delayed HV vaccination and why this delay occurs.
The main objective of this study was to investigate these aspects in the fourth most populous Italian region, Sicily, so to provide with a broader understanding of real word data on HV use in clinical practice.
A retrospective clinical study was conducted in Sicily to investigate HV vaccination schedule completion and timing compliance over a 4-years study period from 2016 to 2019 (birth cohort included in the study from 2014 to 2017) so to provide real world data on HV vaccination clinical practice.
This data and clinical practice mapping will also show any difference in schedule completion and VCR between pre- and post-change of the type of HV vaccination offered to Sicilian Population.
Moreover, this retrospective analysis will contribute to manage potential disruptions (such as catch up as well as interchangeability) due to missed routine vaccination opportunities also pandemic-related.
Methods
A structured questionnaire designed at the Health Promotion Department of Palermo University Hospital and approved by Palermo University Hospital’s Ethical Committee (referent EC for the Palermo’s Province) was initially sent out to the nine Sicilian Local Health Authorities (LHAs) (georeferenced in ): Agrigento (AG), Caltanissetta (CL), Catania (CT), Enna (EN), Messina (ME), Palermo (PA), Ragusa (RG), Siracusa (SR) and Trapani (TP).
Figure 1. Georeferencing of the nine Sicilian Local Health Authorities with the number of newborns in 2017 (in dark gray the five LHAs participating to the project, in light grey the four not participating).
Sicily is the fourth most populated Italian region with 5 million inhabitants (meaning 10% of the national population).
Specifically, from 2014 to 2017 (corresponding to the four birth cohorts considered in the study) an average of 43,083 newborns were born in Sicily, accounting for 9% of total newborns registered in Italy every year.Citation25 Newborn population from the 5 LHAs account for about 70% of the total newborn in Sicily from 2016 to 2019 (corresponding to birth cohort 2014–2017). Details of newborns by province (LHA), also regarding the preterm population collected by the Sicilian Health Department, from 2014 to 2017 are reported in .Citation26 Clinical practices regarding premature infants’ prevention management and co-administration with other pediatric vaccines as indicated in the National Immunization plan were also included in the data collection.
Table 1. HV vaccination schedules (standard and mixed) in 5 LHAs (all combined) in Sicily in 2018–2019 when a new HV vaccine became available.
Data received from each participant LHA (AG, PA, TP, RG and CT) were collected and analyzed at the Hygiene Division of the Department of Health Promotion, Maternal and Infant Care, Internal Medicine and Excellence Specialties of the University of Palermo.
Three different hexavalent vaccines are authorized in Italy providing with options for the National Public Health System so to meet different clinical needs and supply to any HV vaccine potential stock out. Despite, having similar safety, immunogenicity and efficacy profile there are some differences between the HV vaccines available.
These differences mainly include formulation (fully liquid or not), type and number of pertussis antigens, Hib protection timing, other antigens amount content. These three hexavalent vaccines have the same indication of use, including immunization against the six diseases and age of utilization.
The main difference among the hexavalent vaccines is in regards to the preparation that is required for their administration: both Hexyon ® and Vaxelis ® are ready-to-use (RTU) in a pre-filled syringe, whereas for Infanrix Hexa® there is a need-for-reconstitution (NFR) of the Hib antigen with a syringe containing the five other components.Citation8–10
In Sicily, in 2018, a new HV vaccine was introduced (Vaxelis ® - DTaP5-IPV-HB-Hib) following the regional awarder tender, replacing the previous HV (Infanrix Hexa ® - DTaP3-IPV-HB/Hib) which was in use in Sicily over the previous 6 years.
At the end of the data collection period, five LHAs (AG, CT, PA, RG and TP) could collect data on HV vaccination clinical practice during the timeframe 2016–2019.
Data on vaccination adherence in Sicily are available from informatics registry of single LHAs and, as requested by the Italian Ministry of Health, are available from 24 months of age.
For this reason, data collected from the five Sicilian LHAs participating to the study are attributable to the birth cohort from 2014 to 2017.
Preliminary data on study methodology and a data preview were presented at the European Society for Pediatric Infectious Diseases (ESPID) conference 2020 and at the Italian National (held in Lecce in 2021) and Sicilian Regional (held in Lipari in 2022) Public Health congresses.
Data on newborns were obtained from Regional Registries. Vaccination coverage rates (VCR) were obtained from the Local Vaccination registries of the five LHAs, and calculated at 24 months of age.
Currently a Regional Vaccination registry is not available in Sicily, and normally all data from single LHAs were merged at the Regional Epidemiological Observatory of the Sicilian Health Department. Finally, all collected data were send to the Italian Ministry of Health in order to calculate Italian National Vaccination Coverage.
In the present study, quantitative variables were normally distributed and summarized as means with their standard deviations (±SD), while absolute and relative frequencies were calculated for qualitative variables. Chi square for trend was also calculated for the number of children vaccinated above recommended age (from 15 to >36 months) during the study period (2016–2019). The student’s t-test was applied in order to determine if there was a statistically significant difference between the means of age of administration of first, second and third dose of HV vaccines in children that received standard or mixed schedules.
All data were entered into an electronic database created by Excel
16.0 software. Descriptive statistics were performed using EpiInfo ver. 3.5.1 software.
Questionnaire structure and administration
The questionnaires allowed to collect data on each birth cohort from 2014 to 2017. Children age (in months), newborn at term or preterm, number of HV doses administered and completed vaccination schedule. The total number of infants vaccinated with a full series of HV, the average age of infants at dose 1, 2 and 3, and the number of infants vaccinated with only one or two doses of the same HV vaccine (mixed HV schedule) were investigated in response to vaccines availability in Sicily.
For example, the questionnaire referring to year 2018 provided information regarding mixed HV vaccination schedule (i.e. DTaP3-IPV-HB/Hib – DTaP3-IPV-HB/Hib – DTaP5-IPV-HB-Hib or DTaP3-IPV-HB/Hib DTaP5-IPV-HB-Hib – DTaP5-IPV-HB-Hib for the 2016 birth cohort).
HV vaccination started or completed above the age range recommended by the corresponding SumPC was also recorded. Data on co-administered vaccines (anti-pneumococcal conjugate vaccination – PCV and anti-rotavirus vaccination – RV) were also investigated. Specifically, during the study period in Sicily only the PCV13 and the RV1 vaccinations were available in the Vaccination Schedule and co-administration of both vaccines with the HV vaccine at 2 and 4 months of age (and for the PCV13 also with the third dose at 11–13 months of age) as recommended by the Italian NIP.Citation3
In addition, regarding maternal immunization specifically against diphtheria tetanus pertussis (dTpa), the number of pregnant women receiving dTpa booster was also investigated over the study period across the 5 LHAs.
Safety profile information were analyzed through the national pharmacovigilance report released on year basis or in regional database when available.Citation26
All data, once the questionnaires were completed, were automatically recorded on an Excel file (ver. 1997–2003) protected by password and accessible only by the research group, in order to further ensure privacy.
The study was conducted in accordance with the Declaration of Helsinki, questionnaire and informed consent approved by the Palermo Ethical Committee 1 of the University Hospital of Palermo in the session no.7 of July 2020.
Results
Data population overview
Out of 9 Sicilian LHAs initially contacted for this study, only 5 could participate due the possibility to access and recall data required by the questionnaire.
Newborn population from the 5 LHAs account for about 72.5% of birth cohort in Sicily from 2014 to 2017 ranging from 74.9% in 2014, 72% in 2015, 73% in 2016 and 71% in 2017. Population details by provinces/LHAs also regarding the preterm population from the study period (birth cohort 2014–2017) are reported in the . It was not possible to recall preterm population from each LHA, so preterm population was included with the general population analyzed by year.
A total of 123,313 infants, including 9,768 (5.6%) premature newborns, were analyzed during the study period, referring to the birth cohorts from 2014 to 2017 in Sicily.
VCR evolution 2016–2019
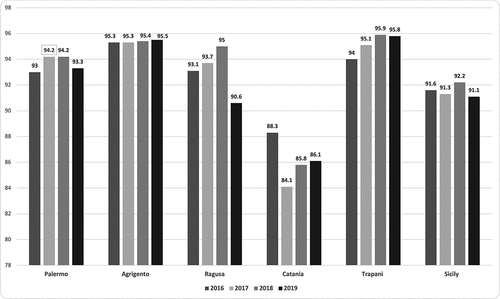
Overall in Sicily VCR for HV (three doses schedule completed) by year was as follow. In 2016 it was 91.6% (40,242), in 2017, VCR was 91.3% (39,441), in 2018 92% (38,188), in 2019 was 91.1% (37,438). Moreover, as reported in , lower VCRs were observed in the LHA of Catania over all the four years of observation.
Full details by year and province (LHAs) are detailed in . In all LHAs but CT, VCRs were maintained above 90% (ranging between 95.9% and 90.6%) during the four-year study period.
In the Catania LHA, HV VCR was lower compared to the other provinces decreasing from 88.3% in 2016 (as the highest VCR over the 4-year study period) to 84.1% in 2017. AG was the only province to maintain HV coverage rates above 95% for the entire study period (2016–2019) ().
Schedule completion and mixed schedules
In 2016 the average age of the infants at dose 1, 2 and 3 of DTaP3-IPV-HB/Hib was 79–155 and 374 days of age, respectively (data not shown in table). In 2017 the average age for the 1st, 2nd and 3rd doses of DTaP3-IPV-HB/Hib was respectively 79–144 and 340 days of age, respectively (data not shown in table).
Mixed HV schedules were reported for 2018 and 2019 as the new DTaP5-IPV-HB-Hib vaccine was introduced at the beginning of 2018 ().
In 2018 a complete vaccination schedule with DTaP3-IPV-HB/Hib, was administered in 19.079 (67.0%) newborns and the mean age (in days of age) at administration was of 73-148-345 respectively at first, second and third dose.
Complete DTaP5-IPV-HB-Hib schedule was administered in 4,334 (15.2%) newborns and the mean age at administration was of 82-156-380 (days of age) respectively at first, second and third dose.
During 2018 mixed schedules were also administered. In particular:
Mixed schedule DTaP3-IPV-HB/Hib -DTaP3-IPV-HB/Hib- DTaP5-IPV-HB-Hib was administered in 4,798 (16.9%) newborns and the mean age (days of age) at administration was 78-170-408 respectively at first, second and third dose.
Mixed schedule DTaP3-IPV-HB/Hib – DTaP5-IPV-HB-Hib – DTaP5-IPV-HB-Hib was administered in 257 (0.9%) newborns and the mean age at administration was 87-320-508 (days of age), respectively at first, second and third dose.
In 2019 no vaccination schedule with three doses DTaP3-IPV-HB/Hib was reported and 22,039 (84.0%) newborns received a complete DTaP5-IPV-HB-Hib schedule (76-157-382 days of age respectively at first, second and third dose).
During 2019 mixed schedules were also administered. In particular:
Mixed schedule DTaP3-IPV-HB/Hib -DTaP3-IPV-HB/Hib- DTaP5-IPV-HB-Hib was administered in 4,097 (15.2%) newborns and the mean age (days of age) at administration was 79-171-402 respectively at first, second and third dose.
Mixed schedule DTaP3-IPV-HB/Hib – DTaP5-IPV-HB-Hib – DTaP5-IPV-HB-Hib was administered in 116 (0.4%) newborns and the mean age at administration was 89-312-499 (days of age), respectively at first, second and third dose.
Mean age at second and third dose of children vaccinated with mixed schedule was significantly higher (p-value <.01) both in 2018 and 2019 in comparison with children vaccinated with standard schedule (data not shown in table).
Older children (vaccinated above recommended age)
In are described the total number of newborns that received vaccination above recommended age.
Table 2. Overall newborns (divided in age classes) vaccinated against HV above recommended age.
In 2016, the total number of children who received at least one dose of DTaP3-IPV-HB/Hib above the recommended age was 7,444 on 29,348 (25.4%) doses administered in the 5 LHAs involved in the study. In particular, a total of 4,376 (58.7%) between 15–24 months of age, 2,776 (37.2%) between 24–36 months of age and 292 (4%) >36 months of age.
In 2017 a total of 7,390 on 28,761 (25.7%) doses administered in the 5 LHAs involved in the study received at least one dose of DTaP3-IPV-HB/Hib above the recommended age, 4,911 (66.4%) newborn (between 15–24 months of age) received at least one dose of DTaP3-IPV-HB/Hib after 15 months of age, counting 1,508 (20.4%) (between 24–36 months of age) and 971 (13.1%) (>36 months of age).
In 2018 the total number of newborns who received at least one dose of DTaP5-IPV-HB-Hib after 15 months of age was 3,999 out of 28,468 total HV administered (14.1%), including 2,116 (53%) (between 15–24 months of age), 977 (24.4%) (between 24–36 months of age) and 906 (22.6%) (>36 months of age).
In 2019, the total number of newborns who received at least one dose of DTaP5-IPV-HB-Hib after 15 months of age was 2,859 out of 26,252 (10.9%) total HV administered, including 2,186 (76.4%) (between 15 and 24 months of age), 569 (20%) (between 24 and 36 months of age) and 104 (3.6%) (>36 months of age).
A significant decreasing trend (chi square for trend: 1,856; p-value <.001) of children vaccinated above recommended age on total HV administered in the 5 Sicilian LHAs involved in the study, was observed from 2016 to 2019.
Over the study period a total of 21,692 infants received their HV doses above the recommended age: the majority (62.6%) completed their schedule within 15–24 months of age, a relevant percentage accounting for a total of 2,273 (10.5%) infants completed the schedule >36 months and 5,830 (26.9%) completed the schedule between 24 and 36 months of age.
Pediatric vaccines co-administration (PCV and rotavirus)
Vaccine co-administration with PCV
Overall in the five LHAs considered, vaccine co-administration with PCV (PCV13) was reported for above 84.2% (peaking up to 88.5% in 2016) during the entire study period.
The higher percentage of co-administration was reported with the first dose of the HV vaccine. Details by the five LHAs involved in the study, year and timing with respective schedule are shown in . These data show that the co-administration trend is consistent with data previously reported for the VCR. In particular, in AG co-administration was maintained high over the study period according to 1st, 2nd and 3rd doses 96.3%, 96.4% and 92.7% in 2016–92.6%, 92.3% and 90.7% in 2019.
Table 3. Pediatric vaccine co-administration HV with PCV13 and RV1in 5 LHAs in Sicily from 2016–2019. (Note: during 2018 and 2019 both HV vaccines were used, coadministration data are reported independently whit HV administered).
In CT the percentage of co-administration were lower as for the VCR reported observed were 90.2%, 89.1% and 88.6% in 2016 and 92.0%, 90.6% and 88.7%.
Vaccine co-administration with RV1
Regarding the co-administration with RV1 in all the Sicilian LHAs considered in the study accounted to 64.7%.
Excepting RG and CT, it was reported around 70% for 1st and 2nd dose. In RG LHA the co-administration was reported around 50% for both doses. In CT percentage of co-administration of HV with RV was considerably lower, around 40%, for both doses in the cohorts considered. Details by province, year and schedule are listed in .
Discussion
Nowadays HV vaccines in Italy and Europe are largely used and several data are available regarding their immunogenicity, safety and tolerability profile. In Italy, three different HV vaccines are currently available. Despite their profile similarities, differences are acknowledged particularly concerning formulation, pertussis number and type of antigens included, adjuvant type, antigens amount as well as Hib early/late response.
Differences can also be observed in clinical practices across geographies depending on several reasons.
In consideration of a specific SumPc, aspects related to administration timing, schedule completion and compliance, age limit administration, other pediatric vaccines co-admin may vary not only at national or regional level but also at LHA level. The above aspects are strictly connected and can affect overall VCR.
In Italy, a mandatory Vaccines Decree (Decree Law No. 73 of 7 June 2017, “Urgent provisions on vaccine prevention,” amended by the Law No. 119 of 31 July 2017) increased the number of compulsory vaccinations in childhood and adolescence, for school attendance, from four to ten antigens: those contained in the HV and those against mumps, measles, rubella and varicella.Citation2
The aim was to address the reported progressive decline in vaccinations coverage rates. In particular, the observed decrease was below the recommended WHO threshold (95%) to ensure ‘herd immunity.’Citation27
Although reporting a VCR above 90% (this article show that VCR of the HV in the region remained above 91% over the study period) this percentage is still far from the aimed optimal VCR as stated above. Noteworthy, in the province (LHA) of AG, VCR resulted above 95% over the entire study period. In the Catania LHA vaccination coverages were lower, but this is in line with a historical trend that shows eastern Sicily always having lower coverages than western Sicily. Some hypotheses can be made on the reasons for this: poor accessibility to vaccination centers and insufficient promotion of vaccination by pediatricians.
Several studies have compared immunization with schedules containing vaccines of different manufacturers, demonstrating good immunological response. Vaccines containing diphtheria, tetanus, poliovirus, HepB and Hib antigens are interchangeable.Citation28
Recent data also support the administration of DTaP5-IPVHB-Hib in a mixed primary series schedule (hexa/penta/hexa) including PRP-OMPC/PRP-T/PRP-OMPC, with no concerns over immunity response or safety.Citation29 Vaccination with different vaccines containing similar antigens is deemed acceptable if the timeliness of the immunization of the child may be affected, or if the vaccine administered previously is unknown. Mixed HV schedules were reported for 2018 and 2019 as a new HV vaccine was introduced. During 2018 mixed schedules were administered in 17% of the children (DTaP3-IPV-HB/Hib -DTaP3-IPV-HB/Hib- DTaP5-IPV-HB-Hib in 16.9% and DTaP3-IPV-HB/Hib – DTaP5-IPV-HB-Hib – DTaP5-IPV-HB-Hib in 0,9%).
In 2019 the 15.4% of the children received mixed vaccination schedule: DTaP3-IPV-HB/Hib – DTaP3-IPV-HB/Hib – DTaP5-IPV-HB-Hib was administered in 15% and DTaP3-IPV-HB/Hib – DTaP5-IPV-HB-Hib – DTaP5-IPV-HB-Hib was administered in 0.4%.
Over the study period a total of 21,692 infants received their HV doses above the recommended age and a relevant percentage accounting for a total of 2,273 infants completed the vaccination schedule >36 months. A study from Stein-Zamir et al. demonstrated that vaccination delay was associated with high child’s birth order, low socio-economic status, ethnicity, season of birth (winter) and delayed receipt of DTaP-IPV-Hib vaccine 1st dose.Citation30
Sicilian data, commonly with previous studies, demonstrated that the general delay in the receipt of the first, second or third dose of HV is the main reasons reported by parents of children vaccinated above recommended age.
It would be interesting to assess further, analyzing the specific data from the parents of children vaccinated above the recommended age, the main reasons for the delay of HV vaccinations in Sicily (also in comparison with Italy), such as the late inscription to kindergarten of the newborns that are typical of Southern Regions of Italy, mainly due to the grandparents role of take care of children during the first years of life.
At the same time, in the five Sicilian LHAs involved in the study, the introduction of the mandatory vaccines decree for school attendance strongly contributed to the anticipation of the hexavalent vaccination schedule, reducing significantly the number of vaccines administered above recommended age.
There is also a substantial experience with HV vaccines co-administered with other pediatric vaccines routinely recommended, thus not affecting immune response and safety profile. These include PCVs, rotavirus, meningococcal conjugate, measles, mumps, rubella, and varicella vaccines.Citation31 In Sicily vaccine co-administration with PCV (PCV13) was reported for above 85% (peaking up to 88.5% in 2016) during the entire study period. Esposito et al. studied the concomitant use of a routine hexavalent vaccine with either PCV7 or PCV13, comparing both groups and the immunogenicity results from this study showed no significant differences between PCV13 and PCV7 in the responses to concomitantly administered hexavalent vaccine.Citation32
Concomitant vaccination with hexavalent vaccines has been implemented in different studies. Vesikari et al. demonstrated that the three hexavalent vaccines have been proved to be not inferior to each other for immunogenicity and safety profile, even in co-administration with anti-pneumococcal and anti-rotavirus vaccination and this co-administration did not impair in any way the immune response to the antigens, with antibody titers concordant with the seroprotective levels.Citation33
Limits of the study
Firstly, data collected from the 5 Sicilian LHAs did not allow to make a complete assessment of the vaccination coverage rates in premature children (9.768 over 4 years) due to the data not reported in the Provincial an Regional vaccination register. However, from a discussion with all the referent for pediatric vaccination of the LHAs involved in the study, we can assume that vaccination coverage at 24 months in preterm infants is in line with the coverage reported for at-term infants and that any differences should be attributed to other factors (delay in the first vaccination, socio-economic status or other that should be further investigated).
Moreover, no certain data on dTpa vaccination among pregnant women in the 5 LHAs were collected through this investigation due to a lack of registration in the Sicilian vaccination recording system.
In any case, we assume that VCR against dTpa during pregnancy is very little, due to the fact that the ministerial decree recommending vaccinations during pregnancy came into force during August 2018.Citation34 Furthermore, the study conducted by Costantino et al. in a selected setting such as pre-natal classes in the years 2019–2020 (after the decree of August 2018) showed that the baseline vaccination coverage in a sample of mother attending childbirth classes in the Province of Palermo (one of the LHAs involved in the study) for dTpa was just over 5%.Citation35
Conclusion
So far, no data have been published specifically addressing HV clinical practice and interchangeability in Europe and in Italy. This retrospective analysis can contribute to manage potential disruptions occurring in specific context (such as catch up as well as interchangeability scenarios) due to missed routine immunization opportunities including also pandemic-related ones.Citation36
Despite the introduction of compulsory vaccination HV vaccination coverage was sub-optimal even before the pandemic as reported above. Now the post-pandemic arises additional challenges for the health care professional and the population.
This data mapping will also show any difference in schedule completion and VCR between pre- and post-pandemic.
This could further support health care decision makers in order to evaluate future switch of vaccination offer against HV at Local, Regional or National context.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Esposito S, Bonanni P, Maggi S, Tan L, Ansaldi F, Lopalco PL, Dagan R, Michel J-P, van Damme P, Gaillat J, et al. Recommended immunization schedules for adults: clinical practice guidelines by the Escmid Vaccine Study Group (EVASG), European Geriatric Medicine Society (EUGMS) and the World Association for Infectious Diseases and Immunological Disorders (WAidid). Hum Vaccin Immunother. 2016;12(7):1–9. doi:10.1080/21645515.2016.1150396.
- Agenzia Italiana del Farmaco. Vaccini Obbligatori e a Offerta Gratuita e Attiva (Legge 119/2017). [accessed 2022 July 5]. https://www.aifa.gov.it/vaccini-obbligatori-e-a-offerta-gratuita-e-attiva-legge-119-2017#: {}:text=ANTI%2DEPATITE%20B,ENGERIX%20B%20uso&text=HEXYON%20(EPATITE%20B%20%2D%20POLIO%20%2D,%2D%20EPATITE%20A)%20%2D%20uso%20intramuscolare.
- Italian Ministry of Health. National vaccination plan 2017–2019. [accessed 2022 July 5]. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Obando-Pacheco P, Rivero-Calle I, Raguindin PF, Martinón-Torres F. DTaP5-HBV-IPV-Hib pediatric hexavalent combination vaccine for use in children from 6 weeks through to 4 years of age. Expert Rev Vaccines. 2019;18(11):1115–26. doi:10.1080/14760584.2019.1690457.
- Block SL, Klein NP, Sarpong K, Russell S, Fling J, Petrecz M, Flores S, Xu J, Liu G, Stek JE, et al. Lot-to-lot consistency, safety, tolerability and immunogenicity of an investigational hexavalent vaccine in US infants. Pediatr Infect Dis J. 2017;36(2):202–08. doi:10.1097/inf.0000000000001405.
- Vesikari T, Becker T, Vertruyen AF, Poschet K, Flores SA, Pagnoni MF, Xu J, Liu GF, Stek JE, Boisnard F, et al. A phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at two, three, four and twelve months. Pediatr Infect Dis J. 2017;36(2):209–15. doi:10.1097/inf.0000000000001406.
- Decker MD. MPH principles of pediatric combination vaccines and practical issues related to use in clinical practice. Pediatr Infect Dis J. 2001;20(11):S10–8. doi:10.1097/00006454-200111001-00002.
- Hexyon®, RCP. AIFA website. https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/farmaco?farmaco=04281.
- Vaxelis®, RCP. AIFA website.
- GlaxoSmithKline. Summary of product characteristics (Infanrix hexa™). Rixensart: GlaxoSmithKline; 2002.
- Tregnaghi MW, Zambrano B, Santos-Lima E. Immunogenicity and safety of an investigational hexavalent diphtheria-tetanus-acellular pertussis-inactivated poliovirus-hepatitis B-Haemophilus influenzae B conjugate combined vaccine in healthy 2-, 4-, and 6-month-old Argentinean infants. Pediatr Infect Dis J. 2011;30(6):e88–96. doi:10.1097/INF.0b013e318212eb80.
- Maman K, Zöllner Y, Greco D, Duru G, Sendyona S, Remy V. The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin Immunother. 2015;11(9):2132–41. doi:10.1080/21645515.2015.1044180.
- Chiappini E, Petrolini C, Caffarelli C, Calvani M, Cardinale F, Duse M, Licari A, Manti S, Martelli A, Minasi D, et al. Hexavalent vaccines in preterm infants: an update by Italian Society of Pediatric Allergy and Immunology jointly with the Italian Society of Neonatology. Ital J Pediatr. 2019;45(1):145. doi:10.1186/s13052-019-0742-7.
- Orsi A, Azzari C, Bozzola E, Chiamenti G, Chirico G, Esposito S, Francia F, Lopalco P, Prato R, Russo R, et al. Hexavalent vaccines: characteristics of available products and practical considerations from a panel of Italian experts. J Prev Med Hyg. 2018;59(2):E107–19.
- European Medicine Agency. Rotatix summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/documents/product-information/rotarix-epar-product-information_en.pdf.
- European Medicine Agency. Rotateq Summary of Product Characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/documents/product-information/rotateq-epar-product-information_en.pdf.
- European Medicine Agency. Prevenar Summary of Product Characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar#product-information-section.
- European Medicine Agency. Prevenar 13 summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar-13.
- European Medicine Agency. Synflorix summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/synflorix.
- European Medicine Agency. Bexsero summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/bexsero.
- European Medicine Agency. Menveo summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/menveo.
- European Medicine Agency. Nimenrix summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/nimenrix.
- European Medicine Agency. Proquad summary of product characteristics. [accessed 2022 Mar 21]. https://www.ema.europa.eu/en/medicines/human/EPAR/proquad.
- Schmitt H-J, Steul KS, Borkowski A, Ceddia F, Ypma E, Knuf M. Two versus three doses of a meningococcal C conjugate vaccine concomitantly administered with a hexavalent DTaP-IPV-HBV/Hib vaccine in healthy infants. Vaccine. 2008;26(18):2242–52. doi:10.1016/j.vaccine.2008.02.041.
- [accessed 2022 Jul 6]. https://demo.istat.it/.
- AIFA - Area Vigilanza Post-marketing. Rapporto Vaccini 2018. Gov.It; 2019 Jul 30 [accessed 2022 Aug 11]. https://www.aifa.gov.it/documents/20142/241056/Rapporto+Vaccini+2018.pdf/62975a0e-a836-da32-f7b9-1e39196374dd.
- Plans-Rubió P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccin Immunother. 2012;8(2):184–88. doi:10.4161/hv.18444.
- Klein NP, Abu-Elyazeed R, Cheuvart B, Janssens W, Mesaros N. Immunogenicity and safety following primary and booster vaccination with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type b vaccine: a randomized trial in the United States. Hum Vaccin Immunother. 2019;15(4):809–21. doi:10.1080/21645515.2018.1549449.
- Martinón-Torres F, Boisnard F, Thomas S, Sadorge C, Borrow R. Immunogenicity and safety of a new hexavalent vaccine (DTaP5-IPV-HB-Hib) administered in a mixed primary series schedule with a pentavalent vaccine (DTaP5-IPV-Hib). Vaccine. 2017;35(30):3764–72. doi:10.1016/j.vaccine.2017.05.043.
- Stein-Zamir C, Israeli A. Age-appropriate versus up-to-date coverage of routine childhood vaccinations among young children in Israel. Hum Vaccin Immunother. 2017;13(9):2102–10. doi:10.1080/21645515.2017.1341028.
- Kwak GY, Kwon HJ, Kim JH, Kim HM, Park JS, Ma SH, Kim JG, Kang JH. The immunogenicity and safety of DTaP interchangeable immunization among Korean children. Vaccine. 2012;30(31):4644–47. doi:10.1016/j.vaccine.2012.04.094.
- Knuf M, Haas H, Garcia-Corbeira P, Turriani E, Mukherjee P, Janssens W, Berlaimont V. Hexavalent vaccines: what can we learn from head-to-head studies? Vaccine. 2021;39(41):6025–36. doi:http://dx.doi.org/10.1016/j.vaccine.2021.08.086.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han H-H, Bouckenooghe A. Immunogenicity and safety of the human rotavirus vaccine Rotarix™ co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine. 2010;28(32):5272–79. doi:http://dx.doi.org/10.1016/j.vaccine.2010.05.057.
- Italian Ministry of Health. Recommended vaccination during pregnant and childbearing age women. 2022 Apr 27. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2019&codLeg=71540&parte=1%20&serie=null.
- Costantino C, Mazzucco W, Bonaccorso N, Cimino L, Conforto A, Sciortino M, Catalano G, D’Anna MR, Maiorana A, Venezia R, et al. Educational interventions on pregnancy vaccinations during childbirth classes improves vaccine coverages among pregnant women in Palermo’s province. Vaccines (Basel). 2021;9(12):1455. doi:10.3390/vaccines912145.
- Guglielmi G. Pandemic drives largest drop in childhood vaccinations in 30 years. Nature. 2022 Aug;608(7922):253. doi:10.1038/d41586-022-02051-w.